CVD is the leading cause of global mortality( Reference Feigin 1 ). Epidemiological and clinical evidence suggests that intake of fish or the long-chain n-3 PUFA (EPA and DHA) from fish may decrease risk of CVD( Reference Mozaffarian and Wu 2 , Reference Chen, Yang and Eggersdorfer 3 ), especially fatal CHD( Reference Del Gobbo, Imamura and Aslibekyan 4 ). The potential mechanisms for protection against CVD include beneficial effects on several CVD risk factors, such as blood pressure, inflammation and platelet aggregation( Reference Mozaffarian and Wu 2 ).
In addition to these CVD risk factors, higher resting heart rate (HR) is associated with higher risk of total and cardiovascular-related mortality( Reference Okamura, Hayakawa and Kadowaki 5 , Reference Legeai, Jouven and Tafflet 6 ). Moreover, epidemiological studies, including the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) from eastern Finland( Reference Savonen, Lakka and Laukkanen 7 , Reference Savonen, Kiviniemi and Laaksonen 8 ), have demonstrated that a low peak HR during an exercise test( Reference Sandvik, Erikssen and Ellestad 9 ) and a delayed HR recovery after an exercise test( Reference Cole, Foody and Blackstone 10 ) are related to higher risk of CVD mortality.
Fish oil supplementation has been reported to reduce resting HR( Reference Mozaffarian, Geelen and Brouwer 11 ), and epidemiological studies have found an inverse association between fish intake or long-chain n-3 PUFA and resting HR( Reference Dallongeville, Yarnell and Ducimetiere 12 – Reference Valera, Suhas and Counil 17 ). Some experimental studies have also investigated the impact of fish oil supplementation on the peak HR during an exercise test and on HR recovery after an exercise test, with inconsistent findings( Reference Macartney, Hingley and Brown 18 – Reference Ninio, Hill and Howe 25 ). To the best of our knowledge, there is no published data from population studies about the association of the long-chain n-3 PUFA with peak HR during exercise and HR recovery after exercise.
In addition to the long-chain n-3 PUFA, fish, especially large and long-living predatory fish, may accumulate methylmercury, an environmental contaminant( Reference Roman, Walsh and Coull 26 ). Methylmercury has been associated with higher risk of CVD and with attenuation of the cardioprotective benefits of the long-chain n-3 PUFA, especially in the KIHD cohort( Reference Virtanen, Voutilainen and Rissanen 27 – Reference Virtanen, Laukkanen and Mursu 29 ), but also in other study populations( Reference Guallar, Sanz-Gallardo and Veer 30 ). Methylmercury is a neurotoxicant, and the autonomic function of the heart is governed by the central nervous( Reference Roman, Walsh and Coull 26 ). Only a few epidemiological studies have evaluated the association of methylmercury exposure with resting HR, with divergent results( Reference Yaginuma-Sakurai, Murata and Shimada 31 – Reference Oka, Matsukura and Okamoto 35 ). However, very little is known whether exposure to methylmercury has an association with HR during and after exercise.
In order to elucidate the relations of the long-chain n-3 PUFA with resting and exercise-related HR and HR recovery, we investigated the cross-sectional associations of the serum long-chain n-3 PUFA with resting HR, peak HR during an exercise test and HR recovery after an exercise test among generally healthy middle-aged and older men from the population-based KIHD cohort. We also evaluated whether high hair Hg concentration, a biomarker for long-term Hg exposure, is associated with these outcomes and whether it could modify the associations with the long-chain n-3 PUFA.
Methods
Study population
We performed a cross-sectional analysis among the participants from the KIHD cohort, a population-based study designed to investigate risk factors for CVD, atherosclerosis, and related outcomes in men from eastern Finland( Reference Salonen 36 ). A total of 2682 men (82·9 % of those eligible) who were 42, 48, 54 or 60 years old and living in the city of Kuopio or its surrounding areas were recruited to the baseline examinations in 1984–1989. The baseline characteristics of the entire study population have been described previously( Reference Salonen 36 ). The KIHD protocol was approved by the Research Ethics Committee of the University of Eastern Finland and complies with the Declaration of Helsinki. All the subjects signed a written informed consent.
From the analyses we excluded participants with a history of CVD (n 1016), those using beta-blockers (n 135) and those with missing data on HR parameters (n 435), serum long-chain n-3 PUFA (n 78) or hair Hg (n 9). We also exclude one participant with resting HR outside of the nomogram range (HR<30 or HR>130 beats/min). After the exclusions, the final study population included 1008 men (online Supplementary Fig. 1).
Assessment of heart rate parameters and other exercise test variables
A maximal, symptom-limited exercise test was performed at baseline using an electrically braked cycle ergometer, as described previously( Reference Lakka, Venalainen and Rauramaa 37 , Reference Laukkanen, Lakka and Rauramaa 38 ). The primary aim was to explore HR data from rest to maximal workload systematically instead of using arbitrarily chosen parts of recorded HR data. HR was recorded from an electrocardiogram (ECG) at rest, at the end of each 60-s interval during the exercise test, at peak exercise and during recovery. Resting HR was expressed as the lowest HR value, whether measured in lying position before the test or while sitting on bicycle at the initiation of the test. During recovery, the workload was set to 0 W and subjects were allowed to continue pedaling at a self-chosen frequency if desired. No predefined pedaling frequency was used during recovery. HR recovery was defined a priori as the reduction in HR from HR peak to HR at 2 min after the exercise test to maximise the number of subjects included in the analyses, because values of HR at 1 min after the exercise test were not available for all men.
Other measurements
Hair and venous blood samples were obtained between 08.00 and 10.00 hours after having abstained from ingesting alcohol for 3 d, smoking for 12 h, and eating for 12 h. After the subject had rested in the supine position for 30 min, blood was drawn with Terumo Venoject VT-100PZ vacuum (Terumo Corp.). No tourniquet was used. A hair sample averaging 40 mg was cut from the scalp hair of the subjects for Hg measurements( Reference Salonen, Seppanen and Nyyssonen 39 ).
Comprehensive description of the determination of serum lipids and lipoproteins, assessment of medical history and medications, smoking and alcohol consumption have been reported previously( Reference Salonen, Nyyssonen and Korpela 40 ). Physical activity was evaluated based on the 12-month leisure-time physical activity questionnaire and expressed as kJ/d (kcal/d)( Reference Lakka, Venalainen and Rauramaa 37 ). The most common leisure-time physical activities were recorded, including the average duration, intensity and frequency of each activity. Hypertension diagnosis was defined as systolic/diastolic blood pressure >140/90 mmHg at study visit, clinical diagnosis of hypertension or use of hypertension medication( Reference James, Oparil and Carter 41 ). Dietary intakes of foods and nutrients were assessed at the time of blood sampling with an instructed 4-d food diary by household measures( Reference Virtanen, Mursu and Tuomainen 42 ). The instructions were given and the completed food records were checked by a nutritionist. Education and annual income were assessed by using self-administered questionnaires.
Serum fatty acid and mercury measurements
Serum fatty acids were determined in one gas chromatographic run without preseparation as described previously( Reference Laaksonen, Lakka and Lakka 43 ). Serum fatty acids were extracted with chloroform-methanol. Chloroform phase was evaporated and treated with sodium methoxide, which methylated esterified fatty acids. Quantification was carried out with reference standards (Check Prep Inc.). Each analyte had individual reference standard, and an internal standard was eicosane. Fatty acids were chromatographed in an NB-351 capillary column (HNU-Nordion) by a Hewlett-Packard 5890 Series II gas chromatograph (Hewlett-Packard Company, since 1999 Agilent Technologies Inc.) with a flame ionization detector. Results were obtained in micromoles per litter and in the data analyses proportion of a fatty acid from the total serum fatty acids was used. The CV was 9·4 % for EPA (20 : 5n-3), 12·7 % for DPA (22 : 5n-3) and 11·9 % for DHA (22 : 5n-3). For the serum total long-chain n-3 PUFA, we used the sum of EPA, DPA and DHA.
Hair Hg was detected by flow injection analysis-cold vapour atomic absorption spectrometry and amalgamation( Reference Salonen, Seppanen and Nyyssonen 39 ). Repeat hair samples were collected from twenty-one subjects in 4 to 9 years (mean, 6 years) after baseline examination to survey the tracking of hair Hg values over time. Pearson’s correlation coefficient between the original and the repeat measurement was 0·91.
Statistical analysis
The univariate associations between the serum total long-chain n-3 PUFA (EPA+DPA+DHA) concentration and demographic, lifestyle and clinical characteristics at baseline were assessed by linear regression for continuous variables and χ 2 test for categorical variables. Correlations between the individual long-chain n-3 PUFA were evaluated by Spearman correlation coefficients. The mean values of resting HR, peak HR during exercise and HR recovery 2 min after exercise in the quartiles of the long-chain n-3 PUFA and hair Hg were analysed using ANCOVA, adjusted for potential confounders. The model 1 was adjusted for age (years) and examination year. The model 2 included the variables in the model 1 plus BMI (kg/m2), type 2 diabetes (yes/no), smoking status (never smoker, previous smoker, current smoker <20 cigarettes/d and current smoker ≥20 cigarettes/d), leisure-time physical activity (kJ/d (kcal/d)), education (years), income (euro), hypertension (yes/no), fasting blood glucose (mmol/l), energy intake (kJ/d (kcal/d)), and alcohol intake (g/week). Additional adjustments for serum TAG, HDL- or LDL-cholesterol concentrations, lipid-lowering medication use or maximal oxygen uptake during the exercise test, did not appreciably change the associations (<5 % change in estimates).
Cohort mean was used to replace missing values in covariates (<0·5 %). Tests of linear trend across categories were conducted by assigning the median values for each category of exposure variable and treating those as a single continuous variable. To assess clinical significance of the findings, we calculated effect sizes based on the Cohen’s d index (the difference between the group means divided by the standard deviation of the comparison category)( Reference Ferguson 44 ). All P values were two-sided (α≤0·05). Data were analysed using the SPSS software version 23 for Windows (IBM Corp.).
Results
Baseline characteristics
The mean age of the participant was 51·4 (sd 5·7) years. The mean serum concentrations, as a percentage of all serum fatty acids, were 4·71 (sd 1·57) % for serum total long-chain n-3 PUFA concentration, 2·48 (sd 0·71) % for DHA, 1·67 (sd 0·89) % for EPA and 0·56 (sd 0·10) % for DPA. The correlations between the individual long-chain n-3 PUFA were 0·70 for EPA and DHA, 0·56 for EPA and DPA, and 0·41 for DHA and DPA. Baseline characteristics of the participants according to quartiles of the total long-chain n-3 PUFA concentration are presented in the Table 1. Men with higher concentration were more likely to have a higher annual income, BMI, fasting blood glucose, hair Hg concentration and alcohol intake. They also had a lower energy intake.
Table 1 Baseline characteristics according to serum total long-chain n-3 PUFAFootnote * (Mean values and standard deviations; percentages)
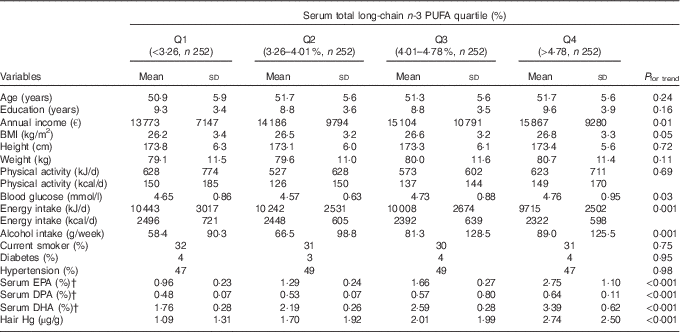
Q, quartiles.
* The univariate associations between the serum total long-chain n-3 PUFA concentration and demographic, lifestyle and clinical characteristics at baseline were assessed by linear regression for continuous variables and χ 2-test for categorical variables.
† Proportion of all serum fatty acids.
Associations with resting heart rate
The mean resting HR was 68·2 beats/min. In the multivariable-adjusted model, higher serum total long-chain n-3 PUFA concentration was associated with lower resting HR (the mean difference between the highest and the lowest quartile −2·17 beats/min; 95 % CI −4·10, −0·24, P for trend=0·02 across quartiles), model 2, Table 2). When the fatty acids were investigated individually, generally similar inverse associations were observed with EPA, DPA and DHA, although the association with EPA appeared slightly stronger than those with DPA and DHA (Table 2). The effect sizes, based on Cohen’s d index, were 0·12 for total serum long-chain n-3 PUFA, 0·07 for EPA, 0·10 for DPA, 0·14 for DHA.
Table 2 Resting heart rate (beats per min) in quartiles of serum long-chain n-3 PUFA and hair mercury among 1008 men aged 42–60 years from the Kuopio Ischaemic Heart Disease Risk Factor StudyFootnote * (Mean values and 95 % confidence intervals)
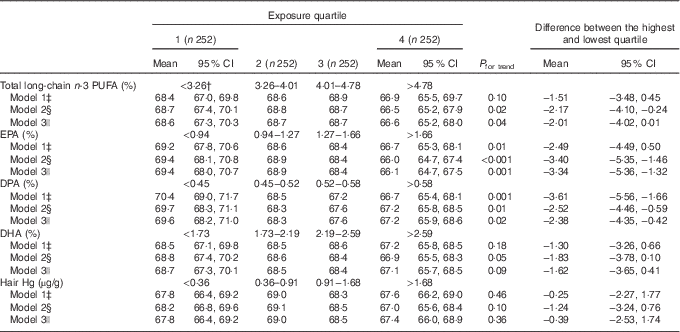
* The association of serum long-chain n-3 PUFA and hair Hg with resting heart rate was analysed using ANCOVA.
† Medians.
‡ Model 1 is adjusted for age and examination year.
§ Model 2 is adjusted for model 1+BMI (kg/m2), diabetes (yes/no), hypertension (yes/no), smoking (yes/no), education (years), income (euro), leisure-time physical activity (kJ/week (kcal/week)), intake of alcohol (g/week), energy intake (kJ/d (kcal/d)) and blood glucose (mmol/l).
|| Model 3 is adjusted for model 2+hair Hg (in analyses with fatty acids) or total long-chain n-3 PUFA (in analyses with Hg).
Hair Hg concentration was not statistically significantly associated with resting HR (Table 2). Additional adjustment of the fatty acid analyses for hair Hg content only slightly attenuated the associations (model 3, Table 2).
Associations with peak heart rate during an exercise test
The mean peak HR during the exercise test was 164·8 beats/min. Serum total long-chain n-3 PUFA concentration or the individual fatty acids were not associated with the peak HR (Table 3). After adjustment for age and examination year, higher hair Hg content was associated with lower peak HR during an exercise test (the mean difference between extreme quartiles −3·55 beats/min; 95 % CI −6·25, −0·85, P for trend=0·001 across quartiles), model 1, Table 3; Cohen’s d=0·10).
Table 3 Peak heart rate (beats per min) during an exercise test in quartiles of serum long-chain n-3 PUFA and hair mercury among 1008 men aged 42–60 years from Kuopio Ischaemic Heart Disease Risk Factor StudyFootnote * (Mean values and 95 % confidence intervals)
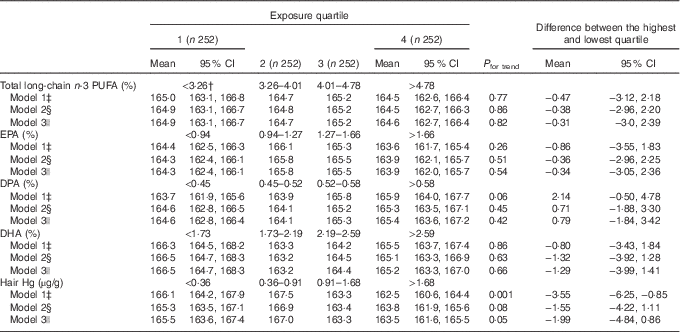
* The association of serum long-chain n-3 PUFA and hair Hg with peak heart rate was analysed using ANCOVA.
† Medians.
‡ Model 1 is adjusted for age and examination year.
§ Model 2 is adjusted for model 1+BMI (kg/m2), diabetes (yes/no), hypertension (yes/no), smoking (yes/no), education (years), income (euro), leisure-time physical activity (kJ/week (kcal/week)), intake of alcohol (g/week), energy intake (kJ/d (kcal/d)) and blood glucose (mmol/l).
|| Model 3 is adjusted for model 2+hair Hg (in analyses with fatty acids) or total long-chain n-3 PUFA (in analyses with Hg).
Further adjustments slightly attenuated the association (model 2), but there was again a trend towards lower peak HR after the serum long-chain n-3 PUFA concentration was adjusted for (P=0·05 for trend across quartiles, model 3, Table 3).
Associations with 2-min heart rate recovery
The mean HR recovery 2 min after the exercise test was 40·6 beats. We did not observe an association between the serum total long-chain n-3 PUFA concentration and 2-min HR recovery. Of the individual fatty acids, only DPA was associated with better HR recovery (the mean difference between extreme quartiles 3·57 beats; 95 % CI 1·52, 5·62, P for trend=0·001 across quartiles), model 1, Table 4; Cohen’s d=0·11).
Table 4 Heart rate (beats per min) recovery 2 min after an exercise test in quartiles of serum long-chain n-3 PUFA and hair mercury among 1008 men aged 42–60 from Kuopio Ischaemic Heart Disease Risk Factor StudyFootnote * (Mean values and 95 % confidence intervals)
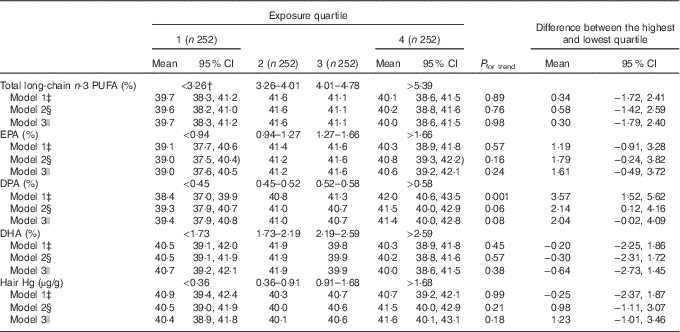
* The association of serum long-chain n-3 PUFA and hair Hg with heart rate recovery was analysed using ANCOVA.
† Medians.
‡ Model 1 is adjusted for age and examination year.
§ Model 2 is adjusted for model 1+BMI (kg/m2), diabetes (yes/no), hypertension (yes/no), smoking (yes/no), education (years), income (euro), leisure-time physical activity (kJ/week (kcal/week)), intake of alcohol (g/week), energy intake (kJ/d (kcal/d)) and blood glucose (mmol/l).
|| Model 3 is adjusted for model 2+hair Hg (in analyses with fatty acids) or total long-chain n-3 PUFA (in analyses with Hg).
Further adjustment for the potential confounders (model 2) and hair Hg (model 3) slightly attenuated the association. Hair Hg concentration was not associated with HR recovery (Table 4).
Discussion
In this study among 1008 generally healthy middle-aged and older men from Eastern Finland, higher serum total long-chain n-3 PUFA concentration was associated with lower resting HR. Generally similar associations with resting HR were observed with the individual long-chain n-3 PUFA EPA, DPA and DHA, although the associations with EPA appeared slightly stronger. No associations were observed with peak HR during exercise or HR recovery after exercise, except for a borderline statistically significant association between serum DPA and better 2-min HR recovery. However, the clinical significance of these associations was quite modest. Hair Hg content was associated with lower peak HR and it only slightly attenuated the associations of the serum long-chain n-3 PUFA.
Previously in this study population, higher serum long-chain n-3 PUFA concentration has been inversely associated with risk of CVD( Reference Virtanen, Voutilainen and Rissanen 27 , Reference Virtanen, Laukkanen and Mursu 29 ) and with CVD risk factors, including C-reactive protein( Reference Reinders, Virtanen and Brouwer 45 ), high blood pressure( Reference Virtanen, Nyantika and Kauhanen 46 ), prolonged QT- and JT intervals( Reference Tajik, Kurl and Tuomainen 47 ), and low exercise cardiac power( Reference Tajik, Kurl and Tuomainen 48 ).
In the present study, we found that higher serum long-chain n-3 PUFA concentrations were inversely associated with resting HR. The extreme-quartile mean difference was 2·2 beats/min. This finding is in line with several supplementation trials and observational studies. For example, according to a meta-analysis of thirty trials, fish oil supplementation (3·5 g/d of EPA+DHA) reduced the resting HR by 2·5 beats/min( Reference Mozaffarian, Geelen and Brouwer 11 ). In population-based studies, among 9758 older men, resting HR was lower in high fish consumers compared with non-consumers( Reference Dallongeville, Yarnell and Ducimetiere 12 ). Similarly, results of two large cross-sectional studies among older adults showed that intake of fish or long-chain n-3 PUFA from fish was inversely associated with resting HR( Reference Mozaffarian, Prineas and Stein 13 , Reference Mozaffarian, Gottdiener and Siscovick 14 ). An inverse association was also observed between erythrocyte concentration of long-chain n-3 PUFA (EPA+DHA) and resting HR in three small cross-sectional population-based studies among middle-aged and older women( Reference Valera, Dewailly and Anassour-Laouan-Sidi 16 ) and in general populations( Reference Ebbesson, Devereux and Cole 15 , Reference Valera, Suhas and Counil 17 ).
Exploration of the mechanism underlying this inverse association is beyond the scope of the current study and future research is warranted. However, impact of the long-chain n-3 PUFA on cardiac autonomic regulation, reduction of sympathetic activity and enhancement of parasympathetic activity, which may lead to a lower myocyte-beating rate( Reference Kang and Leaf 49 ). Other potential mechanisms include the influence of these fatty acids on the function of ion channels (Na+ and Ca2+) in heart cell membranes( Reference Xiao, Sigg and Leaf 50 ).
Regular exercise and physical activity are associated with lower HR( Reference Gregoire, Tuck and Hughson 51 ) and better HR recovery( Reference Darr, Bassett and Morgan 52 ) and with lower risk of CVD( Reference Thompson, Buchner and Pina 53 ). However, in this study population higher serum long-chain n-3 PUFA concentration was not associated with higher physical activity (Table 1), therefore higher physical activity does not explain the findings. Peak HR during exercise and HR recovery after exercise are known predictors of CVD mortality( Reference Sandvik, Erikssen and Ellestad 9 , Reference Cole, Foody and Blackstone 10 ). However, knowledge regarding the association of the long-chain n-3 PUFA and methylmercury with peak HR during exercise and HR recovery is lacking. As far as we know, the current study is the first population-based study to report such associations. In agreement with the lack of association between the long-chain n-3 PUFA and peak HR in the present study, fish oil supplementation has had no effect on peak HR during exercise among healthy men( Reference Macartney, Hingley and Brown 18 , Reference Hingley, Macartney and Brown 19 ), football players( Reference Buckley, Burgess and Murphy 20 ) or men with history of CHD( Reference O’Keefe, Abuissa and Sastre 21 , Reference Vacek, Harris and Haffey 22 ). In contrast, in two small experimental studies, fish oil supplementation decreased peak HR during exercise test among dogs( Reference Billman and Harris 23 ) and well-trained men( Reference Peoples, McLennan and Howe 24 ). The lack of association in the current study and in most other studies may be explained by the fact that peak HR is determined largely by age and genetic( Reference Bray, Hagberg and Perusse 54 ). In the experimental studies also the differences in, for example, the study setting, dosage or length of supplementation period might have had an impact on the outcome of the study. Regarding the HR recovery, a few small supplementation studies have reported a faster HR recovery after an exercise test by fish oil supplementation( Reference Macartney, Hingley and Brown 18 , Reference Hingley, Macartney and Brown 19 , Reference O’Keefe, Abuissa and Sastre 21 , Reference Billman and Harris 23 ), but there are no previous population study data about the associations with the long-chain n-3 PUFA. More studies are needed to evaluate the impact of the long-chain n-3 PUFA, both from natural sources and supplements, on peak HR during exercise and HR recovery after exercise.
In the current study, EPA had a slightly stronger association with resting HR than the other long-chain n-3 PUFA and only DPA was associated with better HR recovery. These findings differ from our previous observations in this study population, where DHA had an inverse association with the risk of atrial fibrillation( Reference Virtanen, Mursu and Voutilainen 55 ) and sudden cardiac death( Reference Virtanen, Laukkanen and Mursu 29 ), whereas EPA or DPA were not associated with these outcomes. This suggests that the stronger inverse associations between DHA and these arrhythmic cardiac outcomes are not explained by its impact on HR in this study population. The potential mechanisms for these different associations of HR with individual fatty acids are not completely clear, although there is some data that EPA and DHA have different effects on the function of membrane ion channels in isolated human arterial myocytes. EPA may be more effective inhibitor of the voltage-gated Na+, whereas DHA mostly inhibits delayed-rectifier K+ current( Reference Li, Sun and Zhang 56 ). It has been reported that structure/function of voltage-gated Na+ is related to the risk of cardiac arrhythmias( Reference George 57 ).
Compared with EPA and DHA, the cardioprotective and mechanistic properties of the mainly endogenously produced long-chain n-3 PUFA, DPA, are less well known. DPA has been found to have a similar inverse association with fatal CHD as EPA and DHA, but a stronger inverse association with total CHD risk( Reference Del Gobbo, Imamura and Aslibekyan 4 ), suggesting that DPA may also have some cardioprotective properties.
Previously in the KIHD cohort, higher hair Hg content has been associated with higher risk of CVD and it has also attenuated the inverse associations of the serum long-chain n-3 PUFA with CVD outcomes( Reference Virtanen, Laukkanen and Mursu 29 ). The exact mechanisms for this are not known, although, for example, reduction in the antioxidative capacity and increasing free radical stress have been suggested( Reference Roman, Walsh and Coull 26 ). Because methylmercury is a neurotoxicant, it is also possible that the adverse cardiovascular effects could be explained by its effects on the autonomic nervous function( Reference Roman, Walsh and Coull 26 ). However, in the current study, we did not observe an association between hair Hg content and resting HR or HR recovery, and adjustment for Hg only slightly attenuated the associations with the long-chain n-3 PUFA. Although we did find that higher hair Hg content was associated with lower peak HR, this finding needs to be interpreted cautiously because, as mentioned, peak HR is mainly determined by age and genetics and there is no clear mechanism how Hg could affect peak HR, but not resting HR or HR recovery. Our result of no association with resting HR is in agreement with the previous findings from an experimental study( Reference Yaginuma-Sakurai, Murata and Shimada 31 ) and from two cross-sectional studies among healthy adults( Reference Valera, Dewailly and Poirier 32 , Reference Valera, Dewailly and Poirier 33 ). In contrast, two studies have found that higher blood methylmercury concentration was associated with increased resting HR among adults( Reference Valera, Dewailly and Poirier 34 , Reference Oka, Matsukura and Okamoto 35 ). These conflicting results might be due to, for example, different methods for measuring the exposure to Hg. Hair Hg concentration is a good marker of long-term Hg exposure, whereas blood Hg reflects relatively short-term exposure to Hg( Reference Roman, Walsh and Coull 26 ).
The strengths of our study include the use of serum long-chain n-3 PUFA and hair Hg as exposures instead of dietary intakes. Because serum fatty acids and hair Hg are objective biomarkers for exposure( Reference Roman, Walsh and Coull 26 , Reference Hodson, Skeaff and Fielding 58 ), their use reduces the bias by misclassification, which would attenuate the associations towards the null. Other strengths include the extensive examination of potential confounders and the large number of participants with the assessment of resting and exercise-induced HR. A potential limitation was that the participants were middle-aged and older men from Eastern Finland, so the findings may not be generalisable to other populations or to women.
In conclusion, higher circulating concentrations of the long-chain n-3 PUFA were inversely associated with resting HR, a finding well supported by the previous research. As higher resting HR is associated with increased risk of CVD( Reference Okamura, Hayakawa and Kadowaki 5 , Reference Legeai, Jouven and Tafflet 6 ), this result could partially explain how long-chain n-3 PUFA may reduce the risk of CVD. Only the minor long-chain n-3 PUFA, DPA, was associated with faster HR recovery and none of the fatty acids was associated with peak HR during exercise. Unlike with the associations with CVD outcomes in this study population( Reference Virtanen, Voutilainen and Rissanen 27 – Reference Virtanen, Laukkanen and Mursu 29 ), Hg exposure only slightly attenuated the associations of the long-chain n-3 PUFA with HR. This indicates that its effects on HR do not explain the negative impact of Hg exposure on CVD health in this study population. More large-scale studies in diverse populations are needed to studies to confirm these findings.
Acknowledgements
The authors thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health, University of Eastern Finland, for data collection.
The study was supported by Olvi Foundation, Antti and Tyyne Soininen Foundation, and Saara Kuusisto and Salme Pennanen Foundation (B. T.).
The authors’ contributions were as follows: B. T., S. K., T.-P. T., K. S. and J. K. V. contributed to the conception and design of the research; S. K., and T.-P. T. acquired data; B. T. and J. K. V. analysed data and interpreted the results; B. T. drafted the manuscript; all authors critically revised the paper and approved the final version.
None of the authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003191







