Hashimoto thyroiditis (HT) is a common autoimmune thyroid disorder characterised by the lymphocyte infiltration of the thyroid follicles, progressively leading to fibrosis and atrophy of thyroid follicular cells. HT is frequently accompanied by elevated levels of serum thyroid peroxidase antibodies (TPO-Ab) and thyroglobulin antibodies (TG-Ab), and hypothyroidism occurs in approximately 20–30 % of HT patients(Reference Ragusa, Fallahi and Elia1).
Oxidative stress promotes autoantibody synthesis through increased formation of reactive oxygen species and contributes to the pathogenesis of autoimmune diseases, including type 1 diabetes, systemic lupus erythematosus and systemic sclerosis(Reference Kurien and Scofield2–Reference Brezovec, Perdan-Pirkmajer and Burja4). To date, only few studies have examined the association between oxidative stress and the progression of HT. A longitudinal study of 80 HT patients revealed that those who developed hypothyroidism exhibited higher levels of oxidative stress index, indicating the potential role of oxidative stress in the development of hypothyroidism in the HT population(Reference Ates, Arikan and Altay5). Another observational study(Reference Ates, Yilmaz and Altay6) showed a continuous elevation of oxidative stress levels during the development of subclinical hypothyroidism and overt hypothyroidism among HT patients. Antioxidants may have therapeutic value in preventing autoimmune thyroiditis by reducing reactive oxygen species generation and intracellular adhesion molecule-1 expression in thyrocytes, which is crucial at an early inflammatory response stage(Reference Burek and Rose7).
Vitamin C is a potent nonenzymatic antioxidant that effectively inhibits lipid peroxidation, thereby minimising reactive oxygen species-induced cellular and organ damage(Reference Monacelli, Acquarone and Giannotti8,Reference Marik9) . A previous prospective study showed that HT patients who received a daily intervention of 500 mg of vitamin C for 3 months had a lower level of serum TPO-Ab compared with placebo(Reference Karimi and Omrani10). An experimental study showed that injection of boldenone undecylenate resulted in elevation of thyroid-stimulating hormone (TSH) and TPO-Ab in rats(Reference El Deib, El-Sharkawy and Beheiry11). However, the detrimental effects of boldenone undecylenate on the thyroid function were ameliorated when treated in combination with vitamin C(Reference El Deib, El-Sharkawy and Beheiry11).
To our knowledge, whether vitamin C intake is associated with hypothyroidism among HT patients is unknown. Herein, we aimed to investigate the relationship between total vitamin C intake and the odds of hypothyroidism in patients with HT in a cross-sectional study based on the National Health and Nutrition Examination Survey (NHANES) database.
Methods
Study population
The NHANES is a nationally representative survey on the health and nutritional status of the general USA population. The National Center for Health Statistics institutional ethics review board approved the protocol of the NHANES. All participants signed the written informed consent. This study was conducted according to the guidelines laid down in the Declaration of Helsinki. This cross-sectional study included participants from three cycles of NHANES from 2007 to 2012, in which complete thyroid function data were available. In these three survey cycles, 30 442 participants completed the interview.
HT was defined as being positive for TPO-Ab or TG-Ab, with TPO-Ab levels exceeding 9·0 IU/ml or TG-Ab levels exceeding 115·0 IU/ml(Reference Li, Ying and Liang12). A total of 1043 participants with HT aged 18 years and above were included in the study. Participants with missing data on dietary vitamin C intake (n 45), incomplete thyroid function (n 2) and variables of interest, such as age, sex, ethnicity, dietary Se intake, dietary vitamin D intake and urinary iodine (n 24), were excluded in the final analysis. Pregnant individuals or those with hyperthyroidism or thyroid cancer (n 32) were also excluded.
Total vitamin C intake assessment
Two 24-h dietary recall interviews have been administered to participants in each NHANES cycle since 2002. Following the initial dietary interview, the second interview was carried out via telephone 3–10 d later. The average of the two recall periods of dietary vitamin C intake was utilised, and if only the first recall data were available, they were used instead of the average. Additionally, participants were interviewed about their vitamin C intake from supplements, with two 24-h recalls being conducted. The average of the two recalls of vitamin C intake from supplements was also calculated if available. The total vitamin C intake was determined by combining dietary and supplement intake.
Thyroid function assessment
Detailed descriptions of thyroid function measurements can be found at http://www.cdc.gov/nchs/nhanes/. According to the official NHANES documentation, the normal reference range for TSH is 0·34–5·6 µIU/ml. Based on this range, participants were categorised as hypothyroid if their TSH values exceeded 5·6 µIU/ml without the use of antithyroid medication or if they were currently taking levothyroxine or liothyronine, regardless of their TSH levels. Participants who were taking methimazole and propylthiouracil, regardless of their TSH values or those with TSH levels less than 0·34 µIU/ml, were classified as hyperthyroidism. Participants with TSH values within the normal range and not receiving thyroid hormone replacement or antithyroid medication were considered euthyroid.
Variables of interest
The categorical variables collected for analysis included sex, age (≥ 60 years and < 60 years), ethnicity, smoking status (never, former and current), alcohol status (never, former and current), educational level (less than high school, high school and more than high school), leisure-time physical activity, diabetes and hypertension. Levels of leisure-time physical activity were classified as none, insufficiently active (participating in leisure-time moderate activity 1–5 times with metabolic equivalents of 3–6 per week or leisure-time vigorous activities 1–3 times with metabolic equivalents > 6 per week) and active (with more activities than insufficiently active)(Reference Ou, Qiu and Geng13). A participant was considered to have diabetes with any of the following: (1) if he or she answered ‘yes’ to the question, ‘Doctor told you have diabetes?’, (2) fasting glucose level ≥ 126 mg/dl and (3) glycated HbA1c ≥ 6·5 %.
Other continuous variables included in the analysis were age, BMI, total dietary and supplemental intake of vitamin D and Se (calculated in the same manner as vitamin C), urinary iodine, total cholesterol, TG, HDL-cholesterol, LDL-cholesterol and creatinine.
Statistical analysis
Continuous variables were presented as means with sd if normally distributed or medians with interquartile ranges if not normally distributed. Categorical variables were expressed as counts with weighted percentages. Wilcoxon rank-sum tests were used to compare continuous variables between the hypothyroid and euthyroid groups, and the Rao-Scott χ 2 tests to compare categorical variables. Total vitamin C intake was categorised into quartiles. Thyroid function was also compared between different quartiles of total vitamin C intake. Total vitamin C intake, TG-Ab and urinary iodine data were log10 transformed due to their skewed distribution. To determine the dose–response relationship between total vitamin C intake and hypothyroidism among HT patients, we employed a restricted cubic spline regression model with four knots (5th, 35th, 65th and 95th percentiles). Extreme values of 1 % were excluded from the model to minimise the potential impact of extreme outliers.
The OR and 95 % CI for hypothyroidism diagnosis were calculated using multivariate logistic regression models according to the total vitamin C intake quartiles among individuals with HT. We constructed three models. No covariates were adjusted in model 1. Model 2 was adjusted for age, gender and ethnicity. Smoking status, drinking status, urinary iodine, TG-Ab levels and diabetes were further adjusted in model 3. In all three models, tests for linear trends were conducted by modelling the categories as ordinal variables. We also carried out subgroup analyses stratified by sex and age (≥60 years and <60 years). We tested the robustness of the results with some sensitivity analyses. First, we excluded those with extreme 1 % values of total vitamin C intake as a sensitivity analysis. Second, a distinct TPO-Ab cut-off point (TPO-Ab > 250 IU/ml)(Reference Karimi and Omrani10) for defining HT was utilised to establish the association between total vitamin C intake and hypothyroidism in HT. A third sensitivity analysis was conducted limited to those with normal TG-Ab levels to eliminate the potential influence of the difference in TG-Ab levels on the results. All statistical analyses were conducted with R software (version 4·2·2). Differences were determined statistically significant by two-sided P < 0·05.
Results
Population characteristics
We included 940 adults with HT in the final analysis (Fig. 1), including 164 (19 %) participants with hypothyroidism. Table 1 summarises the differences in characteristics among participants with hypothyroidism and euthyroidism. Compared with those with euthyroidism, participants with hypothyroidism had a lower level of total vitamin C intake (87 mg/d v. 119 mg/d), more likely to be female (79 % v. 65 %), non-Hispanic White (88 % v. 77 %), had higher levels of TG-Ab (5 v. 1 IU/ml) and TSH (6·37 v. 2·17 µIU/ml) and a lower level of free triiodothyronine (FT3; 2·90 v. 3·09 pg/ml). Participants with lower total vitamin C intake were more likely to have hypothyroidism (online Supplementary File 1: Table S1).
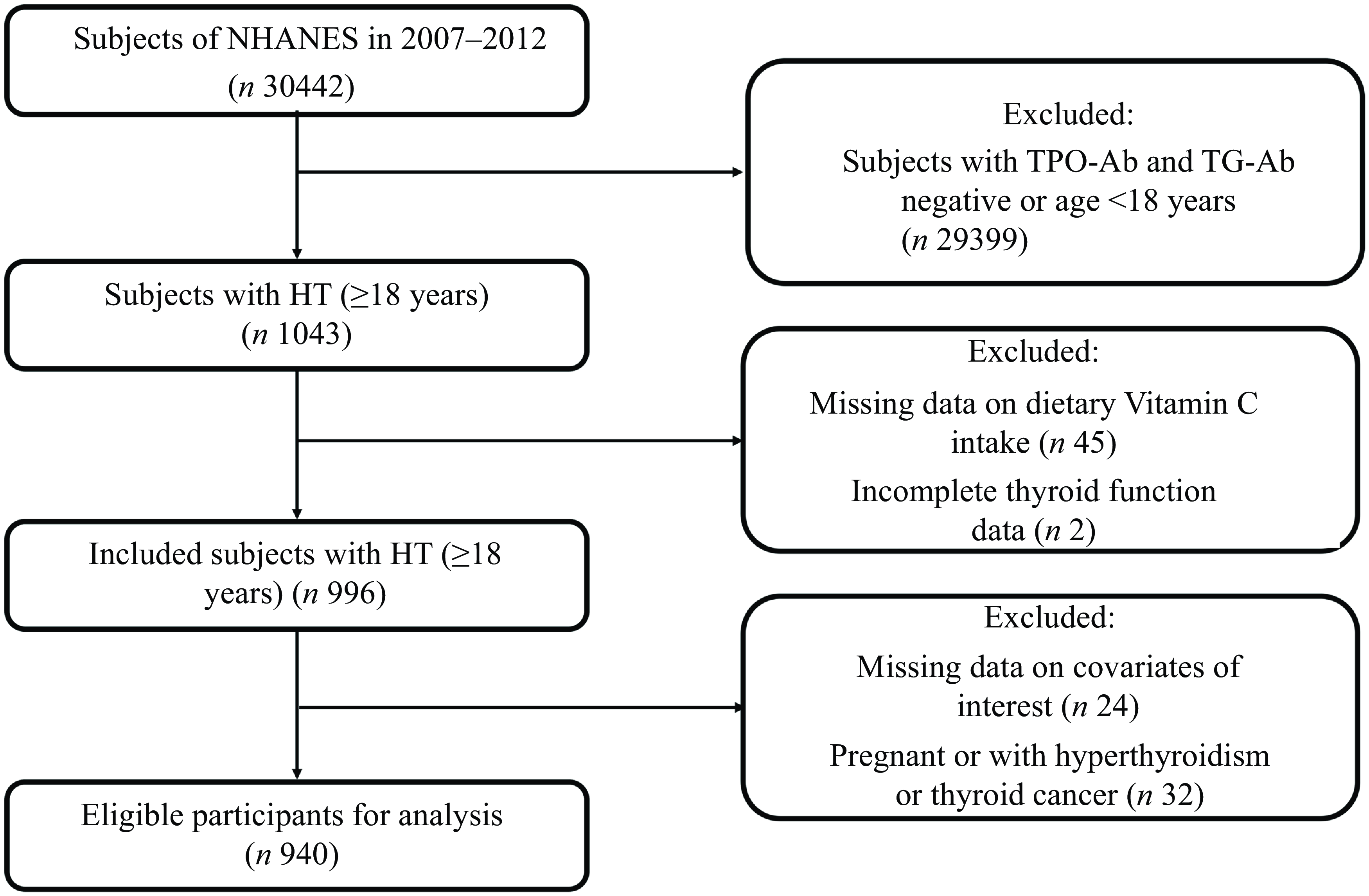
Fig. 1. Flowchart of study selection from NHANES 2007–2012. NHANES, National Health and Nutrition Examination Survey.
Table 1. Baseline characteristics of participants with Hashimoto thyroiditis by hypothyroidism status
(Numbers and percentages; median values and interquartile ranges)
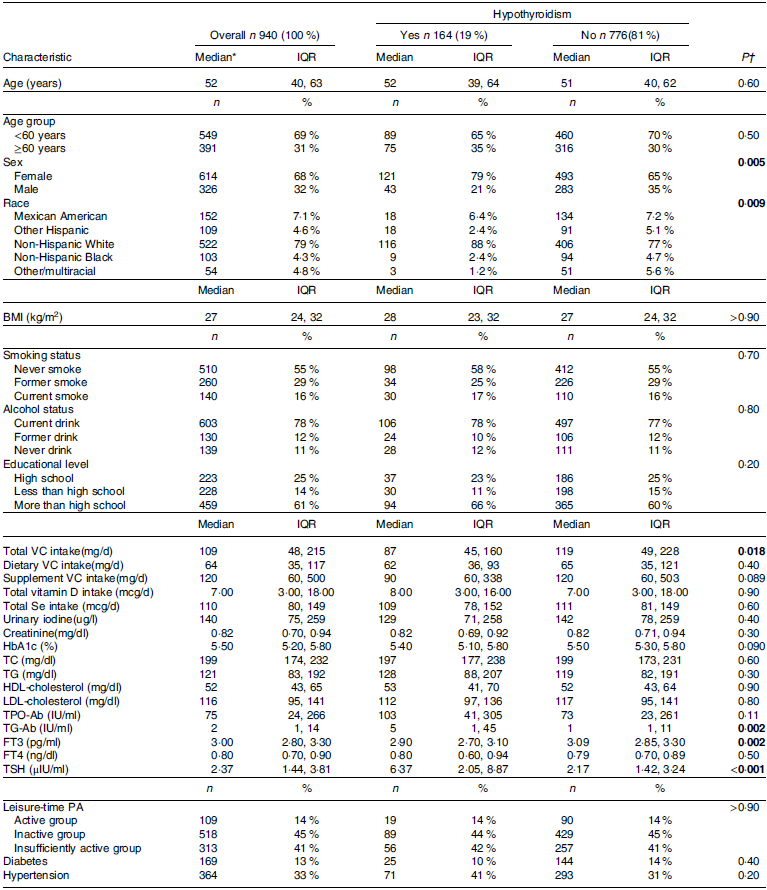
IQR, interquartile range; VC, vitamin C; HbA1c, glycated HbA1c; TC, total cholesterol; TPO-Ab, thyroid peroxidase antibodies; TG-Ab, thyroglobulin antibodies; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; PA, physical activity.
*Values were reported as medians (interquartile ranges) for continuous data and numbers (percentages) for categorical data.
†Wilcoxon rank-sum test for continuous variables and Rao-Scott χ 2 test for categorical variables were conducted between groups.
The bold values represent statistically significant.
Relationship between total vitamin C intake and hypothyroidism in Hashimoto thyroiditis
In restricted cubic spline regression, lower total vitamin C intake (log10-transformed) was significantly associated with increased odds of hypothyroidism after adjustment for potential confounders in HT (P overall < 0·001, P nonlinearity = 0·066, Fig. 2). Table 2 shows the association of increasing total vitamin C intake (lowest quartile as reference) with the odds of hypothyroidism in HT. Univariable analysis showed that a higher total vitamin C intake exhibited a significant association with lower odds of hypothyroidism. This association remained after adjustment for age, sex and race. Further adjustment for smoking and drinking status, urinary iodine (log10-transformed), TG-Ab levels (log10-transformed) and diabetes did not change the association between total vitamin C intake and the odds of hypothyroidism (v. the lowest quartile, the highest quartile, OR: 0·40; 95 % CI 0·18, 0·88, P trend = 0·027).
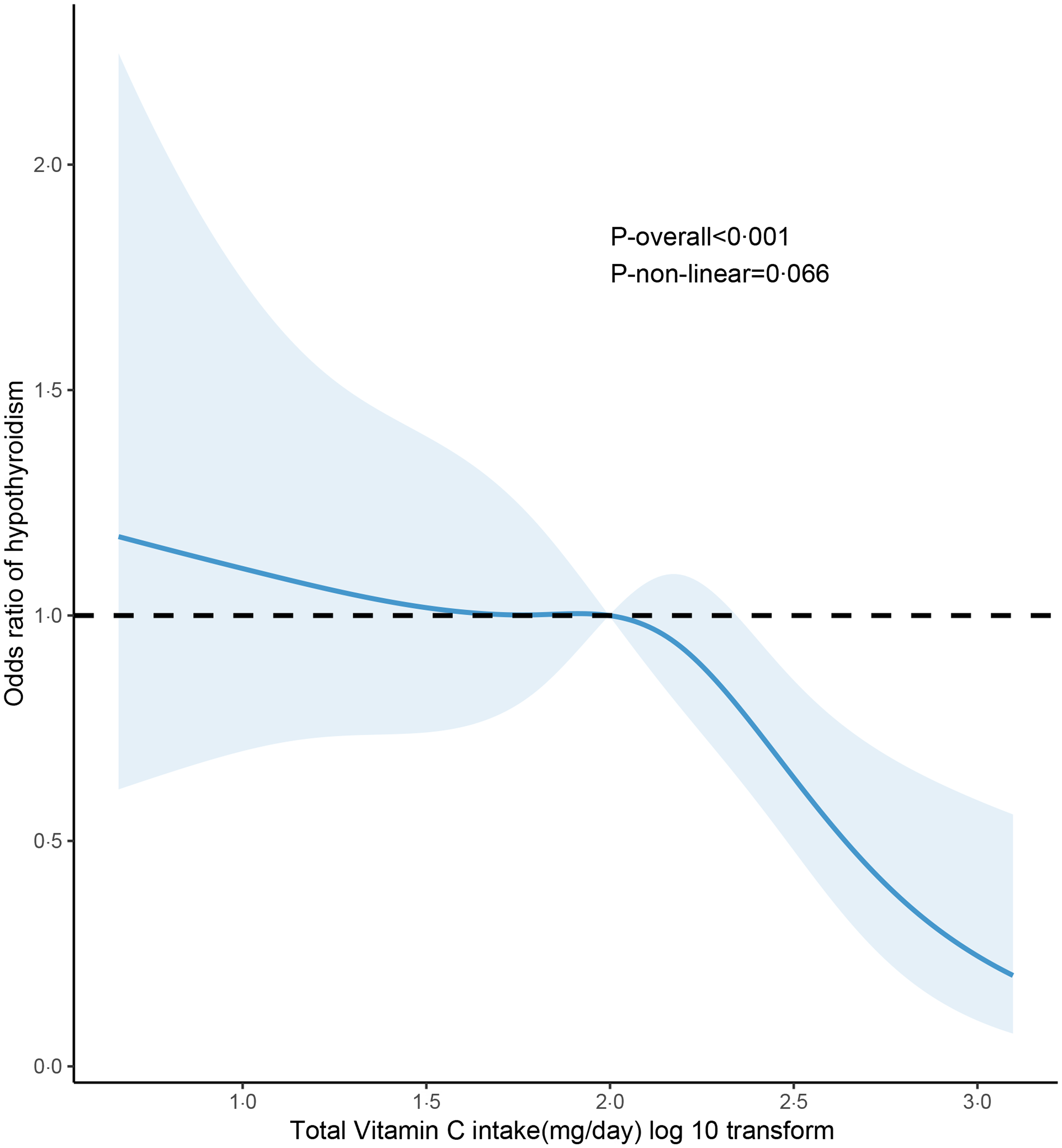
Fig. 2. Association between total vitamin C intake and hypothyroidism among adults with Hashimoto thyroiditis. Restricted cubic spline regression model with four knots (5th, 35th, 65th and 95th percentiles) was employed with age (continuous), sex (male or female), race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black or other race/multiracial), smoking status (never smoke, former smoke or current smoke), drinking status (never drink, former drink or current drink), urinary iodine (continuous, log10 transformation), TG-Ab levels (continuous, log10 transformation) and diabetes (yes or no) adjusted. TG-Ab, thyroglobulin antibodies.
Table 2. Odds ratios (95% confidence intervals) for hypothyroidism according to quartiles of total vitamin C intake among adults with Hashimoto thyroiditis (Odds ratio and 95% confidence intervals)
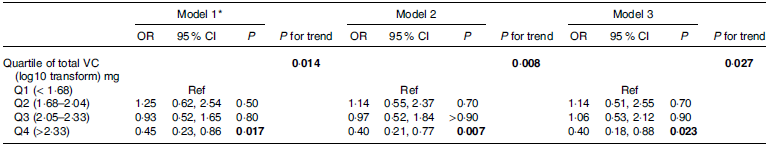
VC: vitamin C; Ref: reference; TG-Ab: thyroglobulin antibodies.
*Model 1: no covariates were adjusted; model 2: age, sex and race were adjusted. Model 3: age, sex, race, smoking status, drinking status, urinary iodine (log10 transformation), TG-Ab levels (log10 transformation) and diabetes were adjusted.
The bold values represent statistically significant.
Subgroup and sensitivity analyses
The relationship between total vitamin C intake and hypothyroidism in HT was consistent in subgroups stratified by sex (P for interaction = 0·084, Fig. 3(a)) or age (≥60 years and <60 years, P for interaction = 0·330, Fig. 3(b)). A sensitivity analysis yielded similar results when excluding participants with extreme 1 % values of total vitamin C intake (online Supplementary File 1: Table S2). This association remained using the cut-off point of 250 IU/ml for TPO-Ab level to define HT in another sensitivity analysis (online Supplementary File 1: Table S3). The results were also unchanged when limited to participants with normal TG-Ab levels (online Supplementary File 1: Table S4).
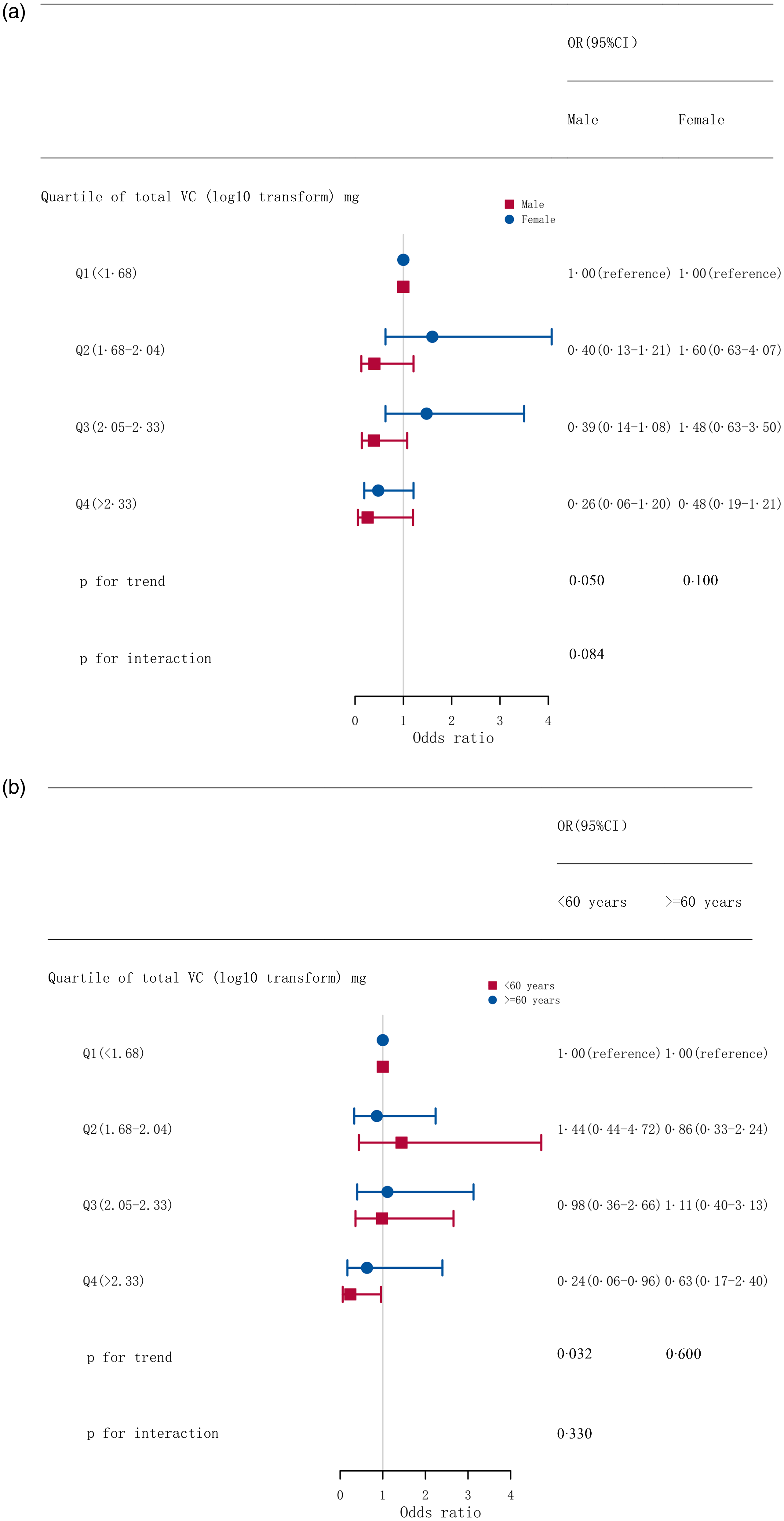
Fig. 3. Stratified associations between quartiles of total vitamin C intake and hypothyroidism among adults with Hashimoto thyroiditis by sex (a) and age (b). Adjusted for age, sex, race, smoking status, drinking status, urinary iodine (log10 transformed), TG-Ab levels (log10 transformed) and diabetes. VC, vitamin C; TG-Ab, thyroglobulin antibodies.
Discussion
This study showed a significant inverse association between total vitamin C intake (log10-transformed) and the occurrence of hypothyroidism in HT patients in a linear dose–response manner. Moreover, this association was consistent across sex and age.
HT is a leading cause of hypothyroidism, and approximately 25 % of patients with HT eventually develop hypothyroidism(Reference Caturegli, De Remigis and Rose14). The percentage of hypothyroidism in HT patients in our study was 19 %, which is comparable with the above-mentioned report. HT is usually treated with synthetic levothyroxine for life to address the resulting hypothyroidism. It is believed that oxidative stress contributes to the pathogenesis of autoimmune disorders and chronic inflammation, as it promotes the synthesis of autoantibodies and inflammatory responses(Reference Mancini, Di Segni and Raimondo15–Reference Baser, Can and Baser17). The potential development of hypothyroidism in HT may be influenced by increased oxidative stress, which has been shown to induce apoptosis in thyroid follicular cells(Reference Rostami, Aghasi and Mohammadi18,Reference Das, Banerjee and Jena19) . Oxidative stress can enhance the immunogenicity of thyroid-specific antigens through the accumulation of excess hydrogen peroxide, thereby intensifying the autoimmune response. Furthermore, it upregulates the expression of intracellular adhesion molecules and facilitates the infiltration of inflammatory cells(Reference Burek and Rose7), ultimately resulting in the apoptosis and necrosis of thyrocytes. Therefore, it is reasonable to imagine that antioxidants may attenuate the deleterious effects of oxidative stress in HT patients.
Vitamin C is a well-known antioxidant due to its robust ability to eliminate free radicals such as hydroxyl, superoxide and peroxyl radicals(Reference Youssef and Salah20,Reference Kietzmann21) . Additionally, vitamin C exhibits anti-inflammatory and immune support properties, which reduces cell and organ damage(Reference Spoelstra-de Man, Elbers and Oudemans-Van Straaten22). In boldenone undecylenate-treated rodent models, there was a significant elevation in serum pro-inflammatory factors, along with observed thyroid dysfunction characterised by elevated TPO-Ab and TSH levels, and reduced FT3 and free thyroxine concentrations. However, the administration of a daily dosage of vitamin C in conjunction with boldenone undecylenate significantly improved immune parameters and thyroid function in rats(Reference El Deib, El-Sharkawy and Beheiry11). Our findings showed that higher vitamin C intakes were significantly associated with reduced odds of hypothyroidism among HT patients, suggesting a potential benefit of vitamin C in this population. Due to the cross-sectional study design, our study did not infer a causal relationship between vitamin C intake and hypothyroidism in HT. In addition, we acknowledge that our study did not test the mechanism by which vitamin C affects thyroid health in patients with HT. There are several possible protective mechanisms of vitamin C in HT as follows: First, activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway can cause reactive oxygen species accumulation. This process has a vital role in the pathogenesis of HT(Reference Feng, Xu and Cai23), whereas vitamin C can inhibit NF-κB activation(Reference Kietzmann21). Second, the role of regulatory T cells (Tregs) in preventing autoimmunity and maintaining immune homeostasis has been well established(Reference Weetman24). In animal model studies, deficiency of regulatory T cells may induce lymphocytic infiltration within the thyroid gland, which was associated with developing hypothyroidism(Reference Hu, Zhang and Chen25). Several studies have shown that vitamin C enhances the immunosuppressive effects of Tregs(Reference Peng, Chen and Wan26,Reference Nikolouli, Hardtke-Wolenski and Hapke27) . Finally, recent studies have shown that T helper 17 cells (Th17 cells) are closely related to the pathogenesis of HT(Reference Salazar-Viedma, Vergaño-Salazar and Pastenes28), and vitamin C can reduce the immune response of Th17 cells and ameliorate the abnormalities of thyroid function in chronic lymphocytic thyroiditis(Reference Wu, Li and Yan29).
TPO-Ab is widely recognised as the most reliable serologic indicator for the diagnosis of HT and is found to be positive in 95 % of HT patients(Reference Caturegli, De Remigis and Rose14). Moreover, this antibody has been identified as a risk factor for progression to overt hypothyroidism over time in HT(Reference Biondi, Cappola and Cooper30). TG-Ab exhibits lower sensitivity and specificity in HT. Our results showed that the serum level of TPO-Ab in participants with hypothyroidism was higher than in those with euthyroidism, but the difference did not reach statistical significance. One plausible explanation is that the hypothyroidism group included individuals with subclinical hypothyroidism, which may reduce the difference in TPO-Ab levels between the two groups. Karimi et al.(Reference Karimi and Omrani10) found a significant reduction in TPO-Ab levels among patients with HT after a 3-month intervention involving a daily intake of 500 mg of vitamin C. In contrast, our study revealed that the titers of TPO-Ab did not exhibit a gradual decline with an increasing intake of vitamin C, although the incidence of hypothyroidism did decrease. Notably, Karimi et al. solely encompassed individuals with TPO-Ab positivity, whereas our study included TPO-Ab- or TG-Ab-positive participants. This discrepancy in inclusion criteria may account for the different findings regarding the association between vitamin C and TPO-Ab. Participants with hypothyroidism had higher TG-Ab levels than those with euthyroidism in our study. Considering the association of total vitamin C intake with hypothyroidism in the HT population may be influenced by higher TG-Ab levels, we not only adjusted for TG-Ab levels in the main analysis but also performed a sensitivity analysis by including only patients with normal TG-Ab levels. The results did not change, suggesting that the relationship between total vitamin C intake and hypothyroidism in HT was not affected by differences in TG-Ab levels.
Previous studies have shown that women with HT were at higher odds of developing hypothyroidism compared with men(Reference Tunbridge, Evered and Hall31,Reference McLeod and Cooper32) . In addition to sex, age and race may also influence thyrotropin levels(Reference Klubo-Gwiezdzinska and Wartofsky33). However, the race variable involved five different strata. If subgroup analysis was performed separately, it would lead to insufficient sample size in each stratum, thus affecting the reliability of the results. Therefore, we did not conduct subgroup analyses for the variable of race. The results of our subgroup analyses suggested that sex and age did not affect the relationship between total vitamin C intake and hypothyroidism among HT.
The median total vitamin C intake (containing dietary and supplement intake) among the HT patients included in our study was 109 mg, slightly surpassing the recommended intake of 75 mg for women and 90 mg for men in the Dietary Guidelines for Americans 2020–2025(34). Our study identified a significant linear inverse association between total vitamin C intake and hypothyroidism in patients with HT, with the amount of total vitamin C intake ranging from 4·57 to 1258·9 mg/d (not exceeding the adult’s tolerable upper intake level of 2000 mg/d(Reference Medicine35)). Based on this, an increase in the intake of total vitamin C within 4·57–1258·9 mg/d may benefit HT. As noted in one review, vitamin C may help counteract oxidative stress and improve the prognosis of HT as an adjuvant therapy(Reference da Silva, Yamauchi and Bagatini36).
The present study had several strengths. First, to our knowledge, this is the first study to explore the association between total vitamin C intake and hypothyroidism in participants with HT. Second, our findings were based on a nationally representative sample and adjusted for potential confounding factors. Additionally, sensitivity analyses were performed by eliminating the influence of extreme vitamin C intake or inclusion of HT defined by a different TPO-Ab cut-off on the results. However, some limitations were also present in our research. First, NHANES did not provide serum vitamin C data for these cycles, and we used the dataset of dietary and supplemental vitamin C intake obtained through two 24-h recall interviews, which may be subject to recall bias. Furthermore, dietary and supplemental vitamin C intake may not always reflect actual serum vitamin C levels because the bioavailability of dietary nutrients varies among individuals. Third, the dataset used in this study was obtained from NHANES 2007 to 2012 cycle, and dietary habits and nutritional intake may have changed over time. Therefore, our study suffered from some temporal bias. Fourth, the definition of HT is currently limited to the presence of serum TPO-Ab and TG-Ab, without information on the appearance of thyroid sonogram. However, approximately 5–10 % of HT cases are seronegative(Reference Klubo-Gwiezdzinska and Wartofsky33), potentially leading to some HT patients’ exclusion, which may result in an underestimation of the relationship between vitamin C intake and hypothyroidism. Finally, the cross-sectional design limited the determination of causal effects. In the future, we can further observe the incidence of hypothyroidism by following up with the HT cohorts (with euthyroidism) with different serum vitamin C levels to test the possible role of vitamin C in HT.
In conclusion, this study showed that increasing total vitamin C intake was associated with lower odds of hypothyroidism in HT. Our findings may provide new insights into the potential protective role of vitamin C in HT. Further studies are needed to examine the benefit of total vitamin C intake in this population.
Acknowledgements
The authors would like to thank the participants and staff who contributed to the NHANES database.
The authors declare that no funds were received to conduct this study.
The authors have no conflicts of interest to declare.
G. C. and L. C. contributed to the conceptualisation and design. L. C. and Y. M. carried out data collection and analyses. L. C. interpreted the findings and wrote the original manuscript draft, and L. C. is the guarantor of this manuscript. All the authors have read and approved the final manuscript.
The National Center for Health Statistics institutional ethics review board approved the protocol of the NHANES, and all participants signed the written informed consent.
This study used NHANES resources, and researchers can access these datasets by logging on to the official website of NHNAES (https://www.cdc.gov/nchs/nhanes/). All data generated or analysed during this study are included in this published article.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524001715








