Chronic exposure to environmental chemicals, adversely affecting the health of large numbers of people, is a global problem. Among the chemicals, lead continues to pose adverse health effects because it is related to a broad range of physiological, biochemical and behavioural dysfunctions(Reference Ahamed and Siddiqui1). Lead induces oxidative stress to the brain, heart, kidney and reproductive organs by affecting membranes, DNA and antioxidant defence systems of cells(Reference Ahamed and Siddiqui1). Aside from lipid peroxidation reactions evidenced by elevations in malondialdehyde, lead appears to enhance oxidative damage to DNA as shown by an increase of 8-hydroxydeoxyguanosine (8-OHdG)(Reference Courtois, Marques and Barrientos2–Reference Dursun, Dogan and Donmez4). In epidemiological studies, blood lead levels (BLL) have been reported to have adverse health impacts such as oxidative stress, mortality and raised homocysteine levels(Reference Lee, Lim and Song5–Reference Menke, Muntner and Batuman7).
Oxidative stress is implicated in the aetiology of many diseases and considered as an important pathophysiological process(Reference Mayne8, Reference Young and Woodside9). Antioxidant nutrients are thought to modulate oxidative damage by influencing a balance between free radical production and antioxidant capacity(Reference Mayne8). A number of studies have shown that antioxidant nutrients such as vitamin C, vitamin E and/or β-carotene may decrease the biomarkers of oxidative stress, although some studies indicate a harmful effect of high-dose antioxidant supplements, particularly β-carotene(Reference Mayne8, Reference Loft, Fischer-Nielsen and Jeding10). The inverse association between vitamin C intake and lead exposure has also been reported(Reference Dawson, Evans and Harris11).
A few studies have examined the interaction between diet and environmental exposure with regard to health. Arora et al. (Reference Arora, Ettinger and Peterson12) found maternal dietary PUFA as a modifier in the transfer of lead from bone to breast milk. A possible modifying effect of arsenic exposure on the relationship between nutrition and arsenic-induced health outcomes has also been suggested(Reference Vahter13). Even though there were a few studies examining the interaction between nutrition and environmental exposure on health outcomes, the relationship between dietary antioxidants and oxidative stress has not been investigated in terms of BLL. The Biomarker Monitoring for Environmental Health was conducted to examine the health effects of environmental chemical exposure in the adult population. As part of the investigation, we evaluated whether the association between dietary antioxidants and oxidative stress in urban adults is modified by the degree of lead exposure.
Materials and methods
Participants and data collection
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of the Ewha Womans University Hospital and Inha University Hospital. Written informed consent was obtained from all participants when they visited the hospitals for health check-ups. Briefly, between April and December 2005, the Biomarker Monitoring for Environmental Health (principal investigator, Y.-C. H.) recruited 1131 adults, 40 years old or more, from two large cities, Seoul and Incheon, in Korea, who agreed to participate. Because we did not limit to one person per family, married couples could enrol into the study. We chose the cities because they are large cities with population sizes over 2·5 million and close enough to share the same urban lifestyle. Seoul is much larger and more metropolitan than Incheon, which is a harbour city on the west coast of the Korean Peninsula.
Of those 1131 participants, we excluded 124 adults with a previous history of disease that could affect the levels of oxidative stress markers, such as cancer (n 31), stroke (n 14), myocardial infarction (n 32), current medication for tuberculosis (n 3), acute hepatitis (n 2), chronic bronchitis (n 9) and arthritis (n 33). We further excluded 292 adults whose BLL were missing, and thirty-two adults whose energy intake was either less than 2092 kJ (500 kcal) or greater than 20 920 kJ (5000 kcal)(Reference Willett and Stampfer14). As a result, a total of 683 adults were included in the data analyses. There was no difference of BLL and dietary intakes between our participants and the 124 adults excluded from the analyses due to their health conditions. The remaining 324 adults excluded due to lack of BLL or inappropriate energy intake values had lower proportions of males, smokers and drinkers and received less education than those included in the analyses.
Trained interviewers collected information on demographics, lifestyle habits, medical history, dietary intake and environmental exposure using a structured questionnaire. The height and weight of each subject were assessed according to standard recommended procedures. After the interviews, fasting blood and single urine samples were collected and stored at − 70°C until analysis. A sample of up to 3 ml of each participant's blood was collected in a BD vacutainer (Becton Dickinson Company) containing K2 EDTA (Becton Dickinson Company) and preserved at − 20°C.
Measurement of biomarkers
As an oxidative stress biomarker, urinary levels of 8-OHdG were measured using the New 8-OHdG Check ELISA kit (JaICA) according to the manufacturer's instructions. The levels were divided by urinary creatinine concentrations to adjust the dilution in urine.
We used whole blood for the measurement of blood lead concentrations. Blood samples were diluted with 0·1 % Triton X-100 solution, and then analysed by atomic absorption spectrometry on an Analyst 100 instrument (Perkin-Elmer) using a graphite tube atomiser (HGA 800; Perkin-Elmer GmbH). Lead concentrations were quantified in 2H background correction mode. The limit of detection was 0·097 μg/dl. The respective intra- and inter-assay CV for the blood lead concentrations were 2·9 and 5·3 % and those for the urinary 8-OHdG concentrations were 5·2 and 7·5 %.
We measured urinary creatinine levels using a HITACHI 7600 instrument (HITACHI). Briefly, 10 ml of urine and 300 ml of picric acid solution (Wako) were mixed at room temperature for 3 min, and absorbance of the solution was measured at 505 nm. Following this, 75 μl of alkaline solution (Wako) were added for 4 min, and absorbance of the solution was measured at 570 nm. The mean of the two values measured at 505 and 570 nm was used as the creatinine level.
Dietary assessment
We assessed usual dietary intake by a validated semi-quantitative FFQ used in the Korean Genome Epidemiologic Study(Reference Ahn, Kwon and Shim15). Respective median correlation coefficients were 0·45 and 0·39 (P < 0·01) for reproducibility and validity of this instrument(Reference Ahn, Kwon and Shim15). The FFQ contains 103 food items with nine non-overlapping frequency response categories, ranging from ‘rarely use’ to have ‘three or more times per day’ in reference to the preceding year. For fruit items, participants were asked to mark one category among four on how long they ate the items: 3, 6, 9 and 12 months. For all foods, the possibility of specifying the use of a small, average or large portion was offered. For easy understanding of portion size, we provided pictures on serving size for food items on their own pages.
The amount of each food item included in the FFQ was converted into grams, from which the daily intakes of nutrients were calculated using DS 24 (Human Nutrition Lab, Seoul National University & AI/DB Lab, Sookmyung Women's University, 1996). For fruit and vegetable intakes, daily servings were calculated based on intake frequencies and portion size options. We divided vegetable intake into kimchi and other vegetables due to the relatively high Na content of kimchi.
Potential confounders
We assessed smoking (never, past smoker, current smoker), alcohol consumption (never, past drinker, current drinker), nutrition supplement intake (yes/no), exercise (yes/no) and educational levels (nine categories from no schooling to graduate school or more) with a structured questionnaire. BMI was calculated using height and weight values.
Statistical analysis
BLL and 8-OHdG values were log10-transformed because of their skewed distribution. Intake variables were log-transformed due to similar reasons. All intake variables were adjusted for total energy by taking the residual from a linear regression model in which total energy intake was the independent variable and the respective intake variable was the dependent variable(Reference Willett and Stampfer14). We evaluated the association between general characteristics and either BLL or 8-OHdG by one-way ANOVA or simple regression. The Scheffé multiple comparison test was performed as appropriate(Reference Cody and Smith16). Partial correlation analyses were used to find the relationship of dietary antioxidants with BLL or 8-OHdG.
We analysed the association between oxidative stress and intake variables in terms of BLL levels using multivariate linear regression models. We obtained the relative change of oxidative stress level by the inter-quartile range of the nutrients and the daily intake frequencies of fruit and vegetables in Q1–Q3 BLL groups as compared to the Q4 BLL group. The analytical models included main and interactive effects of BLL and the intake variable as well as key covariates including age, education, BMI, total energy intake as continuous variables and sex, nutrition supplement, alcohol consumption, smoking and exercise as categorical variables. There was no difference of interactive effects between BLL and dietary variables by sex; and thus we reported data for the total sample. The results were reported as log10 β-coefficients with their standard errors of oxidative stress (8-OHdG). Significance was set at P < 0·05.
Results
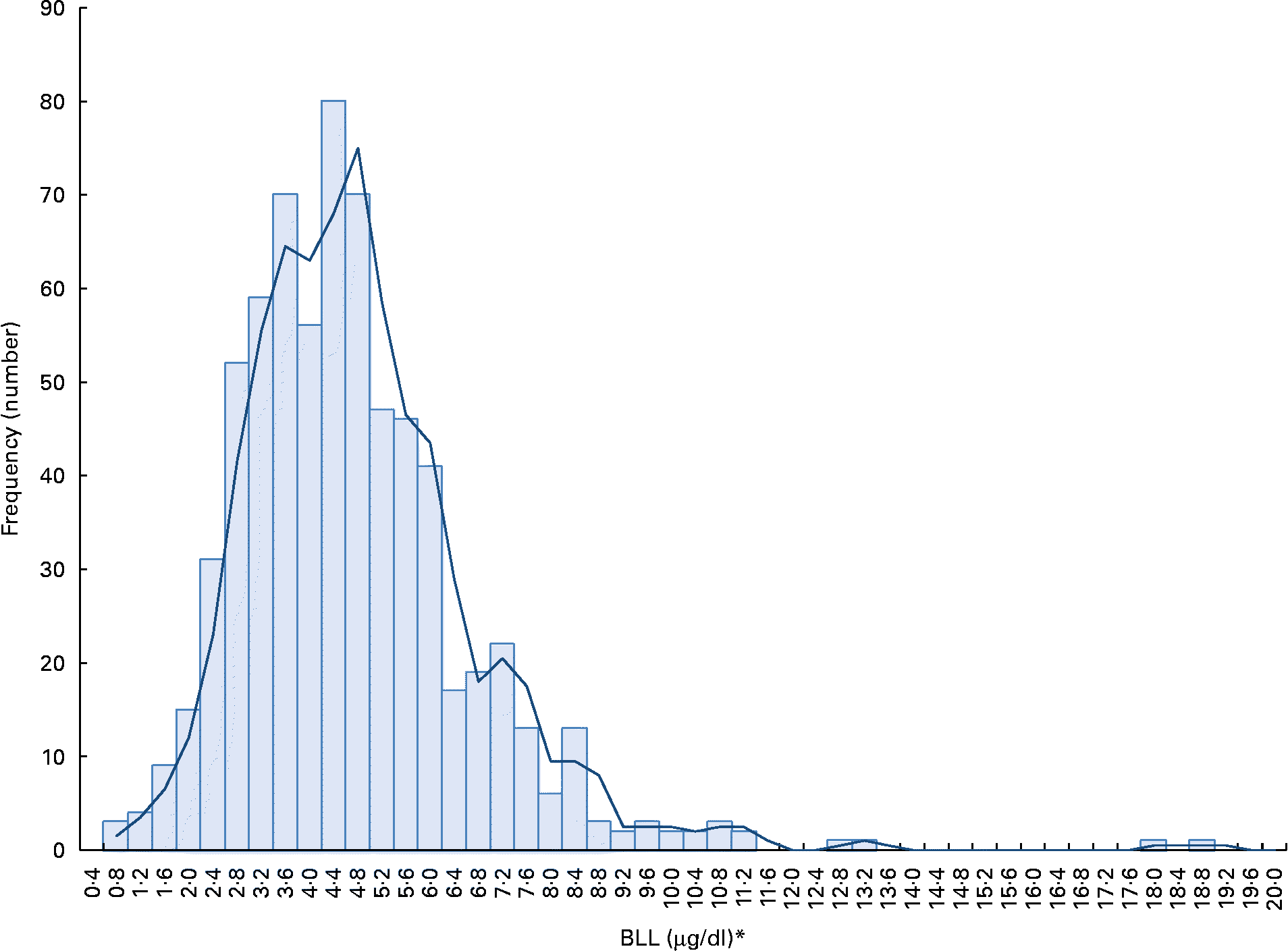
The participants' BLL, except for a few cases, were relatively normally distributed (Fig. 1). The geometric mean of BLL was close to 4 μg/dl (Table 1). The geometric mean of the highest quartile BLL group (6·78 μg/dl) was 2·9-fold higher than the lowest quartile group of BLL (2·36 μg/dl). Cut-off points of BLL were ≤ 3·18 and >5·36 μg/dl for the lowest and the highest BLL groups, respectively. Only 1·0 % had a BLL >10 μg/dl, which is a critical value established by the US Centers for Disease Control and Prevention (CDC)(17). The geometric mean of urinary 8-OHdG was 5·43 μg/g creatinine, showing a wide range of distribution. The level of urinary 8-OHdG was 4·7-fold greater in highest quartile group of 8-OHdG than the lowest 8-OhdG group.

Fig. 1 Distribution of blood lead levels (BLL) by frequency (n 683). * SI unit conversion: BLL (mmol/l) = 0.04826 × BLL (μg/dl). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Table 1 Blood lead levels (BLL) and urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels by BLL groups (Mean values and standard deviations, n 683)

GM, geometric mean; GSD, geometric standard deviation; Q, quartile.
* SI unit conversion: BLL (mmol/l) = 0·04826 × BLL (μg/dl).
† SI unit conversion: 8-OHdG (μmol/mol) = 0·399 × 8-OHdG (μg/g creatinine).
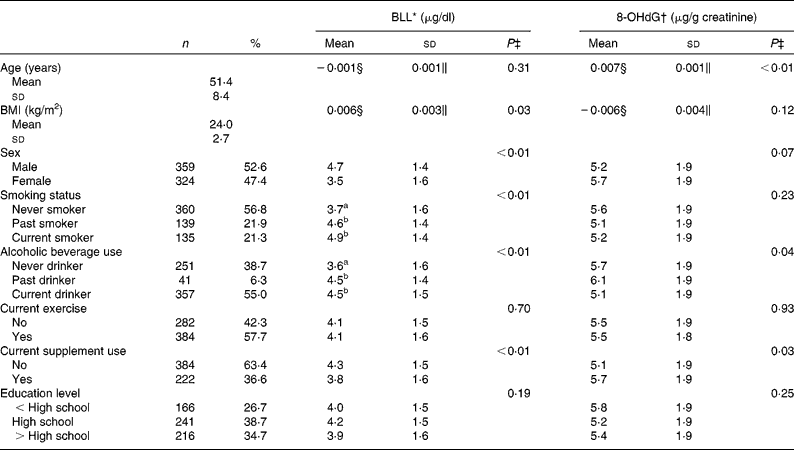
The mean age of the participants was 51·4 years and approximately half of them were females (Table 2). Proportions of current smokers and drinkers were 21 and 55 %, respectively. BLL were higher in participants that were males, smokers, alcohol drinkers or supplement non-users (Table 2). Excretion of urinary 8-OHdG was positively related to age, alcohol consumption and supplement use.
Table 2 Background characteristics and their association with blood lead levels (BLL) and urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels (Number of studies, percentages, mean values and standard deviations, n 683)

a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05, Scheffé multiple comparison test).
* SI unit conversion: BLL (mmol/l) = 0·04826 × BLL (μg/dl).
† SI unit conversion: 8-OHdG (μmol/mol) = 0·399 × 8-OHdG (μg/g creatinine).
‡ Simple regression or one-way ANOVA.
§ Log10 β
∥ Log10 se.
Among the participants, 50 and 65 % met the Korean Dietary Reference Intakes (DRI)(18) for vitamin A and C, respectively (Table 3). Approximately one-third of them had vitamin E and energy intakes above the Korean DRI. Participants consumed 3·3 and 3·5 servings of kimchi and other vegetables, respectively, as well as 1·0 serving of fruits per day, on average. Intakes of antioxidant nutrients and related foods were not associated with BLL and 8-OHdG after controlling for age and sex.
Table 3 Daily antioxidant-related nutrient and food intakes and their associations with blood lead levels (BLL) and urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels (Mean values and standard deviations, medians and ranges, n 683)

KDRI, Korean Dietary Reference Intake; RE, retinol equivalent; TE, tocopherol equivalent.
* Spearman correlation coefficients with adjustment of age, sex and total energy except for total energy that was analysed including age and sex as covariates.
† Average one serving is approximately 50 g for kimchi and 35 g for other vegetables.
‡ Average one serving size is approximately 150 g.
In multivariate analyses, there were significantly greater negative associations of 8-OHdG with vitamin C and E intakes in the lowest quartile group of BLL ( ≤ 3·18 μg/dl) as compared to the highest quartile BLL group (>5·36 μg/dl) after controlling for potential confounders (Table 4). The magnitude of the association was higher for vitamin E than vitamin C. Similarly, the relationship between the vegetable intake, except for kimchi, and the 8-OHdG concentration was greater in the lowest quartile group of BLL than the highest BLL group. The associations between 8-OHdG and the intakes including vitamin A, β carotene, kimchi and fruits did not differ by BLL. There was a main effect of BLL on 8-OHdG when the model included vegetables or fruits as an intake variable.
Table 4 Multivariate linear regression coefficients for the association of urinary 8-hydroxy-2′;-deoxyguanosine (8-OHdG)* with daily antioxidant nutrients and related food intakes in the quartiles (Q1–Q3) of blood lead levels (BLL) as compared to the Q4 of BLL (n 550)

* SI unit conversion: 8-OHdG (μmol/mol) = 0·399 × 8-OHdG (μg/g creatinine).
† SI unit conversion: BLL (mmol/l) = 0·04826 × BLL (μg/dl).
‡ Represents P-values of the covariate-adjusted main effect of each intake (continuous) variable on 8-OHdG.
§ Represents P-values of the covariate-adjusted main effect of BLL (categorical variable) on 8-OHdG.
∥ Represents P-values of the covariate-adjusted relationship between intake and BLL variable with 8-OHdG.
¶ Log10 8-OHdG values corresponding to the inter-quartile range of log nutrient or one serving of vegetable and fruit intake after adjustment for main effects of BLL and intake measure as well as potential confounders such as age, sex, education level, alcoholic consumption, smoking, exercise, supplement use, BMI, and total energy intake.
Discussion
Our cross-sectional study on urban adults showed that BLL modified the relationship between dietary antioxidants and oxidative stress. We found more of a decrease of oxidative stress by the increase of dietary antioxidants only in those from the lowest quartile of BLL ( ≤ 3·18 μg/dl) compared with their counterparts in the highest quartile of BLL (>5·36 μg/dl). These findings show that dietary antioxidant intakes play a role regarding the reduction of oxidative stress, particularly when accompanied with low BLL ( ≤ 3·18 μg/dl) in urban adults. Previous studies have also proposed a stronger effect of antioxidants in persons with low levels of lead exposure(Reference Loft, Møller and Cooke19, Reference Hsu and Guo20); yet none of them have attempted to examine the interaction effect of BLL and dietary antioxidant in relation to oxidative stress like the present study.
Arora et al. (Reference Arora, Ettinger and Peterson12) reported that maternal dietary intake of PUFA modified the transfer of lead from maternal bone to breast milk. Among 301 Mexican women, an increase in patella lead was associated with higher breast milk lead level in the lowest tertile of the PUFA group compared with the highest tertile of the PUFA group(Reference Arora, Ettinger and Peterson12). Previous studies have proposed a stronger effect of antioxidants on BLL in persons with low lead exposure(Reference Loft, Fischer-Nielsen and Jeding10, Reference Hsu and Guo20). Blood nutrient levels were reported to be related to hazardous substances in the high-exposure group(Reference Li, Ekström and Goessler21). In pregnant Bangladeshi women, concentrations of metabolites of inorganic arsenic were inversely associated with plasma folate and Zn levels in only those with high arsenic exposure (>209 μg/l of urinary arsenic)(Reference Li, Ekström and Goessler21).
The modifying role of BLL found in the present study is relevant to the inverse role of BLL and dietary antioxidants regarding oxidative stress. 8-OHdG is one of the most critical lesions generated from deoxyguanosine by oxygen free radicals; and thus urinary 8-OHdG is considered a measure of DNA oxidation in response to free radicals(Reference Arimoto, Yoshikawa and Takano22, Reference Harri, Svoboda and Mori23). Antioxidants are thought to be effective free-radical scavengers while protecting DNA from oxidative damage. Vitamin C has been suggested as a possible chelator of lead, with similar potency to that of EDTA(Reference Goyer and Cherion24). Vitamin E, known to protect biological membranes and lipoproteins from oxidative stress, was reported to prevent lead from affecting the production of free radicals in the liver(Reference Packer25). β-Carotene was thought to mediate lipid peroxidation(Reference Ahamed and Siddiqui1).
Conversely, lead exposure has been shown to induce oxidative stress(Reference Ahamed and Siddiqui1, Reference Lee, Lim and Song5). High to moderate doses of lead exposure appeared to generate free radicals, which in turn result in oxidative damage to critical biomolecules, lipids, proteins and DNA(Reference Ahamed and Siddiqui1). In epidemiological studies, adverse health impacts of BLL were also found not only in high-level but also low-level lead exposure populations. Among adults with low-lead exposure (mean BLL of 2·8 μg/dl), there was a graded positive association between BLL and serum γ-glutamyltransferase, a marker of oxidative stress(Reference Lee, Lim and Song5). BLL was associated with enhanced homocysteine levels, in adults with a mean BLL of 3·5 μg/dl(Reference Schafer, Glass and Bressler6). Considering the links between antioxidant nutrient or related food intakes and oxidative damage(Reference Loft, Møller and Cooke19, Reference Li, Jia and Chen26), it is important that the role of nutrition on health outcomes is modified by the degree of lead levels even in populations with a low-level lead exposure.
We also found a greater inverse relationship between vegetables excluding kimchi and 8-OHdG excretion in the lowest BLL group than the highest BLL group. Kimchi, a pickled and fermented cabbage or radish, is the most important source of vegetables in Korea. Kimchi is also a major intake source of Na, which is a risk factor for hypertension and elevated blood pressure, as is lead exposure(Reference Ahamed and Siddiqui1). Animal studies have shown that high salt intake enhances blood pressure during the development of hypertension via oxidative stress(Reference Koga, Hirooka and Araki27, Reference Ketonen and Mervaala28). For this reason, we divided vegetables into kimchi and other vegetables excluding kimchi. Only the intake of other vegetables showed a greater inverse association with the oxidative stress marker 8-OHdG in participants with the lowest quartile of BLL ( ≤ 3·18 μg/dl) than those in the highest BLL group (>5·36 μg/dl). Thus, it is plausible to assume that the beneficial role of vegetable intake is weakened by its Na content in addition to the extent of lead exposure.
Unlike other vegetable intake, we found a null association between fruit intake and oxidative stress regardless of BLL in multivariate analyses. Similar to our findings, a review of cancer risk also reported that vegetable rather than fruit intake had a more beneficial role with respect to the reduction of cancer risk(Reference Kim29). Besides, a uniformly low consumption of fruit in the participants of this study would be one of the reasons for the null relationship of fruit consumption with outcome measures. Median fruit intake (0·6 servings/d) of our participants was about 10 % of vegetable consumption (5·6 servings/d).
Our study has some limitations. Even though we used a dichotomous measure of nutrition supplement intake, a lack of quantitative assessment of antioxidant nutrient supplements may underestimate true associations of antioxidants with BLL and oxidative stress. As with any other dietary studies using FFQ, recall bias would be a concern. We used the immunoassay methodology utilising primary monoclonal antibody (clone N45·1) to detect 8-OHdG. However, this ELISA assay could lead to overestimation of 8-OHdG because a variety of components in urine, particularly urea, can cross-react with the N45·1 antibody(Reference Song, Li and Ootsuyama30). Therefore, urea removal by enzymatic decomposition by urease would have improved accuracy of the measurements, but we did not treat urease before measurement of 8-OHdG. Finally, the cross-sectional nature of this study may preclude generalising the results of the study to urban adults as a whole.
This is the first study to assess the modifying role of BLL in the association between dietary antioxidants and oxidative stress. Moreover, our study is the largest study available to measure oxidative stress, BLL and dietary intake in the general population in Korea to the best of our knowledge.
In conclusion, the finding of this study that dietary antioxidants more significantly reduced oxidative stress in the low-BLL ( ≤ 3·18 μg/dl) group than the high-BLL (>5·36 μg/dl) group supports a rationale for increasing the intake of dietary antioxidants besides lowering BLL in the urban population.
Acknowledgements
The authors declare no conflicts of interest. Y.-C. H., J.-H. L. and E.-H. H. designed the research and conducted the research; S.-Y. O., S.-O. K., M.-S. P., and H. K. analysed the data; Y.-C. H. and S.-Y. O. wrote the paper. S.-Y. O. had primary responsibility for the final content. All authors read and approved the final version of the manuscript. This study was supported in part by a grant from Kyung Hee University, 2007.