Soya has a high content of complete protein. Its Protein Digestibility-Corrected Amino Acid Score is 0·9–1(Reference Hughes, Ryan and Mukherjea1). It is widely consumed in many Asian countries, especially soya milk, tofu, miso and natto and has increasingly become popular in western countries. In 2018, the average per capita per year soyabean food consumption was 2·06 kg in Asia (7·94 kg in Japan and 3·61 kg in China), compared with 0·21 kg in the USA (0·11 kg in the USA and 1·2 kg in Canada) and 0·28 kg in Europe (0·21 kg in the UK, 0·97 kg in Germany)(2). Soya has been approved as a rich nutrient food containing complex carbohydrates (mainly stachyose and oligosaccharides) that stimulate bifidobacterial(Reference Inoguchi, Ohashi and Narai-Kanayama3). It is a good source of dietary fibre, which helps lower glycaemic indexes(Reference Wolever4), and vitamins and minerals such as vitamin K1, folate, Fe, Ca, Mg and potassium(Reference Rebello, Greenway and Finley5). It stands out from the rest of the legumes for high isoflavone content, a promising agent for cancer chemoprevention and treatment(Reference Sarkar and Li6). The USA 2020 Dietary Guidelines include soya in the vegetables, dairy, protein foods and oil elements(7). It recommends consuming 5 ounces (142 g) of soya protein products per week.
A systematic review of greenhouse gas emissions for fresh food showed that the average global warming potential of soyabean production (0·58 kg CO2-eq/kg) was much lower than beef (28·73 kg CO2-eq/kg), lamb (27·91 kg CO2-eq/kg), pork (5·85 kg CO2-eq/kg) and chicken (4·12 kg CO2-eq/kg) meat. Meat consumption is positively associated with a list of CVD, cancers and diabetes II(Reference Yip, Lam and Fielding8), which are leading causes of death worldwide. Soya products have been increasingly recognised as an alternative to meat intakes to reduce non-communicable diseases and combat climate changes(Reference Rebello, Greenway and Finley5,Reference Kumar, Chatli and Mehta9,Reference Weinrich10) . This scoping review aimed to identify the best-published meta-analysis estimates of observational associations of dietary soya food intakes with CVD, cancers and diabetes II. However, the associations of soya intakes with non-communicable diseases in specific regions or specific groups such as low- and middle-income countries are beyond this study scope.
Methods
This study adopted well-validated systematic review assessment tools, Cochrane Review measures(11), the AMSTAR 2 checklist(Reference Shea, Reeves and Wells12) and a published algorithm specifically designed for conducting a scoping review of similar meta-analyses(Reference Yip, Lam and Fielding8,Reference Yip, Chan and Fielding13) .
Inclusion criteria for studies
In this scoping review, unfermented soya refers to soya food products such as soyabean, soya milk and tofu. Fermented soya refers to soya food products such as miso, natto and tempeh. Total soya refers to a combination of both unfermented and fermented soya. Meta-analyses meeting all the following conditions were included in the scoping review:
-
1. Estimated the direct associations of dietary total soya intakes and the subgroups: unfermented and fermented food intakes, with non-communicable diseases.
-
2. Quantified the pooled relative risks (RR), odd ratio (OR) or hazard ratios (HR) directly associated with dietary soya intakes as in a dietary food group, or as protein or isoflavones estimates from dietary soya food intakes.
-
3. Provided at least the number of individual studies or effects included in the analyses.
Articles that presented conflicts of interest related to the production and commercialisation of soya and its derived products and intervention studies were excluded. Meta-analyses investigating the following were also excluded:
-
1. The associations of diseases with a specific soya product under the fermented or unfermented subgroup such as tofu only or miso only.
-
2. The associations of diseases with biomarkers such as urinary or plasma protein or isoflavones instead of estimates from dietary soya food intakes.
-
3. The associations of disease biomarkers with isoflavones supplements.
-
4. The associations of disease biomarkers with soya food intakes.
Outcome measures
Two major categories of outcome measures were considered. The first category included RR, OR or HR of CVD, cancer and diabetes II incidences and/or mortalities over some time for high v. low food intakes with the 95 % CI provided. The second category included RR, OR or HR per unit of intakes such as per gram(s) or milligram(s), with the 95 % CI provided.
Literature search
Systematic searches were conducted according to Cochrane guidelines(11) in PubMed via Medline, Science Direct, Google Scholar and Cochrane Library databases without limit. A combination of search terms: ‘meta-analysis’; ‘review’; ‘disease’; ‘health’; ‘soy’; ‘isoflavones’ and ‘humans’ were used. Literature searching was last updated in May 2021. Potential abstracts were retrieved and screened. Identified potential full-text articles were then retrieved for further analysis. Full-text articles identified from reference lists of screened reports were also searched, retrieved and screened. All studies meeting the selection criteria were included in the scoping review. Two authors (C. S.C.Y. and W.C.) conducted the literature search and selection independently. Studies were selected based on mutual agreement.
Data extraction from each meta-analysis
Descriptive data: author, year of publication, intake soya group (total, unfermented and fermented), the number of component estimates or studies included in each meta-analysis, publication year range of the included component studies and the association status or direction were extracted for each risk–disease pair. For each identified statistically significant risk–disease meta-analysis estimate, the pooled relative risk, OR or HR at 95 % confidence level, heterogeneity (I2), PHeterogeneity values and significance of publication bias were extracted. For a risk–disease pair, if the pooled case–control studies, cohort studies and case–control and cohort studies combined estimates were provided, the combined estimates were extracted. For each best-identified meta-analysis estimate (BIE), the frequency of geographic regions involved in the estimation, the significant:non-significant ratio of the included component estimates and the total percentage of significant estimates were calculated. Confounding adjustments of each component study for each BIE were also examined. Systematic reviews have shown alcohol, meat, fruit and vegetable intakes were significantly associated with CVD, cancers and diabetes II(Reference Yip, Lam and Fielding8,Reference Yip, Chan and Fielding13–Reference Yoon, Jung and Lee18) . Therefore, the number of component studies adjusted for alcohol, meat, fruit and vegetable intakes in each BIE was also recorded. Full-text component studies were retrieved for any missing variables. Two authors (C.S.C.Y. and Y.C.Y.) conducted the data extraction independently.
Methodologic quality of the included studies
The AMSTAR 2 checklist, a sixteen-item assessment tool with a maximum score of 16, was used to evaluate whether the scientific qualities of the included studies in a meta-analysis study were assessed, whether the methods used to combine the findings of the component studies were appropriate, and whether the conclusions were appropriately formulated(Reference Shea, Reeves and Wells12).
Identification of statistically significant risk–outcome pairs and the best estimates
At the 95 % confidence level, when the P-value of an estimate was < 0·05, the association was regarded as statistically significant. Otherwise, the association was regarded as non-significant. For a risk–disease pair, when only one meta-analysis was found, the estimate from the meta-analysis was taken as the BIE. When multiple meta-analyses were identified, the following rules were applied:
-
1) When all the estimates were significant, the outcome of the meta-analysis that included the most up-to-date component studies was taken as the BIE.
-
2) When both significant and non-significant occurred:
-
i. The outcome of the meta-analysis that included at least 80 % of the most up-to-date component studies captured in all the competing meta-analyses was taken as the best estimate, otherwise,
-
ii. The association was taken as inconclusive.
-
-
3) For 1) and 2)i, priorities were given to the meta-analysis outcome that provided the specific heterogeneity (I2) and Pheterogeneity values and the lower I2 and/or higher Pheterogeneity values.
Summaries of main results
Structured narrative presentation of the results.
Results
Included reports
Initially, 2349 titles and 163 abstracts were identified and screened from database searching, of which 134 abstracts were excluded as irrelevant, due to not meeting the inclusion criteria or were duplicates, and twenty-nine full-text articles were retrieved (Fig. 1). Another eighteen full-text articles were identified from the reference lists of the retrieved articles. A total of forty-seven full-text articles were retrieved and evaluated. Eighteen of them did not fully meet the selection criteria, and another had a critical methodological issue. As a result, nineteen full-text reports were excluded (Appendix A1). The remaining twenty-eight articles(Reference Applegate, Rowles and Ranard19–Reference Yan and Spitznagel46), published between 2000 and 2021, were included in the final review (Appendix A2). Six of them were evaluated as high in quality(Reference Chen, Rao and Zheng21,Reference Li, Ruan and Peng24,Reference Lou, Li and Yan25,Reference Nachvak, Moradi and Anjom-Shoae28,Reference Namazi, Saneei and Larijani29,Reference Zhang, Chen and Liu42,Reference Yang, Va and Wong45) , having AMSTAR scores ≥ 13. Ten of them were evaluated as moderate in quality(Reference Applegate, Rowles and Ranard19,Reference Hwang, Kim and Jee22,Reference Kim, Kang and Lee23,Reference Lu, Pan and Ye26,Reference Myung, Ju and Choi27,Reference Yan, Zhang and Li40,Reference Yu, Jing and Li41,Reference Zhong and Zhang43,Reference Zhu, Sun and Qi44,Reference Yan and Spitznagel46) . The rest were evaluated as low in quality, having AMSTAR scores < 10. Most component studies were conducted in Asia, especially in China and Japan, followed by the USA.
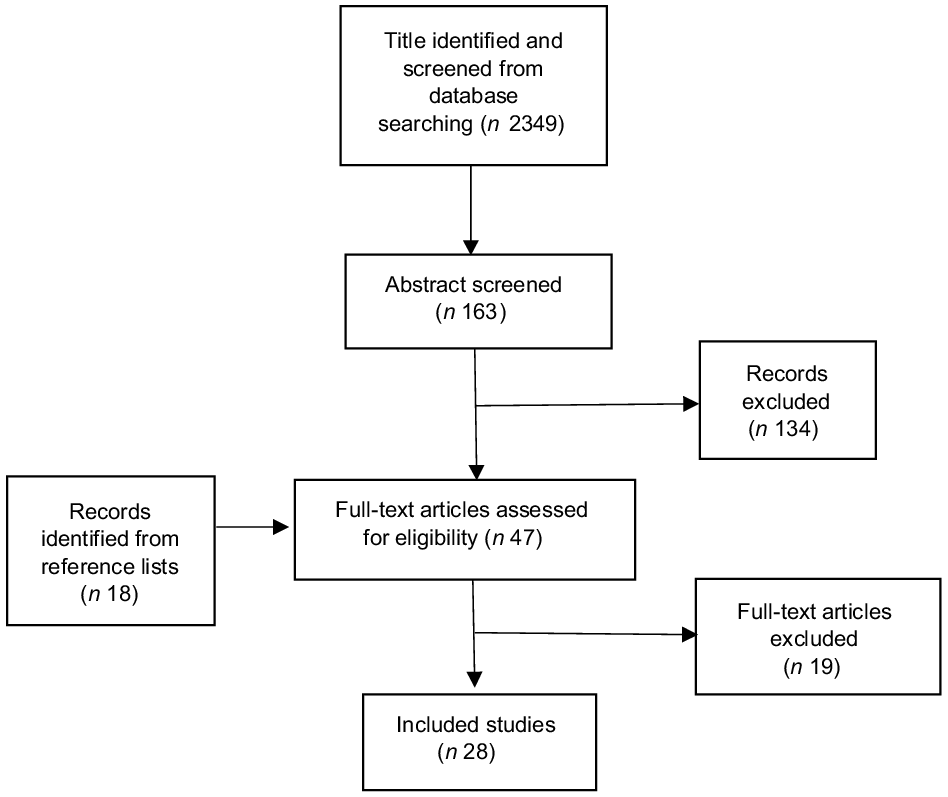
Fig. 1. Literature searching flow chart.
A total of forty-one risk–disease pairs were investigated (Table 1 and Fig. 2). Fifty-four meta-analyses estimated the associations of total soya intakes with twenty-six disease outcomes. Sixteen meta-analyses estimated the associations of unfermented soya intakes with nine disease outcomes, and eleven meta-analyses estimated the associations of fermented soya intakes with six disease outcomes. Most of the meta-analyses estimated the risks of high v. low intakes, only a few evaluated dose–response: 47 v. 7 for total soya intakes, 16 v. 1 for unfermented soya intakes and 11 v. 0 for fermented soya intakes, respectively. It could be because the exposure soya product types and level measures varied among the included component studies in each meta-analysis, and the exact quantities of ‘high’ and ‘low’ intakes in component studies were different. Therefore, standardisation of exposure measures remained a challenge. Among the seventy-four high v. low intake estimates, 57 % were significantly negatively associated, the association of fermented soya and gastric cancer estimate was positive and the rest were non-significant. Among the eight identified dose–response meta-analysis estimates, 50 % were significantly negatively associated, and the rest were non-significant.
Table 1. Selection of best identified meta-analysis studies of the associations of total soya intakes with non-communicable diseases: high v. low intakes

RR, relative risk.
n: number of component estimate.
* Estimates based on information presented in the report.
† Number of included studies might include higher number of component estimate in the analysis. Year: publication years of component studies. NR, Not reported/unclear; NSig, non-significant; Q score, AMSTAR score.

Fig. 2. Number of investigated associations between soya intakes and burden of diseases.
The best-identified meta-analysis estimates for high v. low total soya intake
A total of twenty articles(Reference Chen, Rao and Zheng21,Reference Hwang, Kim and Jee22,Reference Li, Ruan and Peng24–Reference Namazi, Saneei and Larijani29,Reference Qin, Xu and Wang31–Reference Tse and Eslick33,Reference Woo, Park and Oh36,Reference Yan and Spitznagel38–Reference Zhong and Zhang43,Reference Yang, Va and Wong45,Reference Yan and Spitznagel46) estimated the associations of total soya intake with CVD, cancers and diabetes II. Twenty-four risk–disease pairs were investigated (Table 1). No high v. low intake estimate was identified for the total soya intake-breast cancer mortality pair but a dose–response. Multiple meta-analyses were identified for the all-cause, CVD and cancer mortalities; stroke, CHD incidences and gastrointestinal, gastric, colorectal, colon, rectal, endometrial, breast and prostate cancer incidences. Meta-analyses of cancer mortalities; and stroke, CHD and colorectal cancer risks yielded mixed results. The significant negative associations found in Nachvak et al.(Reference Nachvak, Moradi and Anjom-Shoae28), Yan et al.(Reference Yan, Zhang and Li40) and Yu et al.(Reference Yu, Jing and Li41) were evaluated as the BIE for these risk–disease pairs, as they included all or more than 80 % of the component studies captured in the competing meta-analyses.
Estimates from eleven reports(Reference Li, Ruan and Peng24,Reference Lu, Pan and Ye26–Reference Nachvak, Moradi and Anjom-Shoae28,Reference Tse and Eslick33,Reference Yan, Zhang and Li40,Reference Zhang, Chen and Liu42,Reference Zhong and Zhang43,Reference Yang, Va and Wong45,Reference Yan and Spitznagel46) were identified as the BIE for total soya intakes (Table 2 and Fig. 3). The component studies were adjusted for confounding effects ranging from zero to more than forty confounders. But most of them were not adjusted for alcohol, meat, fruit and vegetable intakes. Nachvak et al.(Reference Nachvak, Moradi and Anjom-Shoae28) were identified as the BIE for CHD and cancer mortalities. The study found high intakes might reduce CHD mortalities by 21 %, cancer mortalities by 10 %, gastric cancer mortalities by 51 %, colorectal cancer mortalities by 41 % and lung cancer mortalities by 21 % when compared against low intakes. The heterogeneities were non-significant except for CHD mortality. However, all component studies were conducted in Asia, except for cancer mortalities, where two out of the nine studies were conducted in America. Furthermore, the number of component studies involved in each meta-analysis was unclear. Therefore, the significant-to-non-significant ratios and the significant percentage weightings of the analyses were also unclear. Yan et al.(Reference Yan, Zhang and Li40) were identified as the BIE for CVD, stroke and CHD risks, and Li et al.(Reference Li, Ruan and Peng24) were identified for diabetes II risks. The studies found high soya intakes might reduce CVD incidences by 16 %, stroke incidences by 18 %, CHD incidences by 17 % and diabetes II incidences by 23 % when compared against low soya intakes. However, the heterogeneities were significant, and most of the components studies were conducted in Asia. The ratios of significance among the case–control component estimates were high but very low among the cohort component estimates. The significant percentage weightings for the CVD, stroke and CHD estimates were also low. Most of the component studies for the BIE for lung, gastrointestinal, gastric, colorectal and postal cancers were conducted in Asia. High soya intakes might reduce lung cancer incidences by 23 %(Reference Yang, Va and Wong45), gastrointestinal cancer incidences by 7 %(Reference Tse and Eslick33), gastric cancer incidences by 15 %(Reference Lu, Pan and Ye26), colorectal cancer incidences by 21 %(Reference Yu, Jing and Li41) and prostate cancer incidences by 26 % when compared against low soya intakes. A higher number of component studies done in regions other than Asia were involved in the BIE for endocrine-related gynaecological, endometrial, ovarian and breast cancers. High soya intakes might reduce endocrine-related gynaecological cancer incidences by 39 %(Reference Myung, Ju and Choi27), endometrial cancer incidences by 19 %(Reference Zhang, Chen and Liu42), ovarian cancer incidences by 48 %(Reference Myung, Ju and Choi27) and breast cancer incidences by 14 %(Reference Zhong and Zhang43). However, not all the BIE reported full heterogeneity estimates or reported/applied an appropriate weighting for combining component estimates in the meta-analysis process.
Table 2. The best identified meta-analysis pooled estimates for the associations of soya intakes with non-communicable diseases
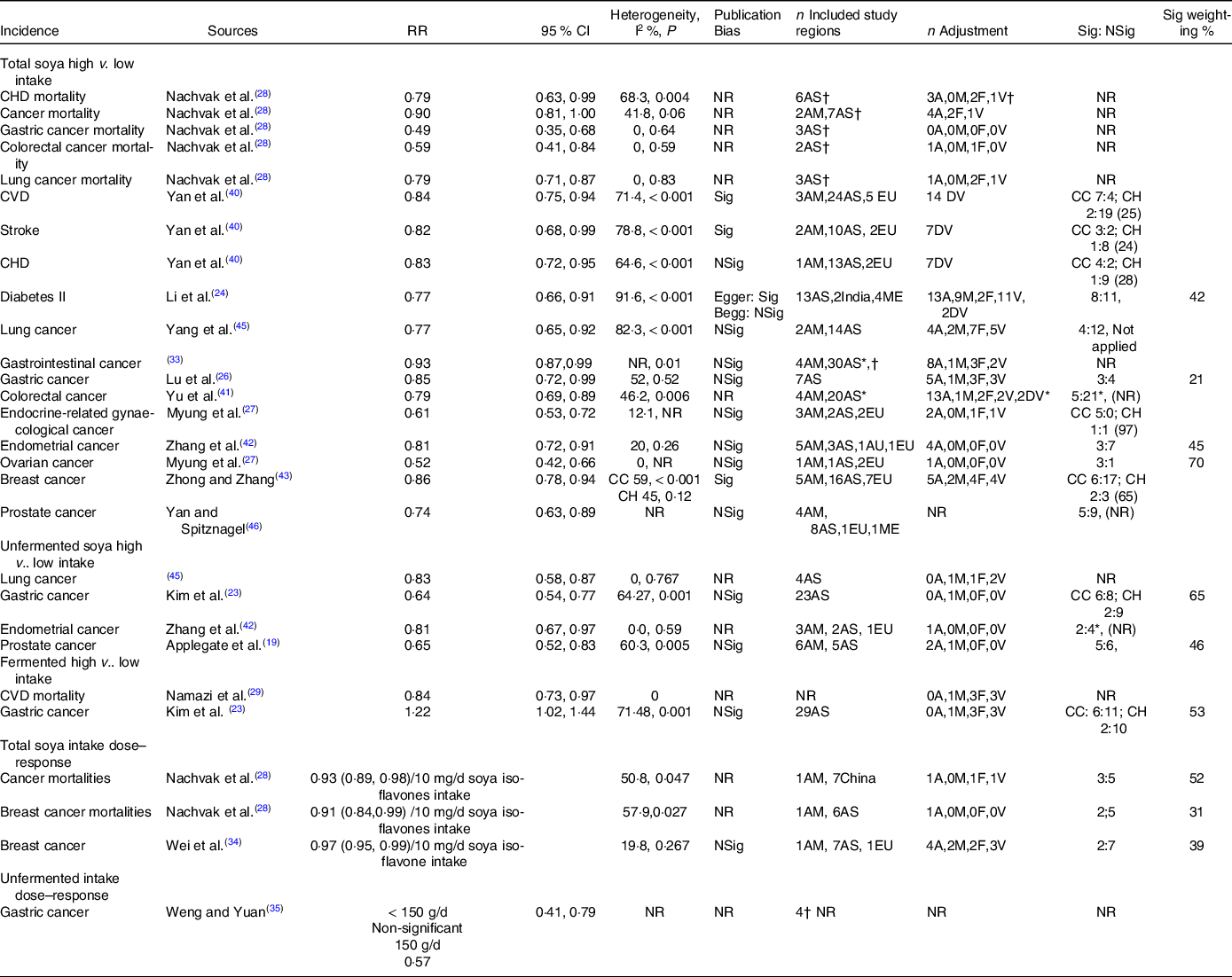
NR, not reported/unclear; AM, America; AS, Asia; AU, Australia; EU, Europe; ME, multi-ethnic; A, alcohol intake; M, meat intake; F, fruit intake; V, vegetable; DV, unspecified dietary variables; CC, case–control study; CH, cohort study; WC, Western country; AC, Asian country; Sig, significant; NSig, non-significant; n, number of component estimate.
* Estimates based on information presented in the report.
† Number of included studies, might include higher number of number of component estimate in the analysis.
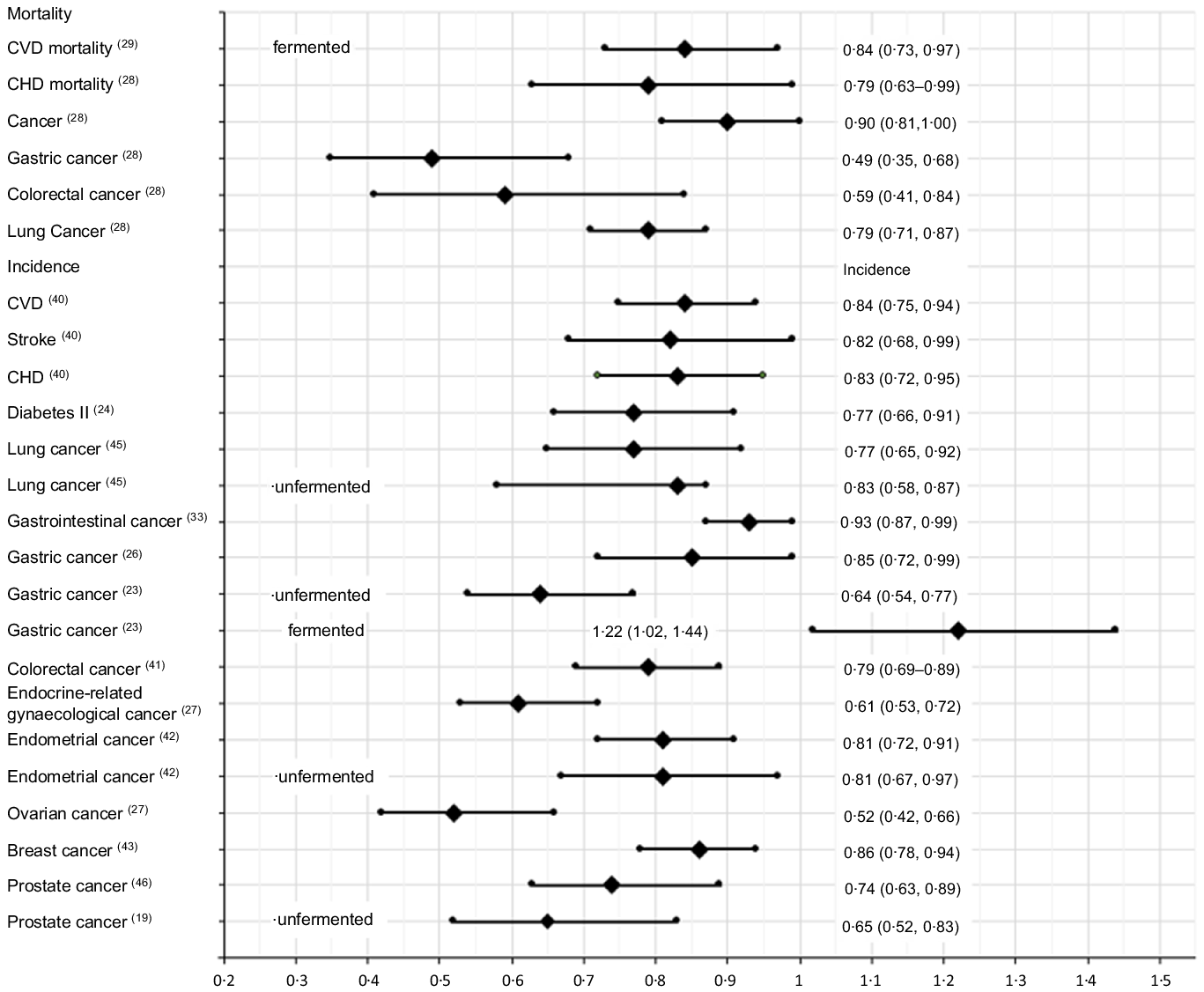
Fig. 3. The best identified meta-analysis estimates of the associations of soya intakes with burden of diseases: high v. low intakes.
The best-identified meta-analysis estimates for high v. low unfermented and fermented soya intakes
A total of fourteen reports(Reference Wolever4,Reference Applegate, Rowles and Ranard19,Reference Becerra-Tomás, Papandreou and Salas-Salvadó20,Reference Hwang, Kim and Jee22–Reference Li, Ruan and Peng24,Reference Namazi, Saneei and Larijani29,Reference Weng and Yuan35–Reference Wu, Yang and Pike37,Reference Zhang, Chen and Liu42,Reference Zhu, Sun and Qi44,Reference Yan and Spitznagel46,Reference Yang, Chen and Xu47) estimated the associations of unfermented and fermented soya intakes with the risks of cancers and diabetes II (Table 1, Appendix A3). Namazi et al.(Reference Namazi, Saneei and Larijani29) found high fermented soya intakes reduced CVD mortalities by 16 %. Multiple meta-analysis estimates were identified for the risks of diabetes II and lung, gastric, colorectal and prostate cancers. Kim et al.(Reference Kim, Kang and Lee23) included more numbers of up-to-date component estimates compared with Weng and Yuan(Reference Weng and Yuan35) and Wu et al.(Reference Wu, Yang and Pike37) and Wu et al.(Reference Wu, Yang and Pike37) also had a low AMSTAR score. Therefore, estimates in Kim et al.(Reference Kim, Kang and Lee23) were identified as the BIE for the associations of unfermented and fermented soya intakes with the risk of gastric cancer. The study found high unfermented soya intakes reduced the risk by 36 %, but high fermented soya intakes increased the risk by 22 %, and the heterogeneities of both of BIE were significant (Table 2 and Fig. 3). Zhang et al.(Reference Zhang, Chen and Liu42) were identified as the BIE for the associations of unfermented soya intakes with the risk of endometrial cancer. The study found high unfermented soya intakes might reduce the risk by 19 %, and heterogeneity was non-significant. Applegate et al.(Reference Applegate, Rowles and Ranard19) were identified as the BIE for the association of unfermented soya intakes with the risk of prostate cancer. The study found high unfermented soya intakes might reduce the risk by 35 %, and the heterogeneity was significant. Zhu et al.(Reference Zhu, Sun and Qi44) and Woo et al.(Reference Woo, Park and Oh36) investigated the association of unfermented soya with the risk of colorectal cancer, which yielded mixed results. As the number of component estimates involved in the meta-analyses in each of the studies was unclear, the association was deemed inconclusive.
Dose–responses best-identified meta-analysis estimates
Four articles provided meta-analysis dose–response estimates(Reference Nachvak, Moradi and Anjom-Shoae28,Reference Pearce, Fanidi and Bishop30,Reference Wei, Lv and Guo34,Reference Weng and Yuan35) (Table 1). Nachvak et al.(Reference Nachvak, Moradi and Anjom-Shoae28) used soya isoflavones intake estimates as a exposure measure. Wei et al.(Reference Wei, Lv and Guo34) converted soya food intakes into soya isoflavones intakes for all component studies. On the other hand, Pearce et al.(Reference Pearce, Fanidi and Bishop30) and Weng and Yuan(Reference Weng and Yuan35) used g/d of soya intake and soya food intake, respectively, but did not clearly define the difference between soya and soya food in the articles. Nachvak et al.(Reference Nachvak, Moradi and Anjom-Shoae28) found each 10 mg/d increase in soya isoflavones intake might reduce cancer mortalities by 7 % and breast cancer mortalities by 9 %, while Wei et al.(Reference Wei, Lv and Guo34) found it might reduce breast cancer incidences by 3 % (Table 2). Weng and Yuan(Reference Weng and Yuan35) found taking 150 g/d of soya food might reduce gastric cancer incidences by 43 % while taking 50 g/d and 100 g/d made no significant difference. But the study did not provide the details of the included studies.
Discussion
Summary of main results
This scoping review identified and evaluated twenty-eight meta-analysis reports of the associations between soya intakes and non-communicable diseases published between 2000 and 2021. It identified eighteen significantly negatively associated risk–disease pairs for total soya intakes, four significantly negatively associated risk–disease pairs for unfermented soya intakes, four significantly negatively associated risk–disease pairs and one significantly positively associated risk–disease pair for fermented soya intakes when compared high v. low intakes (Table 2). The largest significant risk decrease found was gastric cancer mortalities, followed by colorectal cancer mortalities, then ovarian and endocrine-related gynaecological cancers incidences (Fig. 3). Significantly negatively associated risk–disease dose–responses were also identified. However, the estimations might be subject to confounding effects from other factors such as alcohol, meat, fruit and vegetable intakes.
Potential biases in the review process
A recent umbrella review investigated the health outcomes of soya and isoflavone intakes(Reference Li, Wu and Zhuang48). The study included a mixture of published English reports of randomised trials and observational studies of dietary or supplementary soya or isoflavone among healthy or having pre-existing illness humans. The study found that soya and isoflavone intakes were beneficial for a list of CVD and cancer diseases and gynaecological, metabolic, musculoskeletal, endocrine, neurological and renal outcomes but harmful for gastric cancer. However, among the ‘114 identified eligible full texts with meta-analyses’, which the report claimed, the report only presented information and findings from twenty-eight published meta-analysis reports. The evaluation and selection process of the final twenty-eight reports from the ‘114 eligible full texts with meta-analyses’. The titles of the twenty-eight selected reports were not presented in the reference list, and information about the rest of the eighty-six full texts could not be found. Therefore, most of the included studies were unknown. As a result, the relative quality of the meta-analysis outcomes compared with those ‘involved but unknown’ studies remained unknown. Furthermore, mixing randomised trials and observational studies, dietary and supplementary soya and isoflavone and healthy and having pre-existing illness humans in a meta-analysis may create high uncertainty in dietary outcomes and intervention outcomes, therefore, providing little dietary or clinical utility.
This scoping review used a combination of well-validated and published assessment tools, especially the more advanced AMSTAR 2 checklist and the algorithm tailored to capture the specific characteristics of interest in the targeted meta-analyses and minimise potential biases. The assessment algorithm, format and criteria were clearly defined to ensure consistent and systematic evaluation of each meta-analysis and its component estimates. It applied the Cochrane search strategies in a broad range of databases and search terms without the limit to ensure as many relevant studies were identified as possible and minimised exclusion of relevant studies that met the well-defined selection criteria. Hand searching addressed the insufficiencies in electronic database searches. As a result, a total of twenty-eight reports were identified meeting this scoping review selection criteria of dietary soya food product intakes compared with only six articles found by Li et al.(Reference Li, Wu and Zhuang48). Hence, a list of eligible publications might be missing from Li et al.(Reference Li, Wu and Zhuang48)’s umbrella review. Literature search and data extraction were performed by multiple researchers, where inconsistencies were resolved by further investigation and mutual agreement, hence minimising individual personal bias and data extraction errors. Reviewing the direct impacts of dietary soya food product intakes instead of biological components and mortalities and disease incidences instead of biomarker risk factors minimised the uncertainties that other foods’ constituents might be the causal factors and provided daily dietary utility for non-specialists. Additional clearly defined procedures to handle inconsistencies and qualities comparison of competing meta-analyses in a specific risk–disease pair also further ensured the quality of the BIE. Therefore, every effort has been made to minimise potential bias and ensure integrity. Providing the source and quality details of each meta-analysis included in the scoping review ensured the best transparency and minimum effort in future review quality verification, modifications and update work.
Limitations of the scoping review
In addition to the heterogeneities, publication bias and potential confounding effects from alcohol, meat, fruit and vegetable intakes, the BIE were also subject to other limitations and uncertainties presented in the component estimations and the meta-analyses themselves. Some component estimates were not adjusted for any confounding effects. Some BIE might not apply any weighting in the pooled evaluations. Therefore, the BIE were subject to different levels and extent of uncertainties and potential residual confounding effects. However, this would not affect the BIE selection outcomes. It is because high-level duplication of component estimates occurred when multiple meta-analyses existed for a specific risk–disease pair. Additionally, to ensure that the best estimates were selected, the selection process used in this scoping review was also designed to manage such characteristics. All estimates were obtained from case–control and cohort studies, hence subject to uncertainties in natural intervention practices and past dietary patterns. This scoping review reported the BIE for total soya, unfermented and fermented soya intakes. The associations of other subcategories such as tofu, soya milk and miso could be different(Reference Hwang, Kim and Jee22,Reference Qin, Xu and Wang31–Reference Tse and Eslick33,Reference Woo, Park and Oh36,Reference Yan, Spitznagel and Bosland39) .
The ratios of fermented and unfermented soya in the total soya category; the ratios of soya product subcategories under each fermented, unfermented and total soya category and the exact amount of ‘high’ and ‘low’ intakes were generally unclear and non-uniform across the component studies involved in each meta-analysis. Between-study heterogeneities were common in meta-analyses. Heterogeneities could be driven by differences in study designs, combinations of subtypes and combination ratios within each fermented, unfermented and total soya category resulting in different effect sizes, characteristics of the study populations, duration of follow-up, geographic locations, sample sizes and different combinations of confounder adjustments. Attention should also be paid to the followings:
-
1. Regional, population and selection biases
-
2. Where component studies identified in competing meta-analyses were excluded or missing without justification
-
3. High heterogeneities I 2 > 60 % and P < 0·05
-
4. The potential biases in meta-analyses of a small number of component studies (such as n < 5).
Although the benefits of consuming soya products have been reported in many studies, consuming soya products containing phytoestrogens raises concerns(Reference Vieira, Brasiel and Ferreira49). Studies suggest that they may be associated with endocrine-metabolic dysfunctions in adult life(Reference de Almeida Brasiel, Schuchter Ferreira and Vieira50), and maternal consumption of soya protein isolate during lactation worsened the atherogenic indices of the offsprings in adulthood(Reference Ferreira, Luquetti and de Almeida Brasiel51). While soya products are often coming from genetically modified soya seeds whose effect on the body is unknown, more studies are needed to investigate the long-term effects of soya consumption.
Conclusions
This scoping review suggested that soya intakes might potentially reduce cardiovascular, cancer and diabetes II diseases. Being the products with lower greenhouse gas emission intensity, soya products could be the better dietary alternatives to animal products for reducing these non-communicable diseases and helping combat climate change.
Acknowledgements
The authors have no relevant financial or non-financial interests to disclose.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522000691









