Salivary α-amylase (sAA), which is routinely categorised as resting (unstimulated) or stimulated, is the most abundant protein in human saliva( Reference Oppenheim, Salih and Siqueira 1 ), accounting for up to 50 % of salivary protein( Reference Noble 2 ), and is responsible for starch hydrolysis( Reference Lebenthal 3 ). Genetically, sAA production is influenced by individual copy number variations (CNV) of the salivary amylase gene (AMY1), which codes for sAA( Reference Bank, Hettema and Muijs 4 ). sAA concentrations positively correlate with the copy number of the AMY1 gene in adults( Reference Perry, Dominy and Claw 5 , Reference Mandel, Peyrot des Gachons and Plank 6 ). However, a significant amount of variation in sAA levels is not explained by AMY1 CNV( Reference Perry, Dominy and Claw 5 , Reference Mandel, Peyrot des Gachons and Plank 6 ). The AMY1 gene shows extensive variation in copy number( Reference Groot, Bleeker and Pronk 7 , Reference Iafrate, Feuk and Rivera 8 ), with a range of anywhere from two to fifteen diploid copies. sAA levels considerably differ between populations with high-starch diets and those with low-starch diets( Reference Perry, Dominy and Claw 5 ). The patterns of between-population differences have been linked to the CNV of AMY1 gene and seen as an adaptive response to the intake of dietary starch on an evolutionary time scale( Reference Perry, Dominy and Claw 5 ). In addition to gene-dependent variation, differences in sAA levels have been detected in response to various physiological and psychosocial stress conditions( Reference Chatterton, Vogelsong and Lu 9 – Reference Nater, La Marca and Florin 11 ). For example, food intake (mastication) can induce dramatic increase in sAA levels( Reference Behall, Kelsay and Holden 12 , Reference Messenger, Clifford and Morgan 13 ). In addition, gustatory stimulation (by application of citric acid) also stimulates pronounced increase in sAA levels( Reference Froehlich, Pangborn and Whitaker 14 ).
sAA is known to be mainly involved in the initiation of the digestion of starch in the oral cavity. However, the nutritional advantage provided by the amylolytic ‘pre-digestion’ of starch in the oral cavity has rarely been established, since the majority of ingested starch is digested in the small intestine by pancreatic amylase. Several studies, although, have linked sAA levels with the oral perception of textural attributes of starchy foods( Reference Mandel, Peyrot des Gachons and Plank 6 , Reference de Wijk, Prinz and Engelen 15 , Reference Engelen, van den Keybus and de Wijk 16 ), which can explain how sAA benefits nutrition. In a previous study, custards with added α-amylase resulted in increased melting and decreased thickness sensations in the mouth of human subjects, while the opposite effects were observed when custards were added with acarbose (an amylase inhibitor). Then, the authors argued that the effects of amylase on perceived melting and thickness were caused by amylase-induced breakdown of starch( Reference de Wijk, Prinz and Engelen 15 ). Another study has found that subjects with high sAA activity have lower perceived thickness for the starch-based custard and experience decreased creamy after feel in both custard and mayonnaise( Reference Engelen, van den Keybus and de Wijk 16 ). More recently, Mandel et al. ( Reference Mandel, Peyrot des Gachons and Plank 6 ) successfully linked sAA level with the oral perception of starch by introducing time-intensity ratings to track the digestion of starch during oral manipulation. In their study, individuals with high sAA levels reported faster and more significant decreases in perceived starch viscosity than did individuals with low sAA levels( Reference Mandel, Peyrot des Gachons and Plank 6 ). Oral changes in viscosity, or thickness, play an important role in determining individual's liking and preerence for food( Reference Prindiville, Marshall and Heymann 17 ). Collectively, these data suggest that the amylolytic ‘pre-digestion’ of starch-based foods by sAA may be nutritionally important, by influencing the oral perception of textural attributes of starchy foods and thus determining an individual's liking and preference for a starchy food at the preprandial stage, especially for those who traditionally feed on starch-rich foods. Interestingly, Mandel & Breslin( Reference Mandel and Breslin 18 ) raised another possibility that this ‘pre-digestion’ may also benefit nutrition by influencing the plasma insulin concentrations and blood glucose levels at the postprandial stage.
To date, however, few data are available on the direct relationships between sAA levels and individual's nutritional status. It is difficult, although, to link sAA levels with individual's nutritional status directly by the facts that individuals are accustomed to their own idiosyncratic salivary flow rates and sAA levels, and that sAA levels show high variability among and within individuals. More parameters need to be introduced to explain how sAA relates to individual's nutritional status. sAA ratio is a ratio of the stimulated sAA levels to that of resting sAA (stimulated/resting), which reflects the extent to which sAA responds to stimulation. We hypothesised that sAA ratio may be related to individual's nutritional status.
Childhood is a sensitive period of growth and development, which makes children more susceptible to environmental changes than adults. For instance, changes in oral perception, which are associated with sAA levels( Reference Mandel, Peyrot des Gachons and Plank 6 , Reference de Wijk, Prinz and Engelen 15 , Reference Engelen, van den Keybus and de Wijk 16 ), may significantly influence child's liking and preference for foods, and thus play a crucial role in determining their nutritional status, while in adults, it is already shaped by combined actions of innate and environmental factors. We expected that it would be difficult to find direct connections between sAA levels and their nutritional status. Children are expected to have more similar but less psychosocial stress conditions than adults, which would serve to introduce less variations in sAA levels since these stress factors significantly influence individual sAA levels, and adults have unmanageable and varying psychosocial stress conditions. In addition, basal sAA activity in children has reached adult levels( Reference Rohleder and Nater 19 ), and acute sAA responses have also been found in children( Reference Strahler, Mueller and Rosenloecher 20 ). Based on these considerations, in the present study, we investigated whether nutritional status in children is related to (1) sAA levels and (2) AMY1 gene copy number. The present study did not include overweight or obese children, since they were shown to have sAA levels similar to those of normal-weight children( Reference de Campos, Kobayashi and Barbosa 21 ).
Materials and methods
Ethics statement
The present study was conducted according to the guidelines laid down in the declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Academic Ethics Committee of Guangzhou University of Chinese Medicine. Written informed consent was obtained from all subjects/patients. All participants (children) were accompanied by at least one of their parents, who also signed the informed consent and stayed throughout the study.
Participants
Children aged 5–12 years were recruited in Haizhu Maternal and Child Health Hospital. We included Low-BMI children (thinness grade 3) based on BMI cut-offs, which were defined by international survey of six large nationally representative cross-sectional studies( Reference Cole, Flegal and Nicholls 22 ), and Normal-BMI children. Overweight or obese children were excluded based on the criteria established by the Working Group on Obesity in China( 23 ). Individuals using asthma medications, psychotropic substances or painkillers were excluded. Participants were free of psychiatric, sever somatic and oral diseases, as evaluated from an interview by one of the authors (M. Z.). Use of vitamins and natural therapeutics was allowed, and participants were instructed to take their prescribed drugs at least 5 h before intervention. Individuals who reported acute caffeine consumption were excluded, because caffeine ingestion leads to significant increases of sAA activity and output( Reference Bishop, Walker and Scanlon 24 ). Participants were not allowed to eat or drink (anything but water) or to take strenuous exercise at least 1 h before intervention, because these factors significantly modify sAA activity( Reference Rohleder and Nater 19 ). Forty-three children (twenty-three boys and twenty girls) met the inclusion criteria. Of them, twenty-two children (thirteen boys and nine girls) were assigned to the normal-BMI group (healthy) and the remaining twenty-one children (ten boys and eleven girls) were assigned to the low-BMI group (thin).
Stimuli
Our laboratory has used citric acid to stimulate sAA since 1978. We used our standard method of stimulation: pieces of filter paper of fixed size (1 × 1 cm) were soaked in 0·4 mol/l citric acid solution for 10 min, then dried in a drying oven, and finally collected and stored in a small and clean container until their use.
Saliva collection, handling and storage
All the participants were invited to the outpatient department of Haizu Maternal and Child Health Hospital on Saturday between 09.00 and 11.00 hours in the morning. During a 30-min resting period to minimise the impact of physical activity and emotions, height (m) and weight (kg) of all the participants were measured. Immediately after the end of the resting period, unstimulated whole saliva was collected by passive drooling in a fashion similar to that described by Navazesh( Reference Navazesh 25 ). Briefly, participants were instructed to seat with their eyes open and head tilted slightly forward, and to empty their mouths by swallowing all saliva. After that, secreted saliva was allowed to drip off the lower lip into a 5 ml test-tube for 3 min. Immediately after that, stimulated whole saliva was collected by the method described below. Participants were instructed to open their mouths and slightly extend their tongues out, and then a filter paper containing citric acid (described above) was placed on the tongue tip of each participant to stimulate saliva for 1 min, during which the stimulated saliva was collected from under the tongue in a 5 ml test-tube. Participants were required to keep their tongue tips slightly upward when collecting stimulated saliva, so that citric acid in the filter paper would not mix with the collected saliva. The pH values of all saliva samples were determined before storage using pH test paper with a range from 6·0 to 8·0. All saliva samples went through one freeze–thaw cycle to break down mucopolysaccharides that could interfere with pipetting( Reference Shirtcliff, Granger and Schwartz 26 ). Upon thawing at 4°C, saliva was centrifuged at 11 000 g at 4°C for 10 min. Then, the supernatant was aliquoted and stored at − 80°C for subsequent measurements of sAA amount and activity, while the remaining precipitate (containing cheek cells) was collected and stored at − 80°C for subsequent AMY1 gene analysis.
SDS-PAGE and immunoblotting for salivary amylase
sAA amount (μg/ml) was determined by Western blot in a fashion similar to that described by Perry et al. ( Reference Perry, Dominy and Claw 5 ). Briefly, saliva total protein was determined using BCA Protein Assay Kit (CoWin Bioscience). Saliva samples of equal quantity of total protein (5 μg) were prepared by solubilising samples in SDS-PAGE sample loading buffer and heating at 100°C for 5 min. For quantification purpose, a human sAA protein sample (Sigma-Aldrich) of known quantity was run on each gel. Proteins were separated by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Roche) in a transfer buffer for 1·5 h at 200 mA. Membranes were blocked for 2 h at room temperature in blocking buffer (PBS, 0·1 % Tween-20) with 5 % milk. Membranes were incubated overnight at 4°C with rabbit anti-α-amylase (Abcam), diluted 1:5000 in blocking buffer without milk. After washing in phosphate-buffered saline Tween-20 (PBST), membranes were incubated for 2 h in goat anti-rabbit IgG-horseradish peroxidase conjugate (R&D Systems), diluted 1:1000 in blocking buffer without milk. After washing, the membranes were exposed to 3,3′-diaminobenzidine (DAB) substrate (Tiangen Biotech) for 5 min. A ChemiDocXRS+ Chemiluminescence system (Bio-Rad) was used for detection of amylase. Quantification of protein bands was performed using Gel-Pro Analyzer 4.0 software (Media Cybernetics). The test sAA amount was estimated by comparing with the human sAA of known quantity.
Enzymatic activity assay for salivary amylase
Because salivary and pancreatic α-amylase share homology of 97 %, we measured sAA activity according to the method established for pancreatic α-amylase activity assay using a commercially available kinetic reaction assay kit (Kofa Biotech Company) on Hitachi 7180 automatic biochemical analyzer in the clinical laboratory of First Affiliated Hospital of Guangdong Pharmaceutical University. Briefly, diluted saliva (1:200) was incubated with a specific chromogenic substrate, 4,6-ethyliden-G7-PNP, and the auxiliary enzyme α-glucosidase. The substrate was cleaved by sAA into intermediate products, which were further broken down by the auxiliary enzyme into p-nitrophenol (PNP), which absorbed light at a wavelength of 405 nm (yellow), and glucose. The resulting colour change was directly proportional to sAA activity, thus the sAA activity was determined by measuring the light absorbance value, and was expressed as units/ml. Intra- and inter-assay precision expressed as percent CV were below 5 %.
DNA extraction and quantitative PCR for the AMY1 gene
Genomic DNA was extracted from cheek cells by the method described below. Briefly, the frozen salivary precipitate (containing cheek cells) was thawed at 4°C, and then washed and scattered using 0·01 m-PBS. Cheek cells were collected by centrifugation at 2300 g for 10 min. Cell lysis was achieved by adding 50 μl 5 m-KI solution and vortexing for 30 s. The lysate was then added with 100 μl of 0·9 % NaCl solution and subsequently 150 μl chloroform. After vortexing for 30 s and then centrifugation at 11 000 g for 3 min, the supernatant was transferred to a new Eppendorf tube, followed by adding equal volume of isopropanol. After centrifugation at 11 000 g for 3 min, the precipitated DNA was washed once by 500 μl ethanol. Finally, DNA was dissolved by adding 30 μl TE buffer (10 mm-Tris and 1 mm-EDTA) and then stored at − 20°C.
Quantitative PCR (qPCR) was performed to determine diploid AMY1 gene copy number by the method described by Perry et al. ( Reference Perry, Dominy and Claw 5 ). A fragment from the tumour protein p53 (TP53) gene was also amplified to adjust for DNA dilution quantity variation. We used previously published primers for AMY1 and TP53 gene fragment amplification( Reference Perry, Dominy and Claw 5 ). Extracted DNA was quantified using NanoDrop (Thermo Scientific), and concentration of all samples was brought to 10 ng/μl. PCR amplifications were carried out in a reaction volume of 20 μl on a Bio-Rad CFX96 Touch Real-Time PCR Machine. Each qPCR mixture was composed of 10 μl PCR mix (Bio-Rad Super mix SYBR Green), 1 μl of each amplification primer (1 pmol/μl), 7 μl PCR-analysed water and 1 μl DNA extract (0·5 ng/μl). All samples were run in triplicate. Thermal cycling was organised in three repeated steps: the first denaturation step of 3 min at 95°C, followed by thirty-nine repeated cycles of 95°C for 15 s and 60°C for 30 s. Melting curves were obtained by increasing the temperature from 55 to 90°C with a plate reading every 0·2°C. Data were analysed using CFX Manager Software version 2.1 (Bio-Rad). AMY1 diploid copy number was estimated using a standard curve constructed from the reference DNA sample (NA18972; Coriell Cell Repositories), which was previously determined to have fourteen AMY1 diploid copies by qPCR and Fiber FISH (fluorescence in situ hybridisation)( Reference Perry, Dominy and Claw 5 ).
Statistical analyses
Data were tested for normal distribution and homogeneity of variance by the Kolmogorov–Smirnov and Levene's tests before statistical analyses were conducted. These analyses revealed normal distribution of all variables in both groups, with the exception of the resting sAA amount, the stimulated sAA amount and the resting sAA activity of the Low-BMI group (but not Normal-BMI group). Therefore, we log-transformed data for the resting sAA amount, the stimulated sAA amount and the resting sAA activity in each group (the Low-BMI and the Normal-BMI) before analyses, which successfully restored normality of distribution. Data were presented as means and standard deviations. Student's t test was used for comparison of means. Relationships between data sets were analysed using the Pearson correlation coefficient. Data analysis was performed by IBM SPSS statistics version 19.0 (SPSS). For all analyses, a P value (two-tailed) of less than 0·05 was considered to be statistically significant.
Results
Sample characteristics
The main characteristics of the study groups (Normal-BMI children and Low-BMI children) are summarised in Table 1. The results revealed no significant difference in sex, age, weight, height, flow rate of resting or stimulated saliva, and pH value of resting or stimulated saliva between the two study groups, while BMI has been shown to be significantly different between them (t= 6·434; P< 0·0001). We recorded complaints of poor appetite in both groups. Two boys and two girls in the Normal-BMI group, and five boys and seven girls in the Low-BMI group reported poor appetite. All the participants were from southern China, and mainly fed on rice (starch-rich food).
Table 1 Sample characteristics of normal-BMI and low-BMI children (Mean values, standard deviations and ranges)
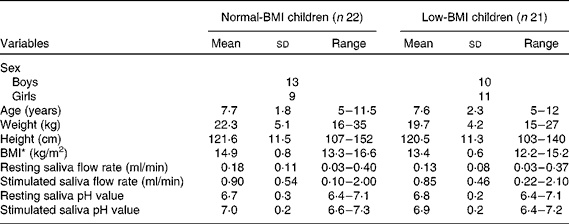
* P< 0·0001 (t test).
Salivary amylase levels
We observed significant individual variation in sAA amount (μg/ml). For the resting sAA, the average amount of the Normal-BMI group and the Low-BMI group were 63·8 (sd 45·9) and 181·0 (sd 230·6) μg/ml, ranging from 10·8 to 174·2 and 17·4 to 842·8 μg/ml, respectively. For the stimulated sAA, the average amount of the Normal-BMI group and the Low-BMI group was 121·2 (sd 86·8) and 225·9 (sd 310·8) μg/ml, ranging from 5·9 to 285·1 and 12·8 to 1064·9 μg/ml, respectively. Both resting and stimulated sAA amounts in the Low-BMI group were not normally distributed. Therefore, for statistical analyses, the resting and stimulated sAA amounts of both groups were log-transformed, which restored normal distribution. There were no significant differences between the two study groups in transformed values for resting sAA amount (t= 1·670; P= 0·103; Fig. 1(a)) and the stimulated sAA amount (t= 0·516; P= 0·608; Fig. 1(b)).

Fig. 1 Comparisons of the resting (a) and stimulated (b) salivary α-amylase (sAA) amount, and the resting (c) and stimulated (d) sAA activity between Normal-BMI and Low-BMI children. For statistical tests, the values for resting and stimulated sAA amount, and the resting sAA activity were log-transformed before analyses, which successfully restored normality of distribution. Values are means, with standard deviations represented by vertical bars. No significant difference was observed in log-transformed values for resting sAA amount, stimulated sAA amount, resting sAA activity or stimulated sAA activity between the two study groups. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
We also observed significant individual variation in sAA activity per unit of saliva. For the resting sAA, the average activity per unit saliva of the Normal-BMI group and the Low-BMI group was 14·3 (sd 8·3) and 32·4 (sd 33·8) units/ml, ranging from 4·4 to 33·8 and 1·4 to 149 units/ml, respectively. For the stimulated sAA, the average activity per unit saliva of the Normal-BMI group and the Low-BMI group was 32·2 (sd 21·3) and 36·1 (sd 19·6) units/ml, ranging from 6 to 77·8 and 1·6 to 66·8 units/ml, respectively. Resting sAA activity in the Low-BMI group was not normally distributed. Therefore, for statistical analyses, the resting sAA activity of both groups was log-transformed, which restored normal distribution. There was no significant difference between the two groups in the transformed values for resting sAA activity (t= 1·838; P= 0·076; Fig. 1(c)). We also observed no significant difference between the two groups in stimulated sAA activity (t= 0·632; P= 0·531; Fig. 1(d)). Together, these results indicate that sAA levels (resting or stimulated) are not directly associated with nutritional status of children.
We also analysed the effects of sex and age on sAA level and found that neither sex nor age were significantly related to sAA levels (resting or stimulated) within or between groups (data not shown).
Because sAA levels have high variability in both previous studies and in the present study, and because individuals are accustomed to their own sAA levels, it is difficult to link sAA levels (resting or stimulated) with individual's nutritional status. We hypothesised that the sAA ratio (the ratio of the stimulated sAA level to that of the resting sAA) may reflect individual's response pattern related to nutritional status. Thus, in the present study, we calculated sAA ratio and analysed its relationship with children's nutritional status. Consistent with our expectation, sAA amount ratio in the Low-BMI children was significantly lower than in the Normal-BMI children (t= 2·727; P= 0·009; Fig. 2(a)). The average sAA amount ratios in the Normal-BMI group and the Low-BMI group were 2·0 (sd 1·2) and 1·1 (sd 0·7), ranging from 0·5 to 5·6 and 0·3 to 2·7, respectively. We also observed a significantly lower sAA activity ratio in the Low-BMI group than in the Normal-BMI group (t= 2·522; P= 0·016; Fig. 2(b)). The average sAA activity ratio of the Normal-BMI group and the Low-BMI group was 2·3 (sd 1·0) and 1·6 (sd 0·8), ranging from 1·0 to 4·9 and 0·4 to 3·2, respectively. These results together suggest attenuated acute sAA responses in Low-BMI children. In line with our expectation, the extent to which sAA respond to gustatory stimulation may be of nutritional importance, at least in children.

Fig. 2 Comparisons of salivary α-amylase (sAA) amount ratio (a) and sAA activity ratio (b) between Normal-BMI and Low-BMI children. sAA amount:activity ratio was expressed as the ratio of stimulated sAA amount:activity to the resting sAA amount:activity. Values are means, with standard deviations represented by vertical bars. * Mean values was significantly different between the two study groups for the sAA amount ratio (P= 0·009). † Mean values was significantly different between the two study groups for the sAA activity ratio (P= 0·016). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
AMY1 gene copy number
DNA samples were analysed by qPCR to determine gene copy number. Values were standardised to a human DNA sample with a known AMY1 gene copy number verified by Fiber FISH. The average number of AMY1 gene copies of all the participants was 7·8 (sd 2·4). In Normal-BMI and Low-BMI children, the number was 7·6 (sd 2·7) and 8·0 (sd 2·1), ranging from 3 to 13 and 3 to 11, respectively. No significant difference was observed in AMY1 gene copy number between the two study groups (t= 0·43; P= 0·670) (Fig. 3). Therefore, AMY1 CNV is not directly associated with child's nutritional status.

Fig. 3 Comparison of AMY1 (salivary amylase gene) gene copy number between Normal-BMI and Low-BMI children. Values are means, with standard deviations represented by vertical bars. No significant difference between the two study groups was observed in AMY1 gene copy numbers. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
We next analysed the relationships between AMY1 CNV and sAA levels in children. Because previous studies have shown that sAA concentration reflects the combined effects of protein secretion and salivary flow rate( Reference Bosch, Veerman and de Geus 27 ), in the present study, we made correction for salivary flow rate when assessing the association between sAA levels (concentration and activity) with CNV of AMY1. We did not observe a significant correlation between AMY1 gene copy number and sAA concentration (r 0·013; P= 0·934) or activity (r 0·029; P= 0·854) in a combined group including all the participants. We also did not observe significant correlations within Normal-BMI group ((r 0·180; P= 0·422) for sAA concentration and CNV of AMY1; (r 0·117; P= 0·604) for sAA activity and CNV of AMY1) or Low-BMI group ((r 0·060; P= 0·796) for sAA concentration and CNV of AMY1; (r 0·014; P= 0·622) for sAA activity and CNV of AMY1). Our observations suggest that AMY1 CNV may influence but do not eventually determine sAA levels in children.
Discussion and conclusion
The present study, for the first time, found attenuated acute sAA responses induced by citric acid in Low-BMI children (thinness grade 3) compared with Normal-BMI children. This finding suggests that sAA responses to gustatory stimulation are of nutritional importance in children. However, we found no relationships of resting or stimulated sAA levels with nutritional status. We also did not observe any associations between AMY1 gene copy number and child's nutritional status. Finally, AMY1 CNV might influence but did not eventually determine sAA levels in children.
Because sAA is increased during stress, i.e. when autonomic nervous system is activated, sAA has been proposed as a marker for activity of the sympathetic nervous system( Reference Nater and Rohleder 28 ). Nevertheless, Bosch et al. ( Reference Bosch, Veerman and de Geus 27 ) considered the idea as too simple, and argued that the parasympathetic nerves also play a significant role in sAA release via several ways, and that the methodology of supporting publications was problematic. This remains to be further clarified. In the present study, however, we focused our attention on reconsidering the main property of sAA, namely its digestive action towards starch-based food and the consequences related to health.
Starch-based foods (e.g. rice) are considered a dominating energy source for those who traditionally feed on them, and thus influence or even determine their nutritional status. Attempts have been made to link sAA levels (resting and stimulated) with individual's preference for a starchy food( Reference Mandel, Peyrot des Gachons and Plank 6 , Reference de Wijk, Prinz and Engelen 15 , Reference Engelen, van den Keybus and de Wijk 16 ). In these studies, researchers found that sAA levels were associated with individual's oral perception of textural attributes of starchy foods. These findings may explain how sAA relates to individual's nutritional status, especially for those who traditionally feed on starch-rich food. These linkages, however, seemed to be not so convincing to come to a conclusion. In humans, sweet taste is a powerful factor influencing food choice and consumption; thus, it may affect individual nutrition. Sugars (e.g. maltose and glucose) are recognised by human's taste system (by interactions with sweet taste receptors expressed on the taste buds) and evoke appetitive consummatory responses( Reference McCaughey 29 ). In the oral cavity, starch-based food is partially hydrolysed by sAA into maltose and glucose after mastication. Both these sugars can act as sweet taste stimuli to interact with taste receptor type 1 (T1R) 2–T1R3 sweet taste receptor, and then evoke pleasant sweet perception and influence ingestive behaviour( Reference Bachmanov, Bosak and Floriano 30 ). In addition to evoking behavioural responses, sweet taste stimuli can elicit preabsorptive cephalic phase responses. For instance, oral glucose can induce a significant preabsorptive elevation of insulin levels, known as cephalic-phase insulin release (CPIR), in rats( Reference Grill, Berridge and Ganster 31 ) and humans( Reference Mandel and Breslin 18 ). CPIR plays an extremely important role in glycaemic homeostasis( Reference Ahrén and Holst 32 ), and thus can affect individual nutrition. Interestingly, CPIR was significantly different between high sAA activity and low sAA activity individuals after starch ingestion but not after glucose ingestion( Reference Mandel and Breslin 18 ), which suggest that CPIR following starch ingestion is dependent on sAA level. Sweet taste stimuli, moreover, can activate endogenous dopaminergic and serotonergic systems( Reference O'Doherty, Deichmann and Critchley 33 , Reference Cooper and Barber 34 ), which play a crucial role in neural basis for food intake and appetite( Reference Baik 35 , Reference Halford and Blundell 36 ). Collectively, the way how sAA levels are related to individual nutritional status is probably following: sAA levels influence the break down of starch-based foods into maltose and glucose in the oral cavity; then the two sugars affect individual sweet taste perception, CPIR, and endogenous dopaminergic and serotonergic systems, which together determine starch-based food consumption (energy intake) and eventually influence individual nutritional status.
Starch-based food intake generally involves mastication, which preferentially stimulates output of sAA( Reference Navazesh and Kumar 37 ). Stimulated sAA might influence individual's appetite( Reference Harthoorn and Dransfield 38 ). Because sAA levels show high variability among or within individuals, and because individuals are accustomed to their own idiosyncratic salivary flow rates and sAA levels, we hypothesised that the degree to which sAA respond to food intake is thus expected to be of nutritional importance. In line with our expectation, we observed a significantly decreased sAA ratio in Low-BMI children when compared with that of Normal-BMI children. In the present study, we received more complaints of poor appetite in the Low-BMI group from children themselves or their parents. Based on the above discussion, the decreased sAA ratio seems to be partially responsible for the poor appetite: the decreased sAA levels may influence individual sweet taste perception, CPIR, and endogenous dopaminergic and serotonergic systems, which together decrease appetite for, and consumption of, the starch-based foods in Low-BMI children. Complaints from parents might be due to their high expectation for children to eat; however, taken together, we had reasons to believe that the Low-BMI children show poorer appetite than did the Normal-BMI children did. There were concerns that a chronic restriction of food intake may have a long-term effect on sAA secretion( Reference Schumacher, Kirschbaum and Fydrich 39 ). However, Rohleder and Nater argued that this effect might be pronounced when examined on an evolutionary time scale( Reference Rohleder and Nater 19 ). In addition, the present study investigated acute sAA responses induced by citric acid, and did not find significant difference in resting or stimulated sAA levels between Normal-BMI and Low-BMI children. Therefore, we argued that the decreased sAA ratio might be partially responsible for the poorer appetite, which, in turn, influenced individual's nutritional status in the Low-BMI children.
In the present study, we found higher number of AMY1 gene copies (average of 7·8 copies) than in previous studies with low-starch populations with the average number of copies 5( Reference Perry, Dominy and Claw 5 ) and 4·4( Reference Mandel, Peyrot des Gachons and Plank 6 ). This suggests that a significant proportion of our subjects' ancestors may undergo positive selection for increased AMY1 gene copy number, rather than this gene CNV evolving neutrally. A significant variation in sAA levels, however, was not explained by AMY1 gene copy numbers in the present study. This lack of relationship might be explained by the following: (1) sAA expression might reflect other genetic influences, such as regulatory region SNP in the AMY1 gene or differences in the transcription or translation efficiency between AMY1 genes in different haplotypes( Reference Bank, Hettema and Muijs 4 , Reference Perry, Dominy and Claw 5 ); (2) non-genetic influences (e.g. physical activity and psychosocial stress conditions) on sAA secretion; (3) protein modifications (e.g. N-glycosylation), which affect enzyme activity( Reference de Barros, do Nascimento Silva and Ramada 40 ). Moreover, we did not observe significant correlation between AMY1 gene copy number and sAA levels in children, while previous work has shown significant correlation in adults( Reference Mandel, Peyrot des Gachons and Plank 6 ). This might be due to subjects' age difference. Development of human salivary glands is morphologically completed in utero; however, they continue to grow during childhood by proliferation of well-differentiated cells( Reference Redman and Sreebny 41 ), and salivary glands function and saliva compositions change with aging( Reference Ghezzi and Ship 42 ).
Mandel et al. ( Reference Mandel, Peyrot des Gachons and Plank 6 ) failed to observe a strong relationship between sAA concentration and the enzymatic activity. Note that the variation in sAA levels in the present study was even more pronounced than in the previous studies, which might be due to age-related and ethnical differences. The present study included children who have higher baseline sAA levels than adults( Reference Strahler, Mueller and Rosenloecher 20 ). In addition, all the participants were Chinese whose ancestors traditionally fed on rice starting from thousands years ago, and thus are generally categorised as a high-starch consuming population.
In the present study, we did not observe associations between AMY1 CNV and child's nutritional status, which might be explained by the following reasons. First, child's nutritional status is more likely to be determined by combined action of various genes, rather than by a single gene. Second, because children are in a sensitive period of growth and development, they may be more susceptible to environmental changes than to genetic factors. For example, changes in oral perception may significantly influence child's liking and preference for foods, and thus play a crucial role in determining child's nutritional status. Finally, AMY1 CNV and individual nutritional status are the most distal levels of analysis in the study; it is not surprising we failed to find a connection between them.
We stimulated saliva production by applying citric acid to the tongue. If citric acid would mix with saliva, this would change salvia pH value, and thus would interfere with assay results. However, we designed our technique to prevent this from occurring. Our laboratory has successfully applied citric acid to stimulate sAA for more than 30 years by using the standardised method described in the Materials and methods section. As a quality control, we measured pH of saliva samples, and a saliva sample would be discarded if its pH value was less than 6·4, which was considered to be the lower limit of saliva pH value. We reported that no saliva samples were less than 6·4 as for pH value in the present study.
The present study has two potential limitations. The first one is a relatively small sample size. Although we could have easily recruited more Normal-BMI children, it was difficult to find Low-BMI children in South China. The second limitation is our ability to accurately evaluate child's appetite. Children typically have difficulties in describing their own appetite partially due to their ignorance, and their parents may have excessively high expectations for their children to eat; together, this may have contributed to variations in evaluating appetite. Thus, findings of the present study should be considered as preliminary until replicated in future research with larger sample sizes.
In conclusion, the present study, for the first time, demonstrated attenuated sAA responses to gustatory stimulation in thin (Low-BMI) children. This suggests that sAA responses to gustatory stimulation may affect individual's nutritional status. AMY1 CNV was not associated with child's nutritional status in our sample. Finally, AMY1 CNV might partially influence but did not eventually determine sAA levels in children.
Acknowledgements
The authors thank all the study participants. They also thank Dr Alexander Bachmanov, Monell Chemical Senses Center, Philadelphia, USA, for his critical review of the present study. The authors also thank Dr Chuan Wei Mo, Guangzhou University of Chinese Medicine, Guangzhou, China, for his assistance in the statistical analyses.
The present study was supported by the National Natural Science Foundation of China (Z. M. Y., grant no. 81102703), the Administration of Traditional Chinese Medicine of Guangdong Province of China (Z. M. Y., grant no. 20123001), the Science and Technology Planning Project of Guangdong Province of China (Z. M. Y., grant no. 2013A032500005) and the Special Funds from Central Finance of China in Support of the Development of Local Colleges and Universities in 2013 (W. W. C., grant no. 338). The funders had no role in the study design, data collection and analysis of the study, in the decision to publish, or in the preparation of the manuscript.
The authors' contributions are as follows: L. H. C. wrote the manuscript, collected data and assisted with the Western blot and qPCR experiments; Z. M. Y. and W. W. C. were the principal investigators and together contributed to the development of the overall research plan, study protocol and study oversight, and analysed the data; J. L. assisted with the Western blot and qPCR experiments; M. Z. was responsible for the participant recruitment, physical examination and saliva sample collection; X. R. Y. performed the enzymatic activity assay; L. B. Z. assisted with participant recruitment and saliva sample collection.
None of the authors has any conflicts of interest.







