Epidemiological studies indicate that a diet rich in plant foods contributes to a decreased risk of cardiovascular disorders and it is also suggested that plant sterols, especially sitosterol, might have protective properties against CVD as well as against different types of cancer(Reference Awad, Roy and Fink1). The anti-atherosclerotic action of phytosterols has been shown in many studies and it has been reported that phytosterols (β-sitosterol) inhibit the growth and development of tumours(Reference Tapiero, Townsend and Tew2, Reference Choi, Kong, Kim, Jung, Kil, Rhee and Park3). One of the postulated and already well-known mechanisms of phytosterol action in cancer cells is activation of the sphingomyelin cycle, thus leading to cell apoptosis. Other studies have shown that phytosterols might mediate apoptosis initiation through caspase activation(Reference Awad, Roy and Fink1). They were also shown to decrease the metastasis process; however, that phytosterol activity was postulated to result from incorporation of sitosterol in the cell-membrane structure resulting in a decreased cholesterol level and consequently leading to cell-cycle arrest in the G2/M phase(Reference Choi, Kong, Kim, Jung, Kil, Rhee and Park3, Reference Awad, Smith and Fink4). It was clearly shown that there are obvious correlations between individual cancer types and population, resulting from different cultural cooking traditions.
However, the mechanism of protective phytosterol action is not yet completely understood. It is supposed that the plant sterols might influence the composition of the cell membrane and its fluidity but they could also interact with the membrane enzymes, intracellular signal-transduction pathways, apoptosis and immunological functions. Moreover, oestrogenic activity of phytosterols is postulated(Reference Awad and Fink5). In many studies an anti-atherogenic action of phytosterols has been shown and been explained by a decreased number of foam cells in the plaque. Interestingly, no cytotoxic activity of phytosterols has been shown considering normal, non-cancer Chang Liver line cells; however, it has been reported that phytosterols and stanols effectively lower the serum level of LDL and thus the atherosclerosis risk(Reference de Jong, Plat and Mensink6). Clinical data have shown that a phytosterol-rich diet, consisting of vegetables, fruits and vitamins C and E, results in reduced breast cancer morbidity(Reference Ronco, De Stefani, Boffetta, Deneo-Pellegrini, Mendilaharsu and Leborgne7).
Phytosterols are known to have protective properties in endothelial cells while their oxy-derivatives are supposed to harm the cells and generate free radical reactive oxygen species(Reference Awad, Burr and Fink8). However, it is postulated that the precursor compounds, phytosterols, might also generate reactive oxygen species during their cell metabolism and thus provoke disruption of cell metabolism. Plant sterols and stanols, being more hydrophobic than cholesterol, may displace cholesterol from mixed micelles, and thus reduce the micellar cholesterol concentrations and consequently lower cholesterol absorption. Finally, plant sterols and stanols, effectively lowering serum LDL-cholesterol levels, may play an important role in atherosclerotic lesion development and plaque regression. However, their pro-apoptotic influence on endothelial cells must also be considered, especially in the context of phytosterol supplementation of hypercholesterolaemic subjects.
The aim of the study was to evaluate and compare the influence of cholesterol, phytosterols and oxysterols on human abdominal aorta endothelial cells HAAE-2 in vitro. We decided to estimate the effect of sterols on normal cells and to study the potential adverse effects of treatment for cancer or hypercholesterolaemia prevention. Sitosterol oxidises to 7α- and 7β-hydroperoxysitosterol, which is reduced to 7α-hydroxysitosterol and 7β-hydroxysitosterol; dehydration of hydroperoxysitosterol leads to the formation of 7-ketositosterol. Epoxidation of the double bond between the C5 and C6 atoms of sitosterol results in 5α,6α- and 5β,6β-epoxysitosterol, which could be converted to sitostantriol. The association of high plant sterol levels in sitosterolaemic subjects and atherosclerosis is documented in the work of Salen et al. (Reference Salen, Horak, Rothkopf, Cohen, Speck, Tint, Shore, Dayal, Chen and Shefer9). These authors confirmed the correlation between the plant sterols and atherosclerosis in an 18-year-old male with sitosterolaemia who died suddenly of an acute myocardial infarction. Their findings indicate that atherosclerosis occurs prematurely in sitosterolaemia and probably results from accelerated plasma sterol levels.
Materials and methods
Reagents
The 1-(4,5-dimethylthiazol-2-yl)-3,5- diphenylformazan (MTT) proliferation assay (Roche Diagnostics, Indianapolis, IN, USA), terminal uridine deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling (TUNEL; Roche Diagnostics), the EnzChek® caspase-3 assay kit no. 2 – Z-DEVD-R110 substrate (Invitrogen, Carlsbad, CA, USA) and trypan blue (0·4 %; Sigma-Aldrich, St Louis, MO, USA) were used.
Cell culture
Human abdominal aorta endothelial cells, HAAE-2, were purchased from the American Type Culture Collection (ATCC no. CRL-2473; Manassas, VA, USA). Cells were cultured in standard conditions providing 100 % humidity and 5 % atmosphere of carbon dioxide at 37°C. Ham's F12K medium containing 2 mm-l-glutamine, sodium bicarbonate (1·5 g/l) (American Type Culture Collection) was supplemented with 10 % fetal bovine serum, heparin (0·1 mg/ml), endothelial cell growth factor (0·03 mg/ml) and gentamycin (100 μg/ml) (all supplements from Sigma-Aldrich).
Study compounds
A β-sitosterol standard containing 13 % of sitostanol (Research Plus, Inc., Bayonne, NJ, USA) diluted in acetone was used for oxidation experiments. For cytotoxicity assays, synthetic β-sitosterol from Sigma was used. Standards of 5α,6α-epoxysitosterol, cholesterol, 5α,6α-epoxycholesterol, β-epoxycholesterol and 19-hydroxycholesterol were obtained from Steraloids Inc. (Newport, RI, USA). The oil extract was an oxyphytosterol mixture (for content, see Table 1) obtained by the thermal processing and oxidation of 1 g crude rapeseed oil. Reagents used for phytosterol oxidation and for their analyses were purchased from Sigma-Aldrich-Fluka (St Louis, MO, USA). For sample clean-up, SPE Sep-Pak NH2 columns (Waters Corp., Milford, MA, USA) were used. TLC separation was performed on Silica gel 60 F254, 20 × 20 cm, 0·25 mm (Merck, Whitehouse Station, NJ, USA).
Table 1 Oxyphytosterol content in crude rapeseed oil after heating at 180°C for 3 d*
(Mean values with their standard errors of three experiments)

nd, Not detected.
* The content of oxyphytosterols was measured as described in Materials and methods.
Identification and quantification of compounds
For the identification of phytosterol oxidation products a Trace 2000 gas chromatograph coupled to a POLARIS Q mass spectrometer (Thermo-Finnigan, Austin, TX, USA) was used equipped with a 50 m DB-5 column (50 m × 0·25 mm × 0·25 μm). The same column was used for the separation of phytosterol oxidation products by GC which was carried out on a Hewlett Packard HP 6890 gas chromatograph with a flame ionisation detector.
Oxidation of β-sitosterol
The standard of β-sitosterol was oxidised according to the method of Fieser & Fieser(Reference Fieser and Fieser10). First, β-sitosterol (2·42 g) was dissolved in 30 ml CH2Cl2 in a 100 cm3 round flask with a magnetic stirrer and kept at 25°C. Then to the flask 1·23 g m-chloroperbenzoic acid dissolved in 12 ml CH2Cl2 was added. The reaction was performed for 2 h. The surplus of oxidising reagent was eliminated by the addition of 10 % sodium sulfate. The mixture was transferred into a separatory funnel and washed twice with 5 % NaHCO3 (25 ml) to remove the m-chloroperbenzoic acid. Then the sample was washed twice with 25 ml saturated NaCl. The organic layer was then evaporated under a stream of N2 and dissolved in chloroform.
A sample of oxidised β-sitosterol was applied to the TLC silica gel plate. Epoxycholesterol was used as a reference compound. The plate was developed in ethyl ether–cyclohexane (9:1, v/v), dried, and then the part containing standards was sprayed with a developing reagent (prepared by dissolving 1 g hydrated phosphomolibdenic acid, 1 g hydrated cerium sulfate and 5·4 ml sulfuric acid in 100 ml water). The sprayed plate was dried at 120°C for 15 min. The zone corresponding to epoxysitosterol was scraped and eluted from the silica using a chloroform–methanol (2:1, v/v) mixture. Finally the solvent was evaporated and compounds identified using GC-MS (Fig. 1). The epoxysitosterol content in this fraction was estimated to be about 80 %.

Fig. 1 Total ion chromatogram of TLC scraped fraction corresponding to epoxysitosterol, which was used subsequently for the cytotoxicity assays. Defined standard of β-sitosterol was oxidised according to the method of Fieser & Fieser(Reference Fieser and Fieser10). The sample was applied to the TLC silica gel plate and epoxycholesterol was used as a reference compound. The zone corresponding to epoxysitosterol was scraped and eluted from the silica using a chloroform–methanol (2:1, v/v) mixture. Finally the solvent was evaporated and compounds identified using GC-MS.
Preparation of oxyphytosterol fraction from heated rapeseed oil
Crude rapeseed oil obtained from ZPT (Warsaw, Poland) was heated at 180°C for 8 h every day for 3 d. The oxyphytosterol content was analysed according to the procedure described by Rudzińska et al. (Reference Rudzińska, Jeleń and Węsowicz11, Reference Rudzińska, Kazuś and Węsowicz12). Briefly, 250 mg rapeseed oil was transesterified with 10 % sodium methanolate and extracted with chloroform. To isolate the oxyphytosterols the lipid mixture was fractionated by solid-phase extraction on 360 mg NH2 columns (Waters Corp., Milford, MA, USA). Isolated oxyphytosterol fractions were silylated with 75 μl SylonTM HTP (Supelco, Bellefonte, PA, USA) for 4 h at room temperature and analysed by GC-MS and GC-flame ionisation detection. As an internal standard, 19-hydroxycholesterol was used. The quantitative results are presented in Table 1. For the cytotoxicity assay the fraction of oxyphytosterols was isolated from 1 ml of heated crude rapeseed oil according to the same procedure. The sample after fractionation on Sep-Pak NH2 (Waters Corp.) was used for the analysis.
Cytotoxicity assay: 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan
Cells (4000) were seeded for 24 and 72 h in Ham's F12K medium, as specified in the Cell culture section, on ninety-six-well titration plates in the presence of test substances dissolved in acetone. At time intervals, 0·5 % MTT was added. After 16 h incubation the solubilisation solution was added (10 % SDS in 0·15 mm-HCl) in order to visualise the metabolised MTT(Reference Carmichael, DeGraff, Gazdar, Minna and Mitchell13). Absorbance intensity was measured by a Labsystems Multiscan RC reader (Labsystems, Helsinki, Finland) at λ = 570 and 690 nm. Proliferation rate was expressed as the absorbance of a sample compared with control cells treated with acetone (1 %) and the half maximal inhibitory concentration (IC50) values were calculated in CalcuSyn (BioSoft, Ferguson, MO, USA).
Cell viability assay
The viability of cells was evaluated after 24 h incubation in the presence of test substances. After treatment, all cells were stained with 0·4 % trypan blue (1:1, v/v) and counted in a haemocytometer with a Neubauer net. The number of cells with a functioning membrane was compared with the total cell number.
Caspase-3 activity
Caspase-3 activity was assessed according to the manufacturer's instructions. Briefly, cells (8 × 105) were incubated with test substances for 24 h, collected and lysed for 30 min at 4°C. After centrifugation at 5000 rpm for 5 min the supernatant fraction was collected, transferred onto a microplate and incubated with Z-DEVD-R110, caspase-3 substrate, for 30 min, followed by fluorescence detection at λ = 496/520 nm (Delfia–Victor-2; Perkin Elmer, Waltham, MA, USA). The concentration of compounds was calculated according to the cytotoxic effect shown in the MTT test and it was half of the IC50 value for sterols and their oxy-derivatives. The concentration of oil extract was standardised against β-sitosterol and its epoxyderivative content. The final concentrations of those compounds in cells treated with oil extract was chosen to be close to the IC50 values, achieved in the MTT test.
Terminal uridine deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling assay
DNA fragmentation was assessed using the In Situ Cell Death Detection Kit (Fluorescein) according to the manufacturer's instructions. Briefly, cells (8 × 105) were incubated with test compounds for 24 h, detached with a 0·5 % trypsin–EDTA solution and collected. The cell suspension was fixed with 4 % paraformaldehyde and permeabilised with 0·1 % sodium citrate. After the reaction mixture was added, cells were incubated for 30 min and samples were analysed by flow cytometry (Becton Dickinson FACScanTM; Beckton Dickinson, Franklin Lakes, NJ, USA). Concentrations of test compounds correspond to the concentrations applied to the caspase-3 assay.
Statistical analysis
All results are means from three to seven separate experiments in triplicate. Statistical analysis was performed by one-way ANOVA followed by Tukey's post hoc test. We used P < 0·05 as the cut-off for significant difference.
Results
Cytotoxicity
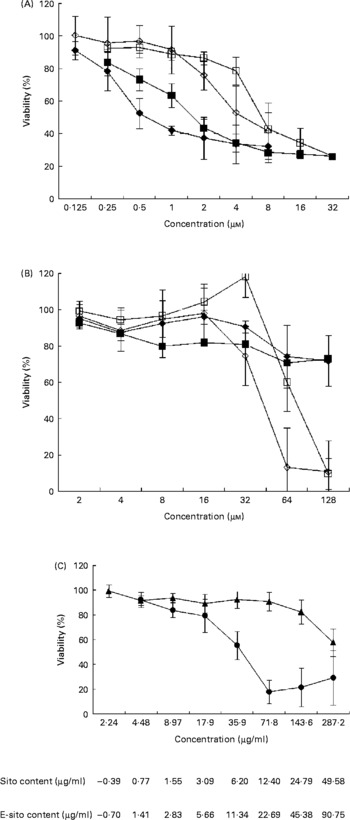
To investigate the effect of sterols and their epoxy-derivatives on human abdominal aorta endothelial cells, HAAE-2 cells were treated with different concentrations of test compounds and cell growth was evaluated after 24 and 72 h (Fig. 2 (A)). β-Sitosterol was shown to be more cytotoxic than cholesterol in almost the whole range of concentrations in both incubation time intervals. The cytotoxic activity of β-sitosterol was revealed even in low concentrations (0·125 μm) while incubation of cells with cholesterol resulted in a significant cell viability inhibition only in almost ten times higher concentration. The cytotoxicity of both test substances increased with incubation time but only in some concentration ranges. β-Sitosterol treatment resulted in cell viability inhibition by 10 % in the concentration of 0·125 μm after 24 h of incubation. The inhibitory effect was more effective at the concentration of 0·25 μm and it was similar for both time intervals. At the concentration of 0·5 μm, β-sitosterol showed 25 % inhibition after 24 h and almost 50 % inhibition after 72 h of incubation. The inhibitory effect was increased with concentration and incubation time; however, at the concentration of 2 μm and higher the incubation time did not significantly change the cytotoxicity. Similarly, cholesterol inhibited cell growth in a time- and dose-dependent manner but significant changes in cell viability were observed at the concentration of 1 μm and higher. Cholesterol showed higher cytotoxicity at 4 μm when cells were incubated for 72 h (50 % growth inhibition) compared with the effect observed at 24 h (20 % growth inhibition). However, β-sitosterol was shown to be more cytotoxic than cholesterol at all concentration and incubation time ranges; both substances finally revealed 70 % cytotoxicity at the concentrations of 8 μm (β-sitosterol) and 16 μm (cholesterol).

Fig. 2 Sensitivity of human abdominal aorta endothelial cells (HAAE-2) to sterols. Cytotoxicity of sterols was evaluated with the 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) test after 24 and 72 h of incubation time. The cells were cultured in Ham's F12K medium, as specified in Materials and methods, on ninety-six-well titration plates in the presence of test substances dissolved in acetone. At time intervals, 0·5 % MTT was added followed by incubation with a solubilisation solution. Absorbance intensity was read by a Labsystems Multiscan RC (Helsinki, Finland) at λ = 570 and 690 nm. Proliferation rate was expressed as the absorbance of a sample compared with control cells treated with acetone (1 %) and the half maximal inhibitory concentration (IC50) values were calculated using CalcuSyn (BioSoft, Ferguson, MO, USA). The data are means from three to seven separate experiments in triplicate, with standard errors represented by vertical bars. (A) β-Sitosterol at 24 h incubation time (■), β-sitosterol at 72 h incubation time (♦), cholesterol at 24 h incubation time (□), cholesterol at 72 h incubation time (⋄). (B) 5α,6α-Epoxy-β-sitosterol at 24 h incubation time (■), 5α,6α-epoxy-β-sitosterol at 72 h incubation time (♦), 5α,6α-epoxycholesterol at 24 h incubation time (□), 5α,6α-epoxycholesterol at 72 h incubation time (⋄). (C) Oil extract at 24 h incubation (▲) and 72 h incubation (●). Standardised values of β-sitosterol (Sito) and 5α,6α-epoxysitosterol (E-sito) content in oil extract are given.
To investigate the influence of sterol epoxy-derivatives, i.e. 5α,6α-epoxysitosterol and 5α,6α-epoxycholesterol, on human abdominal aorta endothelial cell (HAAE-2) growth, cells were supplemented with varying concentrations of test compounds and cell growth was evaluated after 24 and 72 h (Fig. 2 (B)). The analysis demonstrated that 5α,6α-epoxy-β-sitosterol decreased the viability of the cells by about 10–20 % in the whole concentration range, while incubation time and compound concentration slightly increased this effect. It was also shown that 5α,6α-epoxycholesterol in the range of 2–16 μm at the 24 h incubation time did not alter cell viability, while at the concentration of 32 μm at the same time a pro-proliferative effect was observed at the level of almost 20 % compared with control cells. At higher concentrations 5α,6α-epoxycholesterol decreased cell viability by up to 90 % at the concentration of 128 μm. When cells were treated for 72 h the results were similar, showing no effect of 5α,6α-epoxycholesterol in the concentration range of 2–16 μm; however, at the concentration of 32 μm a growth-inhibitory effect was observed at the level of over 20 % and that effect was increased with concentration up to 90 % inhibition at 64 μm.
A comparison of sterols and their epoxy-derivatives revealed that oxy-sterols significantly inhibited cell growth but only in concentrations a few times higher compared with basal substances.
The study of the mixture of oxyphytosterols derived from the oil extract and their influence on cell viability at 24 h of incubation revealed that these compounds slightly decreased cell number in the concentration range of 2·24–71·8 μg/ml by about 10 % (Fig. 2 (C)). This effect was potentiated by higher concentrations, reaching 20 % at the concentration of 143·6 μg/ml and 40 % at the concentration of 287·2 μg/ml. When cells were treated with the oil extract for 72 h with concentrations of 2·24–17·9 μg/ml, cell viability decreased with concentration up to 20 % while further concentration increase showed higher cytotoxic effect, reaching almost 50 % inhibition at 35·9 μg/ml and 80 % at the concentration of 71·8 μg/ml and higher.
Calculation of IC50 values enabled comparison of test substance cytotoxicity. It was revealed that after 24 h of incubation in the presence of β-sitosterol, 50 % of cells were killed by the concentration of 1·99 μm (IC50) compared with incubation with cholesterol when 50 % of cells were killed by the concentration of 8·99 μm (Table 2). Incubation time increase up to 72 h resulted in decreases in IC50 to 1·33 and 5·84 μm respectively (Table 2). Calculation of IC50 values for sterol epoxy-derivative treatment for 24 h did not give results. However, incubation for 72 h showed significant cytotoxicity of the compounds (Table 2).
Table 2 Half maximal inhibitory concentration (IC50) values*
(Mean values with their standard errors of three experiments in triplicate)

* The values of IC50 were calculated using CalcuSyn (BioSoft, Ferguson, MO, USA) and are expressed in μm or μg/ml.
Cell viability in the presence of individual test substances was also assessed using a trypan blue assay (Table 3). Significant viability differences compared with control were observed in cells treated with 2 μm-β-sitosterol and oil extract at 147·6 μg/ml. Other study compounds and their concentrations did not show any significant changes in cell viability.
Table 3 Trypan blue cell viability assay
(Mean values with their standard errors of three experiments in triplicate)

Apoptosis assessment
Influence of sterols on caspase-3 activity
To investigate the contribution of test compounds to the mechanism of apoptosis in human abdominal aorta endothelial cells, HAAE-2, cells were treated for 24 h with test compounds in concentrations close to IC50 and the caspase-3 activity assay was performed (Fig. 3 (A)). It was demonstrated that β-sitosterol significantly increased the enzyme activity by over 60 % compared with control. No significant difference was shown in enzyme activity when cells were treated with epoxy-derivatives of sterols while the cholesterol and oil extract slightly increased the caspase-3 activity by about 15 and 25 %, respectively.

Fig. 3 Apoptosis in endothelial cells provoked by sterols. HAAE-2 cells (8 × 105) were treated for 24 h with test compounds in concentrations close to half maximal inhibitory concentration (IC50) and both the caspase-3 activity assay and the terminal uridine deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labelling (TUNEL) assay were performed. The caspase activity was assessed with an ELISA test at λ = 496/520 nm and DNA fragmentation was evaluated using an In Situ Cell Death Detection Kit by flow cytometry (FACScanTM; Beckton Dickinson, Franklin Lakes, NJ, USA). The data are means from three separate experiments, with standard errors represented by vertical bars. (A) Caspase-3 activity in HAAE-2 cells in the presence of test substances: NEG, negative control (MCF-7 cells, showing no caspase-3 activity); CPT, camptothecin (2·5 μm); C, control untreated cells; sito, β-sitosterol (0·5 μm); e-sito, 5α,6α-epoxy-β-sitosterol (2 μm); chol, cholesterol (0·5 μm); e-chol, 5α,6α-epoxycholesterol (2 μm); oil, oil extract (2·9 μg/ml). (B) DNA fragmentation in HAAE-2 cells in the presence of test substances: NEG, negative control (sample without terminal transferase); CPT, camptothecin (2·5 μm); C, control untreated cells; sito, β-sitosterol (0·5 μm); e-sito, 5α,6α-epoxy-β-sitosterol (2 μm); chol, cholesterol (0·5 μm); e-chol, 5α,6α-epoxycholesterol (2 μm); oil, oil extract (2·9 μg/ml).
Influence of sterols on DNA fragmentation
In order to estimate the contribution of test compounds to the final step of apoptosis, human abdominal aorta endothelial cells, HAAE-2, were treated with concentrations of test compounds close to IC50 for 24 h and a DNA fragmentation assay (TUNEL) was performed (Fig. 3 (B)). The effect of β-sitosterol was over two times higher compared with control while the relative TUNEL positive cells in the samples treated with cholesterol, α-epoxycholesterol and oil extract were almost three times higher compared with control. No DNA fragmentation increase was observed in cells treated for 24 h with α-epoxysitosterol.
Discussion
Within the last few years much information has been discovered and published concerning the biological activity of phytosterols. They have been studied in the context of antiproliferative action on cancer cells(Reference Choi, Kong, Kim, Jung, Kil, Rhee and Park3) as well as protection for normal cells(Reference Awad, Williams and Fink14). However, the data concerning advantages or disadvantages of phytosterol uptake are very diverse. The in vitro studies revealed that β-sitosterol is a strong antiproliferative agent in breast cancer cells T-47D, while an adverse effect was observed in other breast cancer cells, MCF-7, treated with 1 μm concentration of this compound(Reference Mellanen, Petänen, Lehtimäki, Mäkelä, Bylund, Holmbom, Mannila, Oikari and Santti15). This was also confirmed by other researchers showing a pro-proliferative action of β-sitosterol on MCF-7 cells at concentrations of 0·001–150 μm(Reference Ju, Clausen, Allred, Almada and Helferich16). Consequently its oestrogenic potency was suggested. Noteworthy, no effect of β-sitosterol was shown on the expression of Bcl-2 and Ps2 genes, which are engaged in cell-cycle control. On the contrary, it was shown that β-sitosterol was cytotoxic in Caco-2, HepG2 and MCF-7 (IC50 values 20, 53, 71·2 μm, respectively)(Reference Rahmat, Kumar, Fong, Endrini and Sani17).
It is also suggested that β-sitosterol might play an important role in cancer prevention and therapy, which was shown in vitro in HCT116 colon cancer cells. Their viability was inhibited by β-sitosterol by about 50 % at the concentration of 7·5 μm(Reference Choi, Kong, Kim, Jung, Kil, Rhee and Park3). It was also demonstrated that β-sitosterol induced caspase-3 and caspase-9 activity and that this effect was accompanied by a proteolytic cut of poly(ADP-ribose)polymerase. It was also shown that during the apoptosis resulting from β-sitosterol treatment, a decrease in mRNA specific for anti-apoptotic protein Bcl-2 and Bcl-2 protein itself with simultaneous increase in mRNA of pro-apoptotic protein Bax was observed(Reference Choi, Kong, Kim, Jung, Kil, Rhee and Park3). Similarly, because β-sitosterol shows a growth-inhibitory effect and has an influence on apoptosis induction in breast cancer cells MCF-7, it was suggested that it might be an important diet complement in breast cancer therapy(Reference Awad, Chinnam, Fink and Bradford18).
Simultaneously, the influence of β-sitosterol on normal cells and their function in atherosclerosis has been discussed. Awad et al. (Reference Awad, Williams and Fink19) showed that this phytosterol inhibited smooth muscle cell (SMC) proliferation and, thus, inhibited plaque formation. This was shown in in vitro studies, when 16 μm-β-sitosterol inhibited smooth muscle cell proliferation by about 30 %. Since it was described as a proliferation inhibitor, β-sitosterol was not proved to have cytotoxic properties. It was described positively, because cell death is supposed to be one of the reasons for plaque formation or plaque break resulting in an increased coagulation(Reference Awad, Williams and Fink19). The results of the present study reveal strong cytotoxic properties of β-sitosterol in endothelial cells. Even at the concentration of 2 μm, much lower than physiological (about 5 μm) serum concentration(Reference Moghadasian, McManus, Godin, Rodrigues and Frohlich20), after 24 h incubation time β-sitosterol decreased human abdominal aorta endothelial cell (HAAE-2) viability by about 50 %. Noteworthy, treatment of cells with a concentration of oil extract containing a similar level of β-sitosterol demonstrated only a minor cytotoxic effect. Only the concentrations of oil containing higher concentrations of β-sitosterol (>16 μm), remarkably exceeding the physiological value, resulted in a significant cell-growth inhibition (P < 0·05). This might indicate interactions between individual oil extract compounds. Interestingly, 5α,6α-epoxy-β-sitosterol in a wide range of concentrations (2–128 μm) showed a weaker cytotoxic effect than its parent compound, β-sitosterol. We hypothesise that this may be due to the oxidation process that if taking place in cells in vitro can not only produce harmful epoxy forms but also generate free radicals. Otherwise, if epoxy-compounds are delivered into the cells their toxic spectrum is also very broad but deprived of an oxidation stress effect.
Anti-atherogenic effects of plant sterols may be not only due to their cholesterol-lowering activities alone, but also to other properties such as effects on the coagulation system, antioxidant system, and hepatic and lipoprotein lipase activities(Reference Boberg, Pettersen and Prydz21). Furthermore, it has been shown that high β-sitosterol levels (up to 0·7 mmol/l) can cause contraction of human umbilical vein endothelial cells in vitro (Reference Pinedo, Vissers, von Bergmann, Elharchaoui, Lütjohann, Luben, Wareham, Kastelein, Khaw and Boekholdt22). These observations suggest that very high plasma concentrations of β-sitosterol may have potentially cytotoxic effects and may interfere with cellular functions. Moreover, significantly stronger cytotoxic activity of β-sitosterol than cholesterol was observed. This might be an effect of displacing the cholesterol from the membrane and thus disturbing the membrane integrity and eliminating the natural compound.
It was also revealed that 5α,6α-epoxycholesterol in very low concentrations did not significantly decrease the cell viability of HAAE-2 human abdominal aorta endothelial cells, and surprisingly at the concentration of 32 μm at 24 h incubation it induced cell proliferation comparative with control. This might be explained by the mutagenic properties of oxysterols(Reference Guardiola, Codony, Addis, Rafecas and Boatella23). Only the concentrations of 64 and 128 μm were remarkably cytotoxic, showing even 90 % cell growth inhibition at the highest concentration. Rimner et al. (Reference Rimner, Al Makdessi, Sweidan, Wischhusen, Rabenstein, Shatat, Mayer and Spyridopoulos24) showed that 5α,6α-epoxycholesterol was not cytotoxic for human arterial endothelial cells. It was then concluded that the role of α- and β-isoforms of oxysterols in the pathogenesis of CHD is different. In that study, 5β,6β-epoxycholesterol was shown to induce cell death in a concentration-dependent manner while the 5α,6α-derivative had a minor influence on cell death. It was also shown that 7β-hydroxycholesterol was much more cytotoxic than epoxy-derivatives. In another study Lemaire et al. (Reference Lemaire, Lizard, Monier, Miguet, Gueldry, Volot, Gambert and Néel25) observed that the position (α or β) of the hydroxyl radical plays a critical role in the induction of the apoptotic process.
In the study of O'Sullivan et al. (Reference O'Sullivan, O'Callaghan and O'Brien26) the cholesterol derivatives were shown to be cytotoxic and induce apoptosis in bovine aorta endothelium cells and U937 cells (derived from monocytes), confirming the pro-atherogenic action of oxysterols. We showed that the oxysterol mixture could influence cell viability in a different way compared with individual mixture components, which suggests complex interactions. A study of 5α,6α-epoxysitosterol action was also performed by Maguire et al. (Reference Maguire, Konoplyannikov, Ford, Maguire and O'Brien27). It was shown that this compound may be cytotoxic for U937 cells. It was also revealed that 5α,6α-epoxysitosterol could inhibit macrophage growth at a concentration of 200 μg/ml for 120 h(Reference Adcox, Boyd, Oehrl, Allen and Fenner28).
In the present study, in a comparison of results received using two methods, assessing cell viability in the presence of test substances, trypan blue and MTT, significant differences were noticed. It was shown that the same substances were more cytotoxic when analysed by the MTT test than in the trypan blue assay. Thus, we may suggest that the study compounds more efficiently target cell metabolism, including cytochrome activity, than their membrane integrity. It is then supposed that β-sitosterol treatment (IC50 = 2 μm in the MTT assay) might result in an oxidation chain block and cell death, rather than in cell membrane integrity (viability decreased only by about 10 % at the concentration of 2 μm). It was also observed in the trypan blue assay that when cells were treated with the oil extract almost 90 % of cells died, while in the MTT assay the viability of cells was decreased only by about 40 % in the 24 h incubation time at a concentration of 143·6 μg/ml corresponding to β-sitosterol and epoxy-β-sitosterol contents of 24·79 and 45·38 μm respectively. This might suggest that the oxyphytosterol extract influences not only the metabolism of the cells but also their membrane integrity. Thus we conclude that the action of phytosterols and their oxy-derivatives may differ. However, the MTT test enables only primary verification of individual compounds when considering their antiproliferative properties and/or influence on cell viability. But surprisingly for us, on the contrary to other studies and reports, phytosterols significantly decreased normal human endothelial (HAAE-2) cell viability. Additionally, since the MTT cell proliferation assay is a colorimetric assay system which measures the reduction of a tetrazolium component (MTT) into an insoluble formazan product by variable cell enzymes (including also mitochondrial) it can really tell what is the metabolic condition, endocytosis ability and viability or proliferation index of the cells studied. However, we realise that such results can only indicate cell growth inhibition and not apoptosis directly. Thus, further studies were performed, concerning precise description of the study compound effects, i.e. caspases and TUNEL assays.
It is postulated that endothelial cell apoptosis may directly affect the growth of neighbouring cells, including the SMC of vessels(Reference Awad, Smith and Fink4). This could result from the mechanism of releasing factors such as NO and PG that limit cell proliferation. Since apoptosis decreases the number of endothelial cells, their influence is decreased and SMC may overproliferate(Reference Awad, Smith and Fink4). Apoptosis reduces the number of endothelial cells, and may result in blood–muscle cell barrier damage which leads to SMC exposure to risk factors, promoting cell growth. Moreover, apoptotic cells may contribute to increased blood coagulation, which can induce proliferation of SMC. Interestingly, it was also demonstrated that endothelial cell apoptosis causes proliferation of neighbouring SMC inducing a signal to supplement the damaged cell layer(Reference Qin and Liu29). This process might be inhibited by serum β-sitosterol, which was shown to limit SMC proliferation rate(Reference Awad, Smith and Fink4).
In the present study it was shown that β-sitosterol as well as cholesterol caused apoptosis, inducing caspase-3 activity in the cells studied (60 % increase compared with control cells). The observed differences in caspase-3 activation and DNA fragmentation that allows discrimination of apoptosis from necrosis might result from distinct reaction kinetics of both processes(Reference Brecht, Gelderblom, Srinivasan, Mielke, Dityateva and Herdegen30). The present results are consistent with other studies where the pro-apoptotic effect of β-sitosterol in LNCaP cells(Reference von Holtz, Fink and Awad31), MDA-MB-231 cells(Reference Awad and Fink5) and HCT116 cells(Reference Choi, Kong, Kim, Jung, Kil, Rhee and Park3) was revealed, showing caspase-3 as well as DNA-fragmentation increase. However, this compound seems to be not selective only to cancer cells.
On the other hand, Z-DEVD-R110 is a substrate for caspases 3, 6, 7, 8 and 10; however, caspases 3, 6 and 7 predominantly cleave the substrate. Thus, we also analysed the influence of study compounds on the activity of other caspases in MCF-7 cells, showing no activity of caspase-3, and we showed no increase in the activity of any of these caspases in MCF-7 cells (negative control). Additionally we did not observe apoptosis when analysing the influence of phytosterols on MCF-7 cells in cytometric analysis. We realise that this information can be deceptive, because these two cell types can differ strikingly in other metabolic pathways, not only in caspase-3 activity. Anyway, when analysing the properties of the phytosterols studied, we can conclude that they probably do not provoke an increase in activity of any caspases other than caspase-3.
So far, a protective influence of phytosterols on the human organism and atherosclerosis has been suggested. The proposed mechanism of this action was connected with decreased LDL capacity of cholesterol as a result of decreased intestine absorption which might be caused by β-sitosterol(Reference Mayr and Xu32). It seems that in atherosclerosis prevention one of the most important factors is keeping a balance in proliferation and apoptosis of endothelial cells as it happens in SMC(Reference Mayr and Xu32). β-Sitosterol, inhibiting endothelial cell growth, might cause a break in the inner vessel wall and thus the apoptotic signals could induce the neighbouring cells to proliferate including over-proliferation of SMC, without the death of cells(Reference Qin and Liu29).
On the other hand, β-sitosterol has been shown to inhibit proliferation of SMC(Reference Awad, Smith and Fink4) which might probably attenuate its influence on blood vessel condition. Since β-sitosterol is supplied to our body together with food as one of its compounds, it is very difficult to appreciate its role and activity because of the presence of multiple vitamins, antioxidants and other compounds that can modulate the effect of its own. This is consistent with the studies of sunflower-seed oil containing 48·7 % of β-sitosterol; there were no changes in the blood vessels of rats fed with the oil for 90 d(Reference Hepburn, Horner and Smith33). We observed that β-sitosterol decreased cell viability at the concentrations of 0·25 and 0·5 μm while treatment of cells with the oil extract corresponding to the same β-sitosterol concentration (1·42 and 2·84 μm, respectively) did not show any significant effect. An opposite effect was observed in studies of the influence of epoxy-β-sitosterol alone when it decreased cell viability much more effectively if added in an oil extract.
It is then concluded that further studies on phytosterols, including those after thermal processing, and their influence on vessels in vivo is required, especially since it has been revealed that animal-derived sterols as well as phytosterols decrease cell viability in a time- and dose-dependent manner in vitro. There may be a hypothesis that phytosterols undergoing oxidation in our cells can be more dangerous that sterols already metabolised and delivered to our body in that state. This may result from the side effects of oxidation, for example, reactive oxygen species production; however, this hypothesis requires further study.
Acknowledgements
This research was financially supported by a grant from the State Committee for Scientific Research PBZ-KBN-094/P06/2003.
There are no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest. There are no potential conflicts of interest. There is no financial arrangement between any of the authors and any company whose product figures in the paper. All authors read and approved the final submitted manuscript.