The dietary intakes of children in developed nations have been shown to be low in fruit and vegetables (among other core foods) and high in nutrient-poor, energy-dense foods( 1 – Reference Lioret, McNaughton and Spence 3 ). Evidence exists to suggest that this is the case even in young children( Reference Siega-Riz, Deming and Reidy 4 – Reference Duncanson, Burrows and Collins 6 ). In addition, evidence suggests that overweight and obesity in childhood and adolescence have adverse health consequences in adult life and that these health consequences present a significantly greater risk of disease and early mortality( Reference Reilly and Kelly 7 ). Differences in children’s weight gain according to socio-economic indicators such as parental education or occupation( Reference Wijlaars, Johnson and van Jaarsveld 8 , Reference Bouthoorn, Wijtzes and Jaddoe 9 ) and parental BMI( Reference Freeman, Fletcher and Collins 10 ) have been recorded from very early in life. In addition, behavioural determinants of weight status have been noted to track throughout childhood into adulthood( Reference Northstone and Emmett 11 – Reference Craigie, Lake and Kelly 13 ), whereas dietary quality declines over this time( 1 , Reference Cameron, Ball and Pearson 14 ). Children’s learning about food is substantial during early childhood through their observation and imitation of significant others( Reference Campbell and Crawford 15 – Reference Carnell, Cooke and Cheng 22 ). The influence of parents on young children’s diets is considered central, although the majority of research concentrates on maternal influence or combines the data of both parents( Reference Peters, Sinn and Campbell 23 – Reference Sotos-Prieto, Santos-Beneit and Pocock 25 ).
For example, in Rasmussen et al.( Reference Rasmussen, Krølner and Klepp 24 ) review of the determinants of children’s and adolescent’s fruit and vegetable intake, only eleven of the ninety-eight papers included in their review included fathers. The review reported that fathers’ education (as a proxy for socio-economic position (SEP)) was associated with children’s fruit and vegetable intakes. Both mothers’ and fathers’ fruit and vegetable intakes were also reported to be associated with children’s fruit and vegetable intakes. As these results reflect pooled parental data, they do not inform our understanding of the independent effects of mothers and fathers on children’s dietary intakes. A further systematic review and meta-analysis by Wang et al.( Reference Wang, Beydoun and Li 26 ) on the similarities between the diets of parents and their children reported that only six of twenty-four papers included father-specific analyses. Of these papers, only two reported correlations for food intake (the other four reported correlations for nutrients or energy), and of these two papers only one included children under the age of 5 years (up to 18 years). That study, by Beydoun & Wang( Reference Beydoun and Wang 27 ), reported that child fruit intake was associated with parental fruit intake for children aged 2–10 years.
When considering paternal influences on children’s diets, Robinson et al.( Reference Robinson, Rollo and Watson 28 ), in their cross-sectional study, reported positive associations between the dietary intakes of fathers and children for fruit and grains of sixty-six Australian families, whereas Hall et al.( Reference Hall, Collins and Morgan 29 ), in their Australian sample of fifty father–child dyads, reported moderate to strong positive correlations for dietary intakes of fruit (r 0·40), cookie (r 0·54) and potato chip (r 0·33) intakes. Both of these studies were, however, in primary-school-aged children. In addition, Wake et al.( Reference Wake, Nicholson and Hardy 30 ), in their analysis of nationally representative Longitudinal Study of Australian Children data, reported that it was the parenting behaviours and styles of fathers (and not mothers) that were associated with the BMI of their young children. Collectively, these studies suggest that fathers’ eating and parenting behaviours have a moderate influence on their children’s eating behaviours and subsequent health outcomes.
A number of determinants of children’s dietary intakes have been identified previously( Reference Wang, Beydoun and Li 26 ), including parental education (as a proxy for SEP), BMI and age. With respect to fathers specifically, associations between paternal education and children’s diets( Reference Rasmussen, Krølner and Klepp 24 ) and paternal BMI and children’s diets( Reference Freeman, Fletcher and Collins 10 , Reference Brophy, Rees and Knox 31 ) have been reported, although not in the same age group as our study. Associations between maternal age and poor child diet quality have been reported( Reference Giovannini, Riva and Banderali 32 ), but, to our knowledge, paternal age and child diet have not been investigated. With limited data on the diets of fathers and their young children, and established relationships between children’s diets and parental age, SEP and BMI( Reference Cameron, Ball and Pearson 14 , Reference Brophy, Rees and Knox 31 , Reference Smithers, Brazionis and Golley 33 ), the aim of this cross-sectional study was to investigate associations between the dietary intakes of fathers and their children at 20 months of age and to determine whether these associations differed on the basis of fathers’ age, education (as a proxy for SEP) or BMI.
Methods
Study design
The Melbourne Infant Feeding Activity and Nutrition Trial (InFANT) Program was a cluster-randomised controlled trial (RCT) undertaken within pre-existing first-time parent groups and has been described in detail elsewhere( Reference Campbell, Hesketh and Crawford 34 , Reference Campbell, Lioret and McNaughton 35 ). Briefly, sixty-two first-time parent groups were selected using a two-stage random sampling design, from fourteen local government areas (across areas of all SEP) within a 60-km radius of the research centre (Deakin University in Burwood). Random allocation to either intervention or control arms occurred for groups that consented to participate. Inclusion criteria were a minimum of eight parents in the groups consenting to participate (or six parents in low-SEP area groups), and English literacy. Main carers were offered six 2-h dietitian-delivered sessions over 15 months focusing on parental knowledge, skills and social support around infant feeding, diet, physical activity and television viewing. Control group parents received six newsletters on non-obesity-focused themes.
Exclusion criteria for this study included non-first-time parents (n 14), single-parent families (n 8), father as the main carer (n 1), same-sex couple (n 1) and families in which fathers did not complete the baseline questionnaire (n 58). The sample available at baseline consisted of 460 fathers. In all, twenty-nine families were lost to follow-up, and follow-up data were missing for 114 fathers; thus, the final sample at 20 months consisted of 317 fathers (Table 1).
Table 1 Fathers’ characteristics and dietary intakes (Mean values and standard deviations; number and percentages; n 317)
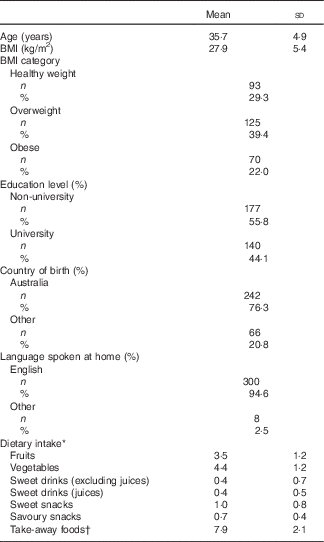
* Intake was measured in g or ml per d for children and serves per d for fathers.
† Take-away foods measured in occasions for fathers.
The Melbourne InFANT Program was approved by the Deakin University Human Research Ethics Committee and the Victorian Government Department of Human Services, Office for Children, Research Coordinating Committee. Informed written consent was obtained from all participants.
Measurements
Dietary data from fathers involved in the Melbourne InFANT Program were collected using the validated( Reference Hodge, Patterson and Brown 36 ) Cancer Council Victoria FFQ, an updated version of the semi-quantitative FFQ developed specifically for the Melbourne Collaborative Cohort Study( Reference Ireland, Jolley and Giles 37 ). The FFQ has ten response options for ninety-eight food items ranging from ‘never’ to ‘three or more times per d’. Fathers were asked to indicate how often they had consumed each food or beverage item over the preceding 12 months. These data were converted into daily frequencies according the Cancer Council Victoria protocol. An additional question was included in the FFQ relating to the amount of diet and non-diet soft drinks consumed (glasses per d).
Demographic and socio-economic variables included fathers’ age, marital status, country of birth, main language spoken at home, employment status and education level. Weight and height were self-reported, with BMI calculated as weight (kg)/height (m2). A dichotomous age variable was created, split at the median age of the fathers in the sample (35·2 years). Paternal education was used as a proxy for SEP and was collapsed into two groups (university education v. non-university education). As the focus of this study was on fathers rather than on mothers, fathers’ education was used as the measure of SEP. Paternal BMI was dichotomised into healthy weight and overweight/obese( Reference Brophy, Rees and Knox 31 ) in order to maximise power available for between-group analyses. Each food/beverage item recorded was matched to an appropriate nutrient composition and quantity, using the 2007 AUSNUT Database( 39 ).
Children’s dietary intakes were assessed when they were 20 months of age by trained nutritionists through a telephone-administered multi-pass 24-h recall with the primary carer (mothers in all but one case)( Reference Blanton, Moshfegh and Baer 40 , Reference Spence, McNaughton and Lioret 41 ). Five passes were undertaken using the same methodology as undertaken in the Feeding Infants and Toddler’s survey( Reference Ziegler, Briefel and Clusen 42 ). Purpose-designed booklets were provided to parents to assist in their assessment of the consumption of foods and drinks considered difficult for parents to quantify. These included examples of measures and photographs of common portion sizes adapted to age. Two or three non-consecutive days of dietary data were collected, including one weekend day; 3 d of data were available for 97 % of the study sample. Dietary outcome variables and analyses selected for this study were based on those reported in the primary outcome paper for the InFANT study (fruit, vegetables, water, non-core drinks, non-core savoury snacks and non-core sweet snacks)( Reference Campbell, Lioret and McNaughton 35 ). The average daily intake (in g/d for children and serves per d for fathers and mothers) of fruit (excluding juice), vegetables, non-core sweet snacks (e.g. chocolate, candy, cakes), non-core savoury snacks (e.g. crisps, savoury biscuits), take-away foods and non-core sweet drinks (i.e. fruit juice, soft drinks) were calculated( Reference Campbell, Lioret and McNaughton 35 ).
Statistical analyses
Associations between fathers’ and children’s fruit, vegetable, non-core snack and non-core drink intakes were assessed using linear regression analyses. All analyses were adjusted for intervention status and the cluster-based sampling design (first-time parent groups) by using the vce (cluster) command in Stata (release 12; StataCorp. LP). The significance level was set at 5 %. To determine any influence of maternal intake on the associations between father and child intakes, mediation analyses (as described by MacKinnon et al.( Reference MacKinnon, Fairchild and Fritz 43 )) (Fig. 1) were conducted. Moderation by paternal age, education and BMI was assessed using regression models containing interaction terms for paternal age/education/BMI, as well as the dietary outcome variable of interest. In cases in which the coefficient of the interaction term was significantly different from zero( Reference Whisman and McClelland 44 ), further regression models were conducted stratified by the relevant moderator. As testing for moderation/interaction can be very sensitive to both sample size and sample distributions( Reference Fairchild and MacKinnon 45 ) and the purpose of moderation analysis in RCT is hypothesis generation, as well as hypothesis testing( Reference Kraemer, Wilson and Fairburn 46 ), an indicative P value of 0·2 or below was used to indicate likely moderation and an interaction worthy of closer consideration for future studies. It should be noted that the indicative P value of 0·2 was not used to indicate significance, and results close to this value were still considered to be worthy of further consideration. Analyses were conducted using Stata software.

Fig. 1 Mediation model. X, Predictor variable; Y, outcome variable; M, mediator variable; a, association between predictor (X) and potential mediator (M); b, association between potential mediator (M) and outcome variable (Y); c, overall association between predictor variable (X) and outcome variable (Y); c', mediator adjusted effect of predictor variable (X) on outcome variable (Y).
Results
Table 1 presents the demographic characteristics of participating fathers for this study. Mean paternal age was 35·7 years and mean BMI was 27·9 kg/m2. English was the main language spoken at home, with 78·5 % of the sample born in Australia and more fathers being non-university educated (73·8 %). There were no differences in baseline characteristics between those lost to follow-up or excluded from analyses and those retained at 20 months. Mean child age was 20 (sd 2·4) months. As there were no differences in the outcomes of interest between intervention and control groups (online Supplementary Table S1), data from each group were pooled. In analyses adjusted only for intervention status and clustering (Table 2), associations were found between fathers and children’s consumption of fruit (P=0·001), sweet snacks (P=0·03) and take-away foods (P=0·05). After additional adjustment for mothers’ intake for each food category (Table 2), the associations remained for fruit (P=0·008) and sweet snack intakes (P=0·04). Mediation analyses to assess the influence of maternal intake indicated that maternal intake partially mediated the associations between father and child consumption of fruit (mediation effect 2·64 (95 % CI 0·09, 5·17); % mediated=22·92) and vegetables (mediation effect 3·4 (95 % CI 1·17, 5·64); % mediated=69·51) (Table 2).
Table 2 Associations between fathers’ and children’s dietary intakes after adjustment for intervention status and maternal intake including assessment of mediated effect of maternal intake (β Coefficients* and 95 % confidence intervals)

* Coefficient values taken from stratified regression model (no interaction term included).
† Intake was measured in g or ml per d for children and serves per d for fathers.
‡ Take-away foods measured in occasions for fathers.
Moderation analyses were conducted for paternal BMI, age and education level as a proxy for SEP (Table 3). Positive associations were observed for fruit intake of children and fathers for all categories except overweight/obese fathers. A positive association was also observed for vegetable intake of children and older fathers. Positive associations were observed for take-away food intake of fathers and young children among University-educated, overweight/obese and younger fathers. A positive association was observed between non-university-educated fathers and their young children for sweet snack intake, whereas an inverse association was observed between fathers with a healthy BMI and their young children for savoury snack intake.
Table 3 Fathers’ and children’s dietary intakes* according to paternal BMI, education and age† (β Coefficients and 95 % confidence intervals)

* Intake was measured in g or ml per d for children and serves per d for fathers.
† All variables adjusted for intervention status and clustering. Model includes term for interaction between BMI, education or age and the dietary variable of interest in order to test for moderation.
‡ Take-away foods measured in occasions for fathers.
Discussion
This study provides important insights into the associations between the dietary intakes of fathers and their 20-month-old toddlers and suggests that associations exist in early childhood. To our knowledge, this has not been examined before in father–child dyads for this age group. Research has demonstrated that parents influence young children’s lifestyle behaviours through their own behaviours and eating-related parenting practices( Reference Brown and Ogden 47 ), and our study adds to the small number of studies that have examined the influence of fathers specifically.
Our observation of an association between fathers’ and children’s fruit intake is consistent with the results of two separate reviews by Rasmussen et al.( Reference Rasmussen, Krølner and Klepp 24 ) and Wang et al.( Reference Wang, Beydoun and Li 26 ). The only identified study in either review that reported dietary associations between parents and very young children was that by Beydoun & Wang( Reference Beydoun and Wang 27 ) who reported in a US sample of 1473 households that fruit intakes were correlated between parents and their 2- to 10-year-old children( Reference Beydoun and Wang 27 ).
Our observation of a relationship between fathers’ and children’s sweet snack intake is consistent with results from Hall et al.( Reference Hall, Collins and Morgan 29 ) in their study of overweight fathers, in which they observed moderately strong correlations between fathers’ and children’s intakes of cookies( Reference Hall, Collins and Morgan 29 ). Although their findings were observed in primary-school-aged children, it is one of only a few studies that has investigated the associations between paternal and child dietary intakes. In the current study, both fruit and sweet snack associations were maintained even when controlling for maternal intake, and although mother’s intake did partially mediate the association found for fruit this only accounted for approximately 23 % the observed relationship. Previous work has demonstrated associations between the dietary intakes of both mother–father and mother–child pairs( Reference Rasmussen, Krølner and Klepp 24 , Reference Robinson, Rollo and Watson 28 , Reference Longbottom, Wrieden and Pine 48 – Reference Lioret, Cameron and McNaughton 51 ), and thus the finding of partial mediation is not surprising. This has important implications for the design and delivery of future family-based dietary interventions.
Our analysis of paternal BMI categories presented differences in the associations between the dietary intake of fathers and their 20-month-old children depending on paternal weight status. The association between fathers’ BMI and children’s weight status has been previously reported( Reference Freeman, Fletcher and Collins 10 , Reference Brophy, Rees and Knox 31 ). Brophy et al.( Reference Brophy, Rees and Knox 31 ) reported associations between father’s BMI and children’s weight status in 11- to 13-year-olds, and Freeman et al.( Reference Freeman, Fletcher and Collins 10 ) reported that children with an overweight or obese father (not mother) at 4 years of age were at a higher risk of becoming obese in later childhood. Our own observations indicate that shared dietary patterns in children and their fathers may cause such associations to commence at a very early age, which is of particular concern given that the majority of pre-pubertal weight gain has been reported as occurring before the age of 5 years( Reference Gardner, Hosking and Metcalf 52 ).
In addition to moderation by paternal BMI, the relationships between fathers’ and children’s intakes of savoury snacks and take-away foods were also moderated by paternal education (as a proxy for SEP). Two investigations of the relationship between SEP and young children’s diets that are relevant to our findings include those by Smithers et al.( Reference Smithers, Brazionis and Golley 33 ) and Cameron et al.( Reference Cameron, Ball and Pearson 14 ). Smithers et al.( Reference Smithers, Brazionis and Golley 33 ) reported associations between children’s non-core snack intake and lower maternal education in children aged 6 months and again at 15 months. Cameron et al.( Reference Cameron, Ball and Pearson 14 ) observed greater consumption of non-core snacks in 2- to 8-year-old boys whose secondary carer (90 % fathers) had a lower education level. These studies suggest that parental education (as a proxy for SEP) is associated with infant diets.
The moderation effect of paternal education on take-away food intake is of interest but not unexpected. Reports by van der Horst et al.( Reference van der Horst, Brunner and Siegrist 53 ) on the associations between higher income (a common proxy for SEP) and the consumption of fast-food and take-away foods suggest that higher income was likely to be related to demanding work conditions, which in turn influenced the consumption of take-away foods. This is supported by Devine et al.( Reference Devine, Farrell and Blake 54 ) who reported that long work hours and non-standard work hours were associated with fathers’ intake of take-away foods. Our findings support those previously reported, but are of particular interest as they have not been reported with respect to fathers of children as young as those in our cohort.
We observed moderation by paternal age for vegetable intake, which, to our knowledge, has not been investigated previously. This result has similarities to those from Giovannini et al.( Reference Giovannini, Riva and Banderali 32 ) who reported a link between poorer infant diets and lower maternal age. That study was based on maternal rather than paternal age, with the age of younger parents in that study <30 years. Given the known dietary associations between mothers and fathers( Reference Lioret, McNaughton and Crawford 50 ), our results suggest agreement across studies for this finding.
Our investigation was novel in its focus on fathers and young children. Study strengths included the sampling of fathers from all SEP categories reflecting national data; the large proportion of fathers who completed data collection despite their partners being the point of contact for the study; and the investigation of moderation and mediation effects on the dietary relationships observed. There were some limitations to the study that should also be noted. The cross-sectional study design does not allow causation to be inferred. Dietary data were self-reported for fathers and proxy-reported for children (by mothers) and is therefore susceptible to social desirability bias. However, as it is the association between father and child data that is the focus, associations should still be apparent even in the presence of a bias towards socially desirable reporting. The FFQ used for collection of these data has been previously validated( Reference Hodge, Patterson and Brown 36 ,55), but it is nonetheless an inferior method of dietary assessment in comparison with the best practice 3×24-h dietary recalls used to assess infant’s diets in this study. It is also acknowledged that the use of two different methods for parental and child dietary intakes may pose some limitations when investigating dietary associations. Finally, the study focused on first-time fathers, and the results might not be generalisable to fathers with more than one child.
Conclusion
Our results show associations between fathers’ and children’s fruit, sweet snack and take-away food intakes at 20 months of age. Our results also demonstrate that paternal BMI, education and age moderate these associations. Although the associations between mothers and children’s diets are well established, these findings highlight the potentially important role that fathers’ diets may have in determining child dietary intakes. Family-based interventions that evaluate the impact of specifically targeting fathers are recommended to help elucidate the role of fathers. That paternal BMI, education and age moderated these associations suggests fathers who are younger, overweight and from low-SEP backgrounds may have a stronger influence on their children’s diets than their counterpart fathers. Future research on child diet could include examination of paternal, as well as maternal, factors. The use of longitudinal designs that allow determination of how dietary associations between fathers and their children may change over time are warranted.
Acknowledgements
This study was funded by an Australian National Health and Medical Research Council Project (grant no. 425801). A. J. C. is supported by a training fellowship (APP1013313) from the Australian National Health and Medical Research Council (NHMRC) and is a researcher within a NHMRC Centre for Research Excellence in Obesity Policy and Food Systems (APP1041020). K. D. H. was supported by a National Heart Foundation of Australia Career Development Award. D. C. and K. J. C. were supported by fellowships from the Victorian Health Promotion Foundation.
The authors’ contributions were as follows: A. D. W. drafted and edited the manuscript, contributed to the interpretation of the results and had primary responsibility for the final content. A. J. C. guided the statistical analysis, contributed to the interpretation of the results and edited the manuscript. K. D. H. designed and led the Melbourne InFANT Program and edited the manuscript. D. C. contributed to the interpretation of the results and edited the manuscript. K. J. C. was the principal investigator on the Melbourne InFANT Program. She designed and led that study, conducted the dietary data collection, contributed to the interpretation of the results and edited the manuscript. All authors read and approved the final manuscript.
There are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114515002755







