Childhood obesity is emerging as a global problem and concern( Reference Lobstein, Baur and Uauy 1 – Reference Wang, Beydoun and Liang 3 ). The results of a worldwide survey demonstrated that almost one-fifth of children were above normal weight (Wt)( Reference Ng, Fleming and Robinson 4 ). In 2010, the prevalence of childhood obesity in Europe, Southeast Asia and the Americas was 10, 5 and 15 %, respectively( Reference Wang and Lobstein 5 ). Prevalence rates are reported from Iran( Reference Kelishadi, Ardalan and Gheiratmand 6 ) similar to many other developing countries( Reference Kelishadi 7 ). Childhood obesity represents a range of disability and is a risk factor for many chronic disorders of adulthood such as osteoarthritis, cardiovascular and gall bladder diseases, as well as diabetes( Reference Tsiros, Coates and Howe 8 – Reference Wang, McPherson and Marsh 10 ).
In obesity, concentrations of biomarkers of low-grade systemic inflammation such as C-reactive protein (CRP), TNF-α, IL-6 and IL-8 are increased( Reference Das 11 – Reference Kim, Park and Kawada 15 ). Several studies revealed positive correlation between inflammatory biomarkers and anthropometric indices of obesity( Reference Kim, Park and Kawada 15 – Reference Caminiti, Armeno and Mazza 25 ). A positive association between CRP and BMI or waist circumference (WC) is seen in several observational surveys( Reference Kim, Park and Kawada 15 , Reference Ford 18 – Reference Huffman, Whisner and Zarini 23 ). Moreover, this association was observed between CRP and waist circumference:height ratio (WHtR)( Reference Malshe and Udipi 24 , Reference Caminiti, Armeno and Mazza 25 ).
Components of diet can affect systemic inflammation. Results of surveys indicate that high consumption of whole grains, olive oil, fruits, vegetables and fish, and low consumption of butter and red meat and low/moderate intake of wine (Mediterranean dietary pattern) are accompanied by reduced levels of inflammation. By contrast, the Western dietary pattern (rich in refined grains, red meat and high-fat dairy products) has been associated with high levels of inflammatory biomarkers( Reference Estruch, Martínez-González and Corella 26 – Reference King, Egan and Geesey 28 ). Numerous studies have illustrated that nutrients such as fibre( Reference Ma, Griffith and Chasan-Taber 29 ), complex carbohydrates( Reference Kitabchi, McDaniel and Wan 30 ), moderate consumption of alcohol( Reference Avellone, Di Garbo and Campisi 31 ), n-3 PUFA( Reference Ferrucci, Cherubini and Bandinelli 32 ), vitamin C( Reference Wannamethee, Lowe and Rumley 33 ), vitamin E( Reference Bertran, Camps and Fernandez-Ballart 34 ), Mg( Reference King, Mainous III and Geesey 35 ) and β-carotene( Reference Erlinger, Guallar and Miller 36 ) have some anti-inflammatory effects, while SFA and sugar tend to have pro-inflammatory effects( Reference Huang, Sjögren and Ärnlöv 37 , Reference Gu and Lambert 38 ).
Several dietary indices exist for evaluating the quality of an individual’s diet( Reference Puchau, Zulet and de Echávarri 39 – Reference Guenther, Kirkpatrick and Reedy 41 ). One of these indices is the Dietary Inflammatory Index (DII®), which was first described in 2009( Reference Cavicchia, Steck and Hurley 42 ) and updated in 2014( Reference Shivappa, Steck and Hurley 43 ). The DII is used to evaluate the inflammatory potential of diet based on pro- and anti-inflammatory properties of specific food items, spices, macronutrients, micronutrients and flavonoids( Reference Shivappa, Steck and Hurley 43 ). In two observational studies, a significant association was demonstrated between DII score and anthropometric indices in adults( Reference Sokol, Wirth and Manczuk 44 , Reference Ruiz-Canela, Zazpe and Shivappa 45 ). Results of a cohort in the USA indicated the relationship between maternal prenatal and early childhood DII with mid-childhood WC and BMI z-score( Reference Sen, Rifas-Shiman and Shivappa 46 ).
Given that there is no study focusing on the association between the DII and anthropometric indices in children and adolescents in Middle Eastern countries, the present study was conducted to evaluate this relationship using data derived from the fourth survey of an Iranian national surveillance programme entitled the Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable Diseases (CASPIAN-IV). The hypothesis of the present study is that high inflammatory potential of diet, indicated by higher DII score, is related to higher levels of anthropometric indices.
Methods
Ethical statement
The study protocol was approved by the Ethics Committee of Isfahan and Alborz Universities of Medical Sciences (code: 194049). The objectives of the study were explained to the students and their parents, and the informed consent form and oral assent were obtained from eligible parents and students who wished to participate. The study was conducted according to the guidelines of the Declaration of Helsinki (Seoul, 2008).
Study design and population
The present study is based on data obtained from the CASPIAN-IV study. This nationwide cross-sectional survey was performed among students aged 6–18 years, from urban and rural areas of thirty provinces of Iran in 2011–2012. Students were selected by multistage, cluster sampling. Stratification was done in each province according to the number of students (proportional to size) in each residential area (urban/rural) and education grade (elementary/intermediate/high school) with equal numbers of boys and girls. Cluster sampling with equal clusters (eighty-three clusters) was used in each province to reach the necessary sample size. Clusters were defined at the level of schools and ten students were selected in each cluster. The sampling frame was defined based on the information bank of Ministry of Education. In each province, schools were categorised by type and name of school and the number of students was added cumulatively in each province. A total of ten students were randomly selected from clusters when the clusters were defined in each province. Finally, eighty-three clusters were selected and a total of 25 000 students were selected. In each province approximately a quarter of clusters, a total of 6505 students, were selected randomly for dietary assessment. Among 6505 invited students for dietary assessment, students following special diets (104 students); those having a history of chronic diseases, for example, type 1 diabetes, the metabolic syndrome (MetS), CVD (212 students); those using medications such as glucose-, lipid- and blood pressure-lowering medications (eighty-eight students); and those with incomplete dietary data (674 subjects) were excluded from our analysis. A total of 5427 students were included in the present study. None of these students consumed fortified foods. The methodology of the present study was published previously( Reference Kelishadi, Ardalan and Qorbani 47 ).
Demographic information
Demographic variables were collected using a validated questionnaire provided based on the WHO Global School-based Student Health Survey( Reference Kelishadi, Motlagh and Bahreynian 48 , Reference Kelishadi, Majdzadeh and Motlagh 49 ). The data were obtained from all participants through conducting an interview with one of their parents in the sampled classes of the selected schools. The variables related to the family consisted of family history of chronic diseases (diabetes, hypertension, obesity and dyslipidaemia), level of parental education (the total years of schooling), having a family private car, type of home (rented/owned), dietary behaviours, physical activity (PA) and sedentary lifestyle behaviours.
Dietary assessment
Assessment of usual dietary intakes for students was conducted using a validated 168-item semi-quantitative FFQ( Reference Esfahani, Asghari and Mirmiran 50 , Reference Mirmiran, Esfahani and Mehrabi 51 ), which included a description of standard serving sizes. This questionnaire was validated for children and adolescents before (Cronbach’s α coefficient = 0·96)( Reference Kelishadi, Majdzadeh and Motlagh 52 ). The FFQ for children <10 years old was completed by the student’s parent; students ≥10 years old completed the form by themselves. Reports consisted of the standard portion size and frequency of consumption of each food item during the previous year on a daily, weekly, monthly or yearly basis, as appropriate. Frequencies of consumption of foods for each person were converted to daily frequencies and then the gram amount consumed daily for each food item was computed using household scale guide. For nutrient analyses, Nutritionist 4 software (First Databank; Hearst), including the United States Department of Agriculture (USDA) food composition data, was used. Other food items, which were not included in this database, were analysed by means of Iranian food composition table( Reference Azar and Sarkisian 53 ).
Anthropometric measurements
Expertly trained staff conducted all anthropometric measurements in the selected schools. Body Wt was evaluated to the nearest 0·1 kg without shoes and wearing only light clothing. Height (Ht) was assessed to the nearest 0·1 cm with each participant standing without shoes and the shoulders positioned against a stadiometer. Wt and Ht were used to calculate the BMI (BMI = Wt (kg)/Ht (m)2)( Reference Kelishadi, Marashinia and Heshmat 54 , Reference Khashayar, Heshmat and Qorbani 55 ). Parental Wt and Ht also were measured. BMI z-scores of the students for age and sex were computed based on WHO growth reference standards for children aged between 5 and 19 years.
Students’ WC was measured to the nearest 0·1 cm in the midpoint between of the lowest rib and the iliac crest, with the students standing and breathing out (i.e. expiring). Hip circumference (HC) was assessed to the nearest 0·1 cm at maximum level of the ileac crest without any pressure to body surface. Neck circumference (NC) was evaluated underneath the Adam’s apple at a comfortable position to the nearest 0·1 cm. Wrist circumference was assessed on the dominant arm to the nearest 0·1 cm. The participants were asked to hold their arm on a flat surface such as a table. The superior border of the tape measure was placed just distal to the prominences of radial and ulnar bones. All assessments were carried out using a non-elastic tape. Waist:hip ratio (WHR) and WHtR were calculated by dividing WC to HC and WC to Ht, respectively( Reference Kelishadi, Ardalan and Qorbani 47 ).
Socio-economic status
Principal components analyses were used to provide a summary measure of socio-economic status (SES) of students. Relevant questions entered in this analysis included type of home, parental education, parent’s job, possessing private car, school type (public/private) and having personal computer. These variables were incorporated as a unit index( Reference Caro and Cortés 56 ), and finally this index was categorised into three grades (low, moderate and high). Questions about SES were asked to the student’s parent for children <10 years, while students ≥10 years old completed the questionnaire independently.
Physical activity and screen time
The Physical Activity Questionnaire for Adolescents (PAQ-A) was used to evaluate the levels of PA in children and adolescents. In this questionnaire, the students’ 7-d recalls of sports or activities were assessed by a self-administrated questionnaire. The validity and reliability of the questionnaire has been tested in the Iranian paediatric population( Reference Kelishadi, Majdzadeh and Motlagh 52 ). The questions included sports or activities that caused the participants to sweat or feel tired in their legs or exercises that made them breathe with difficultly, for example, running, climbing and playing on rope. The questionnaire also collected information regarding PA during the physical education period, leisure time, lunch time and after school, in the evenings and during weekends( Reference Adeniyi, Okafor and Adeniyi 57 , Reference Kowalski, Crocker and Donen 58 ). The total score of PAQ-A questionnaire was 1–5, and PA was categorised as low (students with scores between 1 and 1·9) and high (students with scores between 2 and 5) levels( Reference Kowalski, Crocker and Donen 58 , Reference Copeland, Kowalski and Donen 59 ).
In the present study, the average number of hours per day in which the students spent watching personal computer, television and electronic games was used to estimate the screen time (ST) behaviour during the entire week. According to the international ST recommendations, this variable was categorised into two: low (<2 h/d) and high (2 h/d or more) grades( Reference Salmon, Campbell and Crawford 60 , Reference Bar-On, Broughton and Buttross 61 ). Parents of children <10 years of age completed questions about PA and ST behaviour of students, while students ≥10 years old completed their questionnaire independently.
Dietary Inflammatory Index
The DII® was developed to determine the inflammatory potential of the diet. Development of this index has been described completely elsewhere( Reference Shivappa, Steck and Hurley 43 ). Briefly, 1943 papers published from 1950 to 2010 that assessed the effect of whole foods, spices and nutrients (forty-five dietary factors) on specific inflammatory markers (IL-1β, IL-4, IL-6, IL-10, TNF-α and CRP) were reviewed and scored. Each dietary factor that was accompanied by a significant higher values of IL-1β, IL-6, TNF-α or CRP or lower values of IL-4 or IL-10 was considered a pro-inflammatory component (+1 score), and each dietary factor that was associated with significant lower amounts of IL-1β, IL-6, TNF or CRP or higher amounts of IL-4 or IL-10 was considered as an anti-inflammatory component (–1 score); 0 was obtained for items that had no significant effect on six inflammatory biomarkers.
First, global mean intake of each dietary factor was subtracted from dietary intake of it, and then this value was divided by world standard deviation intake of that factor as derived from the data sets of eleven countries (i.e. to obtain the z-score). To minimise the effect of ‘right skewing’, dietary factor-specific z-scores were converted to a proportion (i.e. with values from 0 to 1). Next, these values were multiplied by 2 and then 1 was subtracted to achieve a symmetrical distribution with a range of –1 to +1 and centred on 0. To obtain the factor-specific DII score of each food, the amount for each dietary factor was multiplied by the overall dietary factor-specific inflammatory effect score. Finally, all dietary factor-specific DII scores were summed to calculate the overall DII score for each student.
Data on twenty-five items of forty-five possible dietary factors that could be used to calculate the DII score were available in this study. Energy, carbohydrate, protein, total fat, cholesterol, SFA, vitamin B12 and Fe were the pro-inflammatory components, and MUFA, PUFA, fibre, folic acid, niacin, riboflavin, thiamin, vitamin A, vitamin C, vitamin D, vitamin E, vitamin B6, Zn, Se, Mg, β-carotene and caffeine were the anti-inflammatory items. The remaining components of DII were not collected as part of the FFQ.
Statistical analyses
Data analyses were performed using STATA® software (version 11). Means and standard deviations and percentages were used to illustrate quantitative and qualitative variables, respectively. DII score and intake of energy and macronutrients were presented as medians and interquartile ranges in both sexes of students. The independent-samples t test was performed for comparison of the means between two groups. One-way ANOVA was used to compare the trend of mean values of the anthropometric indices across DII quartiles. The Mann–Whitney U test was applied for comparing medians of energy, macronutrients and DII score in two groups of students. The percentages of categorical variables were compared using Pearson’s χ 2 test. For evaluating the association between anthropometric indices (dependent variables) and DII score (categorised and continuous independent variables), linear regression models were used. There was no adjustment for covariates in one of the models (model I), while age, sex, place of residence, ST and PA and SES were adjusted in the other model (model II). The first quartile of DII score was considered the referent. A cluster sampling procedure was applied in all analyses. All P values were corrected using the Benjamini–Hochberg correction method to control the false discovery rate due to the multiple comparison problem( Reference Benjamini and Hochberg 62 ). The adjusted P value <0·05 was considered as statistically significant after the multiple comparison correction.
Results
In this national survey, 5427 students (53·2 % boys) with a mean age of 12·61 (sd 3·23) years were included. The anthropometric indices and other characteristics of participants were presented by sex (Table 1). Among anthropometric indices Ht, wrist circumference, NC, WC and WHR were significantly higher in boys than in girls (P for all <0·001). Girls had significantly higher BMI z-score and HC compared with boys (P for all <0·001). The BMI of girls’ parents was slightly higher than boys’ parents (27·40 (sd 5·32) v. 27·06 (sd 5·05) kg/m2) (P = 0·01).
Table 1 Characteristics of the study population according to sex in the weight disorders survey of the Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable Disease-IV study (Mean values and standard deviations)
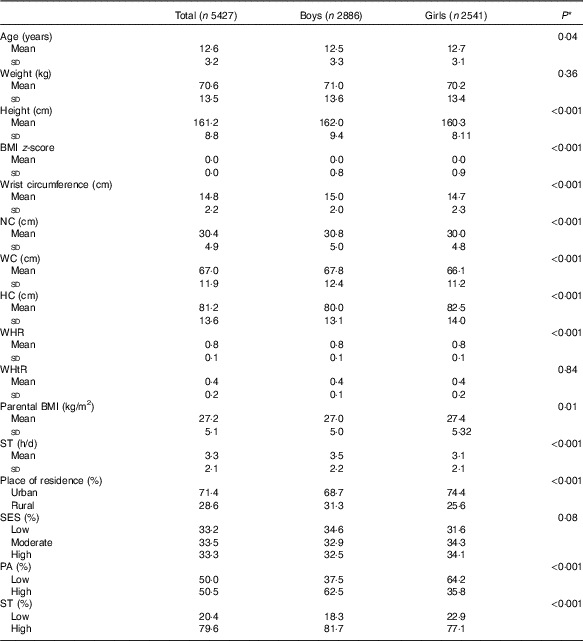
NC, neck circumference; WC, waist circumference; HC, hip circumference; WHR, waist:hip ratio; WHtR, waist:height ratio; ST, screen time; SES, socio-economic status; PA, physical activity.
* P values for the comparisons between quantitative variables were derived using the independent-samples t test, while for the comparisons of categorical variables the χ 2 test was used.
Most participants lived in urban areas (71·4 %). In general, no significant association was found between sex and SES (P = 0·08). However, more boys had high PA levels than that observed in girls (62·5 v. 35·8 %) (P <0·001) (Table 1). Also, ST was significantly higher in boys than in girls (81·7 v. 77·1 %) (P <0·001).
Table 2 shows sex-specific means of DII, intake of energy and macronutrients. All differences among both sexes were not statistically significant.
Table 2 Dietary Inflammatory Index (DII) and intake of energy and macronutrients according to sex in the weight disorders survey of the Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable Disease-IV study (Medians and interquartile ranges (IQR))
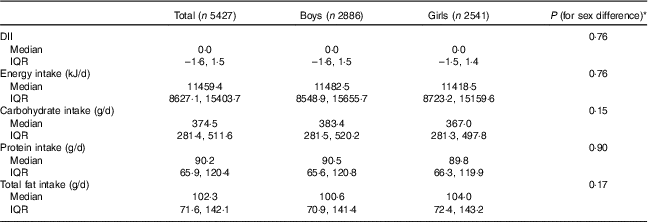
* The Mann–Whitney U test was used.
Table 3 presents means and standard deviations of anthropometric indices according to the DII quartiles. The lower and upper limits of the DII for each quartile are provided. Overall, higher means of BMI z-score, NC, wrist circumference, HC and WC were associated with higher DII score (P trend <0·001 in all comparisons). Anthropometric indices in boys had a significant escalating trend across DII quartiles for all parameters except for WHR and WHtR (P trend in all comparisons was significant). In girls higher DII score was associated with higher BMI z-score, HC and WC (P trend for all comparisons <0·05).
Table 3 Anthropometric measures according to quartiles (Q) of the Dietary Inflammatory Index (DII) in the weight disorders survey of the Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable Disease-IV study (Mean values and standard deviations)
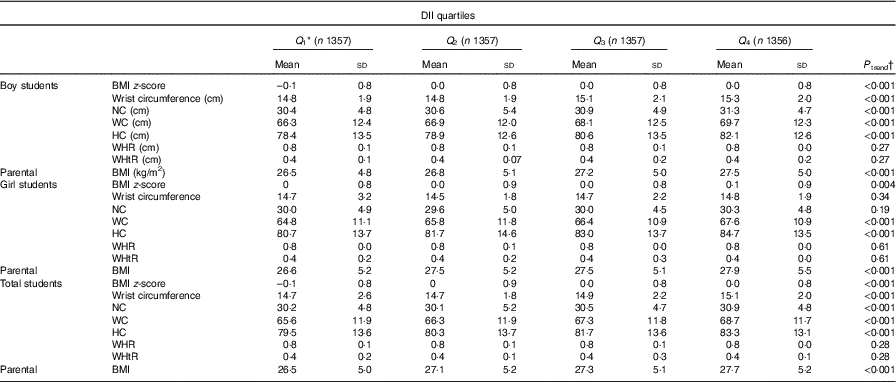
NC, neck circumference; WC, waist circumference; HC, hip circumference; WHR, waist:hip ratio; WHtR, waist:height ratio.
* Range of quartiles for total participants: Q 1 = (–4·42, –1·63), Q 2 = (–1·62, –0·05), Q 3 = (–0·04, 1·51) and Q 4 = (1·50, 4·26).
† One-way ANOVA was used.
Table 4 presents results from the linear regression models fit to describe the association between anthropometric indices and quartiles of DII score as categorical and continuous variable. In the categorical form of DII quartile, in the multivariate model (after adjustment for potential confounders), students in the highest quartile of DII had higher BMI z-scores compared with those in the first quartile as reference (P <0·05). With respect to WC, individuals in the highest quartile of the DII had higher level of WC compared with those in the lowest quartile (P <0·05). Moreover, students in the highest quartile of DII had higher level of HC compared with the individuals in the first quartile (P <0·05). Students’ parents in the second, third and highest quartiles of the DII had higher BMI compared with those in the lowest quartile (P <0·05). When we considered DII quartile as a continuous variable, a significant association was observed between the DII quartile and WC, HC and parental BMI (P <0·05). In the crude and multivariate model, the association of WHR and WHtR with DII quartiles was not statistically significant.
Table 4 Associations between the Dietary Inflammatory Index (DII) and anthropometric measures in the weight disorders survey of the Childhood and Adolescence Surveillance and Prevention of Adult Non-communicable Disease-IV study (β Estimates and 95 % confidence intervals)
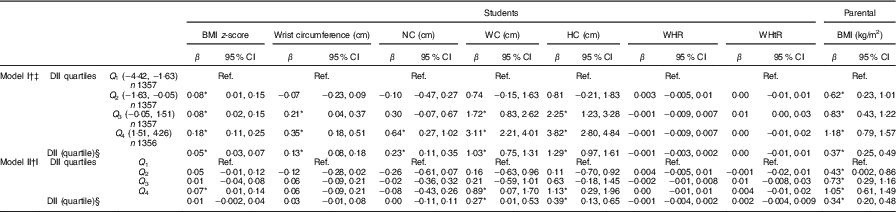
Ref., referent values; NC, neck circumference; WC, waist circumference; HC, hip circumference; WHR, waist to hip ratio; WHtR, waist to height ratio; ST, screen time; PA, physical activity; SES, socio-economic status.
* Statistically significant.
† Linear regression was used.
‡ Crude association.
§ DII quartile considered as continuous variable.
‖ Adjusted for age, sex, place of residence, ST, PA and SES.
Discussion
In this nationwide study performed on Iranian students, the association between overall inflammatory potential of diet, estimated by DII score, and anthropometric indices was assessed. Higher DII scores were associated with higher BMI z-scores, wrist circumference, NC, WC and HC in all students and BMI of their parents. These associations were observed among boys, whereas higher DII score was associated with higher BMI z-score, WC and HC in girls. Results that included positive relationship between DII and BMI z-score of students, HC, WC and parental BMI persisted after adjusting for potential confounders, including age, sex, place of area, ST, PA and SES.
Higher DII score was associated with general obesity (assessed by BMI z-score) in the students. This finding might be associated with the consumption of more pro-inflammatory diets (e.g. fast foods, cookies and crackers), which tend to be energy dense( Reference Bowman, Gortmaker and Ebbeling 63 , Reference Borges, Marchioni and Levy 64 ). This result is line with the study of Ramallal et al. ( Reference Ramallal, Toledo and Martinez 65 ) that showed consumption of an anti-inflammatory diet (lower DII) was associated with lower BMI; with the corollary that participants with pro-inflammatory diet (higher DII) tended to be overweight or obese. The findings of the present study in children and adolescents are consistent with the results of a Spanish study conducted in 7236 adult participants( Reference Ruiz-Canela, Zazpe and Shivappa 45 ). An observational study on 430 young Americans, aged 21–35 years, indicated no significant association between DII and BMI( Reference Wirth, Hébert and Shivappa 66 ). This discrepancy may have resulted from the small sample size of that study. In line with the present study, a meta-analysis was performed on three cross-sectional studies, indicating higher protein (a pro-inflammatory dietary factor) consumption in obese v. non-obese subjects( Reference Kaartinen, Knekt and Kanerva 67 ). A cross-sectional study conducted in 2013–2014 found that high fat (a pro-inflammatory dietary factor) intake is associated with higher BMI, and individuals with normal BMI consumed higher amounts of fibre compared with the obese persons( Reference Little, Humphries and Patel 68 ).
The present finding on the association between DII and WC is consistent with a previous research conducted on older adults( Reference Ruiz-Canela, Zazpe and Shivappa 45 ). Similarly, a case–control study performed on Cuban-American population with and without type 2 diabetes and aged >30 years indicated a direct association between WC and CRP( Reference Huffman, Whisner and Zarini 23 ). Likewise, another observational study observed this positive relationship in participants with abdominal obesity( Reference Faam, Zarkesh and Daneshpour 69 ). It was hypothesised that visceral fat is the main anatomic site for secretion of IL-6( Reference Fontana, Eagon and Trujillo 70 ), and this inflammatory biomarker could stimulate CRP production by liver( Reference Weisberg, McCann and Desai 71 ). A cross-sectional study on 722 adolescents aged 10–19 years, indicated that participants in the highest quartile of healthy eating index-2010 had lower risk of central obesity than those in the lowest quartile( Reference Mohseni-Takalloo, Hosseini-Esfahani and Mirmiran 72 ). The results of a cohort study conducted with the aim of examining the association of Dietary Approaches to Stop Hypertension (DASH) and the MetS on 425 Iranian children and adolescents demonstrated higher adherence to DASH diet was associated with lower abdominal obesity( Reference Asghari, Yuzbashian and Mirmiran 73 ).
WHtR is one of the anthropometric indices associated with body fat( Reference Nambiar, Truby and Abbott 74 ). In a large cross-sectional study conducted with the aim of determination of the association between DII and anthropometric indices in Spain, the findings demonstrated a positive relationship between the inflammatory potential of diet and WHtR( Reference Ruiz-Canela, Zazpe and Shivappa 45 ), which is not in agreement with the outcome of the present study. In this study, the DII score was calculated from only twenty-five dietary factors; whereas in the previous report data for thirty-three food components were available for calculating the DII score. Therefore, this discrepancy may be the reason for the difference in the results. However, we have shown previously that there is no drop off in predictability in going from 44 to 27 parameters used for DII calculation( Reference Shivappa, Steck and Hurley 75 ). On the contrary to our study, in a cross-sectional survey conducted on British children and adolescents, the dietary glycaemic load in adolescents was independently associated with higher WHtR( Reference Murakami, McCaffrey and Livingstone 76 ). This dietary index was positively associated with carbohydrate (a pro-inflammatory dietary factor)( Reference Buyken, Dettmann and Kersting 77 ). This disagreement may be associated with missing data on twenty dietary factors in the DII calculation. However, it should be noted that the range of DII score (from –4·42 to 4·26) is typical of what we see in many of our studies( Reference Sokol, Wirth and Manczuk 44 – Reference Sen, Rifas-Shiman and Shivappa 46 , Reference Wirth, Hébert and Shivappa 66 ). An observational study on 347 Italian children and preadolescents indicated high consumption of protein was associated with greater WHtR( Reference Del Mar Bibiloni, Tur and Morandi 78 ). It must be kept in mind that the nutritional requirements of children are different than those of adults, owing largely to the metabolic requirements for growth( Reference Goodhart and Shils 79 , Reference Hebert 80 ). In particular, we believe that it is important to point out that protein intake may be particularly important, especially for children during growth spurts and those who are involved in extreme athletic activities (e.g. weightlifting, marathon running)( Reference Petrie, Stover and Horswill 81 , Reference Dewey, Beaton and Fjeld 82 ). So even though protein is pro-inflammatory, the need for growth and development may outweigh its effect on inflammation. This is a matter separate and distinct from energy balance, which may be the main driving force in this population of children( Reference Shivappa, Hebert and Marcos 83 , Reference Sen, Rifas-Shiman and Shivappa 84 ).
No association was observed between DII and WHR. This lack of association may be due to the relatively small number of participants with abdominal obesity. Similarly, a survey on Finnish elderly aged ≥90 years indicated no association between WHR and levels of TNF-α, CRP and IL-6 in both sexes( Reference Lisko, Tiainen and Stenholm 85 ). On the contrary, in a study conducted on 377 adolescents and young adults in India, high WHR was associated with high levels of CRP( Reference Tsai and Tsai 86 ). A cross-sectional study that assessed the relationship between inflammatory biomarkers and obesity on young adults aged 18–30 years showed that WHR was positively associated with the levels of IL-6 and CRP( Reference Hermsdorff, Zulet and Puchau 87 ). In another observational study conducted on 1248 Taiwanese participants aged 65 years or older, a positive significant association was found between WHR and the levels of CRP( Reference Vikram, Misra and Dwivedi 88 ). The results confirmed that inflammatory biomarkers can be produced in visceral fat( Reference Fontana, Eagon and Trujillo 70 , Reference Matsuzawa 89 , Reference Berg and Scherer 90 ).
The results of the present study indicted the DII was positively associated with parental BMI. In an extensive study carried out among Korean households, the significant correlations were observed between the pattern of vitamin intake (B1, B2, B3, C and A), Fe, Ca and P in parents and their offsprings( Reference Lee and Park 91 ). In another study conducted on 294 families, the findings demonstrated the significant positive association between consumption of total carbohydrate, SFA, PUFA, energy and cholesterol in children and adolescents and their parents( Reference Laskarzewski, Morrison and Khoury 92 ). Vauthier et al. ( Reference Vauthier, Lluch and Lecomte 93 ) show that there is association between consumption of energy and macronutrients in parents and their children and adolescents in French families. Recently, a cross-sectional study carried out among 401 child/parent pairs in New Zealand represented an inverse association between parental diet quality and snack consumption pattern in their preadolescents( Reference Davison, Saeedi and Black 94 ). These findings reinforced the correlation between parent’s and children’s diet in one family.
Strengths
This national report covered a large group of Iranian children and adolescents as well as their parents. Using a validated dietary assessment instrument and PA questionnaire, applying an updated and validated tool for evaluating the inflammatory effect of diet and estimating the relationship between DII and anthropometric indices after adjustment for potentially confounding factors are the other strengths of the present study.
Limitations and other considerations
The present study was carried out on school students and their parents; hence, the results cannot be generalised to all Iranians or to children in other countries or those of different SES. Moreover, information bias could result from using the FFQ for dietary assessment. Therefore, misclassification is a potential problem common in epidemiologic reports. The questionnaires of children under 10 years of age were completed by one of their parents; this may bias the results. Another limitation is non-availability of data on twenty dietary factors, which may affect the results, primarily by underestimating the DII (as most missing dietary factors are anti-inflammatory though most are probably consumed only infrequently). So, these dietary factors may have no great effect on the DII scoring. Another consideration for the present study is its sample size. Although large sample size is one of the main strengths of this study, having such a large sample size may lead to observing statistically significant association between the DII score and a variety of outcomes. Therefore, we should consider this point when considering the clinical significance of results.
Conclusion
The present investigation observed a positive association between pro-inflammatory diet and greater indices of generalised and abdominal obesity in young children and adolescents. Prospective studies are warranted to confirm our findings.
Acknowledgements
This large observational study was performed with the cooperation of the Ministry of Health and Medical Education, Ministry of Education and Training, Child Growth and Development Research Center, Isfahan University of Medical Sciences and Alborz University of Medical Sciences. This study was supported by Isfahan University of Medical Sciences and Alborz University of Medical Sciences.
N. S. and J. R. H. supported the study by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the DII from the University of South Carolina to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. N. S. is an employee of CHI.
M. Q., R. K. and M. E. M. formulated the research questions and designed the study. H. A. and A. M.-G. carried it out the interviews. M. Q. analysed the data and Z. A., J. R. H. and N. S. computed the DII score and contributed to writing the article.
The authors declare that there are no conflicts of interest.







