Fat-soluble vitamins, including vitamins A, D and E, play an important role in the growth and development of humans. They include vitamins A, E, D, etc. Deficiency of fat-soluble vitamins may cause adverse pregnancy outcomes. In pregnant women, vitamin A deficiency causes night blindness and may increase the risk of maternal mortality(1). Vitamin E deficiency has been postulated to play a role in pre-eclampsia(2). Low concentrations of blood vitamin D in pregnant women have been associated with pregnancy complications(Reference Palacios, Kostiuk and Peña-Rosas3). It is common that mothers have deficient or excessive amount of fat-soluble vitamins during pregnancy. Vitamin A deficiency affects about 19 million pregnant women, mostly in Africa and South-East Asia, causing night blindness(4). Maternal vitamin D deficiency has been a crucial issue globally, as it can lead to a series of perinatal complications and subsequent fetal growth(5). In contrast, a survey of vitamins A and E of pregnant women in six provinces of northern China found the common situation of excess vitamin E(Reference Li, Ni and Zheng6).
Low birth weight (LBW), defined as infant birth weight <2500 g, is one of the adverse birth outcomes, which may lead to higher infant mortality, dysplasia(Reference Zhan7) and non-communicable diseases such as hypertension(Reference Goldenberg, Cutter and Hoffman8), diabetes(Reference Barker and Lackland9) and stroke(Reference Barker10). Macrosomia, defined as infant birth weight ≥4000 g, is increasing in developing countries and is associated with childhood obesity(Reference Liu, Li and Cheng11). Evidence about the effect of maternal fat-soluble vitamins during pregnancy on infant birth weight is inconsistent in the literature. Some studies found that maternal vitamin A and vitamin E concentrations at 18–30 gestational weeks were not associated with birth weight or fetal growth retardation(Reference Tamura, Goldenberg and Johnston12). However, Cohen et al. revealed that vitamin A (retinol) concentration during mid-pregnancy was positively associated with the risk of small for gestational age (SGA, i.e. birth weight below the 10th percentile for the same gestational age)(Reference Cohen, Kahn and Platt13). Scholl et al. found that vitamin E (i.e. α-tocopherol) at 28 gestational weeks was positively correlated with birth weight and reduced the risk of SGA(Reference Scholl, Chen and Sims14). In a meta-analysis of sixteen studies, maternal vitamin D deficiency had an increased risk of LBW (OR 2·39, 95 % CI 1·25, 4·57)(Reference Kehong, Yuna and Min15). Similar findings have been reported in an umbrella review of meta-analyses of observational studies(Reference Theodoratou, Tzoulaki and Zgaga16). However, in an overview of forty-two systematic reviews, mixed results were presented for the associations between maternal vitamin D and LBW(Reference Bialy, Fenton and Shulhan-Kilroy17). Current literature relating maternal vitamin D to birth weight is inconclusive.
Studies exploring the association between fat-soluble vitamins and birth weight are rare in China, and the existing ones were poorly designed (e.g. having a relatively small sample size, measuring vitamins at or about partum only, not matching between observation and control groups, not controlling for covariates, not detailing the study design, etc.)(Reference Zhao18–Reference Zhao, Li and Wang20). Therefore, the present study was conducted to explore the association between maternal serum vitamin A, E and D concentrations during pregnancy and birth weight, based on a large-scale retrospective study among the Chinese population.
Methods
Study design and setting
A retrospective study was conducted in the Tongzhou Maternal and Child Health Hospital of Beijing (Tongzhou hospital), China, from July 2015 to January 2018. Tongzhou is a district located in the southeast of Beijing, with a population of 1·58 million residents by the end of 2018. The district is positioned as the subsidiary administrative centre of Beijing, and its industry mainly focus on culture tourism and technology innovation.
Study population
Pregnant women who had prenatal care in Tongzhou hospital between July 2015 and December 2017 were provided to have routine serum vitamin A (retinol), E and D measurement. The study participants were women who had vitamin A, E and D measurement during pregnancy, had given birth in the Tongzhou hospital and whose complete background information was available (n 19 851). Women who had multiple fetuses (n 153), fetal death (n 52), stillbirth (n 5) or incorrectly reported infant birth weight (n 1; 353 g) were excluded from the study. The final study population were 19 640 (online Supplementary Fig. S1). Oral informed consent was obtained from the participants before any measurement.
Vitamin A, E and D measurement
Venous blood samples were taken from the participants in each trimester of pregnancy (first trimester ≤13 weeks, second trimester 14–27 weeks and third trimester ≥28 weeks). The serum vitamin A and E concentrations were measured in all trimesters, while vitamin D concentration was not measured in the first trimester. It should be noted that not all participants had the vitamin A and E tests in each trimester.
The serum samples were kept away from light since collection and stored at −80°C. In the pre-processing, proteins and impurities were removed from samples by centrifugation. Vitamins to be measured were extracted by using extraction agent, and supernatant liquid was re-dissolved by mobile phase (methanol and pure water). Vitamins A and E were measured by high-performance liquid chromatography (Agilent) with a flow rate of 1·0 ml/min. Vitamin D (sum of 25-hydroxyvitamin D2 and D3) was measured by liquid chromatography mass spectrometry (LC–MS/MS 6340, Agilent) with a flow rate of 0·3 ml/min. The standard curve equation was made according to the measured concentrations of the standard substance, and results of quality control samples and testing samples were calculated from the equation. When the quality control values were all in the range of mean ± 2 sd, then the batch could be regarded as in control, and the test results of samples could be reported.
Data collection
Data were collected from the hospital’s electronic information system, including the results of vitamin A, E and D measurement, sociodemographic information, maternal pre-pregnancy BMI, gestational weight gain, folic acid usage (between 1 to 3 months pre-pregnancy and 3 months post-conception), gestational weeks at the time of vitamin tests in each trimester, delivery and neonatal outcomes (including gestational age, newborn sex, birth weight, fetal death and stillbirth). The information of vitamin supplementation was not collected, as we paid more attention to the internal exposure of vitamin concentrations that represented by serum concentration.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board (IRB 00001052-19005) of the Peking University Health Science Centre. All data used for analysis were anonymous.
Variables and definitions
The outcome variable was birth weight, measured immediately after delivery by midwives. Birth weight <2500 g was defined as LBW(21), while birth weight ≥4000 g was defined as macrosomia(Reference Boyd, Usher and McLean22). Birth weight between 2500 and 4000 g was considered as normal birth weight.
Serum vitamin A, E and D concentrations were independent variables. According to the WHO(4), concentrations of serum vitamin A (retinol) were categorised into deficient (<0·7 μmol/l), insufficient (0·7–1·05 μmol/l), adequate (1·05–2·45 μmol/l) and excessive (≥2·45 μmol/l). Vitamin E concentrations were classified into three groups, deficient (<5·0 mg/l), adequate (5·0–20·0 mg/l) and excessive (≥20·0 mg/l)(23). There is not yet broad consensus on what constitutes vitamin D deficiency, and we defined 25-hydroxyvitamin D concentration <20 ng/ml as vitamin D deficiency, 20–30 ng/ml as insufficiency, 30–70 ng/ml as adequacy and ≥100 ng/ml as excess according to the Endocrine Society and the American Association of Clinical Endocrinologists(Reference Holick, Binkley and Bischoff-Ferrari24,Reference Camacho, Petak and Binkley25) .
Pre-pregnancy BMI was calculated with self-reported weight and height before pregnancy and classified into four groups (<18·50, 18·50–23·99, 24·00–27·99, ≥28·00 kg/m2). Gestational weight gain was calculated from prenatal weight (no more than 4 weeks before delivery) and pre-pregnancy weight. Preterm birth was defined as live births with <37 gestational weeks(Reference Goldenberg, Fculhane and Iams26).
Statistical analyses
Frequencies or proportions were presented as descriptive statistics for categorical variables. The concentrations of vitamins during each trimester were assessed for normality by the Shapiro–Wilk test. The median (quartile 1, quartile 3) was used to describe the central tendency and dispersion tendency of vitamin concentrations. Differences of the proportion of LBW/macrosomia in the maternal characteristics and vitamin status were explored by χ 2 tests. Multiple logistic regression analysis was further performed to assess the independent effect of different concentrations (deficiency, insufficiency, sufficiency and excessiveness) of vitamins in each trimester on LBW/macrosomia. To maximise the statistical power and minimise bias that might occur if missing vitamins and gestational weight gains data were excluded, we performed multivariate multiple imputation with chained equations to impute missing values. To solve the possible problem of separation in logistic regressions, we used Firth’s bias reduction method; the R studio software and ‘logistf’ package were used for data analyses. Variables with a P < 0·10 in χ 2 tests were included in the regression model as potential confounders. Pre-pregnancy height and examination season were additionally adjusted in vitamin D analyses. Considering the relatively low prevalence of vitamin A and E deficiency and excess in Tongzhou district, and in order to further examine their impacts on birth weight, vitamin A, E and D concentrations in each trimester were normalised and we treated the Z-scores as the crucial explanatory variables. The relationship between Z-score of vitamin concentration and LBW/macrosomia was then tested by logistic regression analysis. Considering the predominant influence of preterm birth and to further verify the difference of vitamin concentrations in different birth weight groups, we performed a stratified analysis by preterm birth using Student’s t test. A P value of <0·05 was considered as statistically significant. Statistical analyses were mainly performed by the SPSS software (version 20; IBM SPSS Statistics).
Results
General characteristics of participants
Of the total 19 640 women included in this study, the majority were between 21 and 30 years (64·9 %), had high school education and/or above (78·6 %), of Han ethnicity (94·0 %), were primiparous (60·3 %), had pre-pregnancy normal weight (BMI, 18·50–23·99 kg/m2, 63·1 %) and had used folic acid between 1–3 months pre-pregnancy and 3 months post-conception (91·2 %). The prevalence of preterm birth in the study was 3·9 %. The proportion of LBW and macrosomia was 2·6 and 8·2 %, respectively (Table 1).
Table 1. Maternal characteristics and birth outcomes (n 19 640)
(Numbers and percentages)

Maternal vitamin status
There were 16 703, 5520 and 2192 women having the vitamin A and E measurements in the first, second and third trimesters, respectively. There were 11 634 women having vitamin D measurement in the second trimester and 6609 women in the third trimester. Table 2 presented the median concentrations of serum vitamins A, E and D during three trimesters. The proportion of vitamin A deficiency was relatively low during three trimesters (no more than 0·1%). However, a growing tendency of vitamin A insufficiency from the first to third trimesters was seen. Reversely to that of vitamin A, excess vitamin E increased from 0·2% in the first trimester to 13·7% in the third trimester. The deficiency (second: 29·6%, third: 29·8 %) and insufficiency (second: 32·9%, third: 32·6%) of vitamin D remained to be stably high during pregnancy (Table 2).
Table 2. Vitamin A, E and D concentrations during three trimesters
(Median values and quartile 1, quartile 3 (Q1, Q3); numbers and percentages)
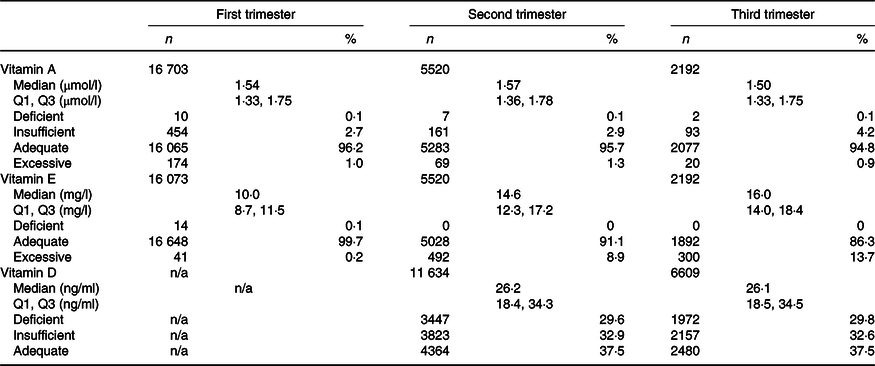
n/a, Not available.
Association between maternal serum vitamin concentrations and birth weight
Table 3 illustrated the univariate association between maternal characteristics and LBW or macrosomia. Table 4 showed the association between vitamin status and LBW and macrosomia by univariate analysis. Tables 5 and 6 present the independent effects of vitamin status in different trimesters on LBW and macrosomia, respectively. As seen in Table 5, there was no significant effect of different vitamin A, E or D concentrations on LBW. As seen in Table 6, excessive vitamin E concentration in the second trimester was associated with a higher risk of macrosomia (OR 1·30, 95 % CI 1·07, 1·59). There was no other statistically significant association of vitamin concentration with the risks of macrosomia, after controlling for potential confounders (Table 6).
Table 3. Association between maternal characteristics and low birth weight (LBW) or macrosomia by univariate analyses (n 19 640)
(Numbers and percentages)
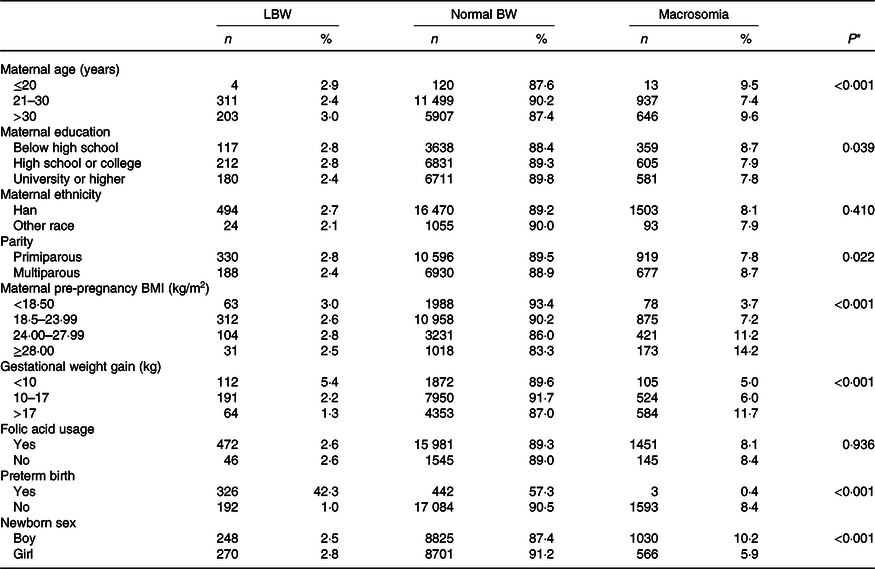
* The P value is reported from χ 2 test.
Table 4. Association between vitamin A, E and D status and low birth weight (LBW) or macrosomia by univariate analysis
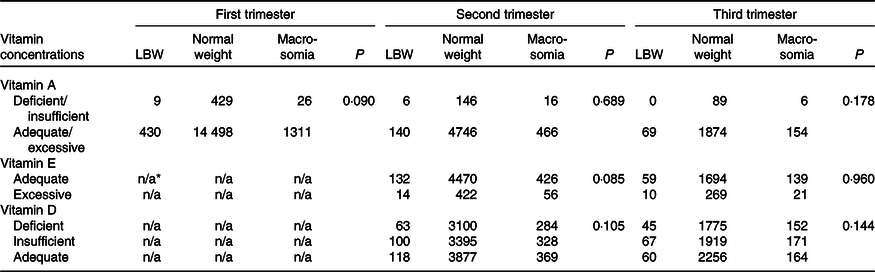
n/a, Not available.
* Number of women with vitamin E deficiency or excessive in the first trimester were not sufficient to perform a χ 2 test.
Table 5. Association between vitamin A, E and D concentrations and low birth weight (LBW) by multiple logistic regressions*
(Numbers and percentages; odds ratios and 95 % confidence intervals)

n/a, Not available.
* Maternal age, preterm birth, sampling time, gestational weight gain were adjusted in the regression models. Pre-pregnancy height and examination season were additionally adjusted for vitamin D analyses.
† Number of women with vitamin A deficiency or insufficiency in third trimester were not sufficient to perform regression analyses. Vitamin E in the first trimester was bypassed for similar reasons.
Table 6. Association between vitamin A, E and D concentrations and macrosomia by multiple logistic regressions†
(Numbers and percentages; odds ratios and 95 % confidence intervals)

n/a, Not available.
* P < 0·05.
† Maternal age, education level, parity, preterm birth, newborn sex, sampling time, pre-pregnancy BMI, gestational weight gain were adjusted in the regression models. Pre-pregnancy height and examination season were additionally adjusted for vitamin D analyses.
‡ Numbers of women with vitamin A deficiency or insufficiency in third trimester were not sufficient to perform regression analyses. Vitamin E in the first trimester was bypassed for similar reasons.
Association between vitamin (A, D or E) concentration (Z-score) and birth weight
The higher vitamin A concentrations in the first trimester (OR 1·14, 95 % CI 1·01, 1·29), second trimester (OR 1·31, 95 % CI 1·05, 1·63) and third trimester (OR 2·00, 95 % CI 1·45, 2·74) were associated with the higher risk of LBW. Vitamin A was negatively associated with macrosomia in the second trimester (OR 0·79, 95 % CI 0·70, 0·89) and third trimester (OR 0·77, 95 % CI 0·62, 0·95). Vitamin E was positively associated with macrosomia in the first trimester (OR 1·07, 95 % CI 1·00, 1·14), second trimester (OR 1·27, 95 % CI 1·11, 1·46) and third trimester (OR 1·28, 95 % CI 1·06, 1·54). No significant association between maternal vitamin D and macrosomia was found in the second trimester, and vitamin D was negatively associated with macrosomia in the third trimester (OR 0·87, 95 % CI 0·77, 0·98) (Table 7). Stratified analysis showed that, among women of preterm delivery, those who had LBW infant had higher vitamin A concentrations in the second and third trimesters than those who had normal birth weight infant. Among women of non-preterm delivery, vitamin A concentrations in the first and third trimesters of those who had macrosomia infant were higher than those who had normal birth weight infant; vitamin E concentrations in all trimesters of those who had macrosomia were higher than those who had normal birth weight infant (Table 8).
Table 7. Association between vitamin A, D and E concentrations (Z-score) and low birth weight (LBW) and macrosomia†
(Odds ratios and 95 % confidence intervals)
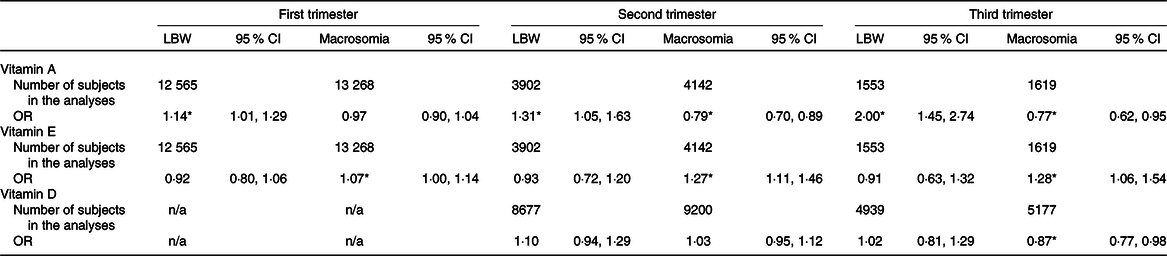
n/a, Not available.
* P < 0·05.
† Maternal age, preterm birth, sampling time, gestational weight gain were adjusted when taking LBW as outcome. Maternal age, education level, parity, preterm birth, newborn sex, sampling time, pre-pregnancy BMI, gestational weight gain were adjusted when taking macrosomia as outcome. Pre-pregnancy height and examination season were additionally adjusted for vitamin D analyses.
Table 8. Association between vitamin A and E concentrations and low birth weight (LBW) and macrosomia, by stratified analysis

n/a, Not available.
* No woman had a preterm birth and macrosomia infant in the third trimester.
Discussion
The study found that the prevalence of LBW and macrosomia were 2·6 and 8·2 %, respectively. Maternal vitamin D deficiency and excess vitamin E were relatively severe during the mid- and late pregnancy. Excess vitamin E was relevant to higher risk of macrosomia compared with adequate vitamin E concentration in the second trimester. Vitamin A (Z score) was positively associated with LBW in all trimesters and negatively associated with macrosomia in the second and the third trimesters. Maternal vitamin E status correlated with the risk of macrosomia positively in all trimesters. Among women of non-preterm delivery, vitamin A concentrations of those who had LBW infant were higher than those who had normal weight infant in the first and third trimesters, and vitamin E concentrations of those who had macrosomia were higher than those who had normal weight infant.
A study of 13 701 infants in a hospital in Beijing from 2012 to 2017 found the prevalence of LBW was 5·3 %, and there was no difference between years(Reference Hu, Zhao and Zou27). The hospital was next to the railway station geographically so that it received patients with complex disorders nationwide, under which condition the prevalence of LBW might be high. Actually, a previous study had found the prevalence of LBW between 2013 and 2017 in the same hospital as our study, and the ratio of singleton pregnancy women delivering a baby with LBW was 2·79 %(Reference Meng, Jin and Wang28), almost the same as ours. The prevalence of macrosomia during 2013–2017 in Hebei province was 6·3 %(Reference Jin, Wang and Zhao29), slightly lower than our result, reflecting the development level between two areas, in spite of similar geographic environment and eating habits.
Previous studies referring to the association between LBW and vitamins A, E or D did not reach an agreement(Reference Eggemoen, Jenum and Mdala30–Reference Miliku, Vinkhuyzen and Blanken38). The results of our study (Table 5) were similar to a prospective cohort study in Ethiopia, which detected no association between vitamin A deficiency and LBW in neither the second nor the third trimester(Reference Gebremedhin, Enquselassie and Umeta39). However, we speculate the insignificance was due to the low prevalence of LBW and relatively low ratio of vitamin A deficiency, insufficiency and excess in our study. In terms of the association between vitamin E and LBW, our result was similar to a recent research in Brazil, which detected no association between vitamin E concentrations of umbilical cord serum and weight to gestational age at birth(Reference Silva, Medeiros and Lima40). As for vitamin D, our finding was similar to a mother–offspring cohort in Singapore that maternal vitamin D status in pregnancy did not influence infant birth outcomes such as SGA(Reference Ong, Quah and Tint36). However, our results were inconsistent with two former studies in China, which reported relatively severe vitamin D insufficiency (<30·0 ng/ml) (73·65 and 96·41 %)(Reference Wang, Xiao and Zhang31,Reference Chen, Fu and Hao37) . We believe that the relatively small sample size of the former two studies (n 2374 and 1326), and not taking gestational weight gain as consideration might account for the inconsistency in results between our study and the former studies.
Regarding the outcome of macrosomia, we found no significant association between any maternal vitamin deficiency or excess during pregnancy and macrosomia, except that there existed a tendency that excess vitamin E would lead to macrosomia more easily than vitamin E adequacy (Table 6). Our finding was consistent with a prospective study in Hunan province, China, among 1999 pregnant women, which found that excess vitamin E in the third trimester was related to higher risk of macrosomia(Reference Lu41). In a mice experiment, prohibitive intake of high-fat foods during pregnancy also brings abundant vitamin E for mothers, in which case fetus live in an environment of higher nutrition, then macrosomia come into being(Reference Schaiff, Knapp and Barak42). Besides, another prospective cohort study conducted in South Korea demonstrated that birth weight were highest when the concentrations of both vitamins C and E in the second trimester were high(Reference Lee, Hong and Lee43). The author explained that vitamin E acted as an antioxidant, thus defensed against oxidative stress and impairment to fetal growth. Similarly, Scholl et al. (Reference Scholl, Chen and Sims14) related vitamin E at 28 weeks to the risk of large-for-gestational-age births positively. Vitamin E can potentiate the synthesis of prostacyclin, which has a vasodilatory effect(23). Prostacyclin/thromboxane A2 balance regulates maternal and fetal vascular function during pregnancy. Decrease in maternal prostacyclin:thromboxane A2 ratio may contribute to intrauterine fetal growth restriction, owing to inadequate blood flow between placenta and fetus(Reference Majed and Khalil44). In all, we believe that the physiological function of vitamin E itself and/or its catalysate contribute to the association between the excess of vitamin E and macrosomia.
When regarding Z score of vitamin concentrations as explanatory variable, we observed the positive association between vitamin A concentrations in three trimesters and higher risk of LBW (Table 7). And in the further stratified analysis, women with LBW infants have higher vitamin A concentrations than those with normal weight infants except for the first trimester in preterm group (Table 8). Our result was similar to Cohen et al. that elevated vitamin A in mid-pregnancy might be associated with an increased risk of SGA(Reference Cohen, Kahn and Platt13). And in later pregnancy, serum vitamin A was negatively related with fetal growth(Reference Mathews, Youngman and Neil45). The association could be explained by inadequate haemodilution or defective transport(Reference Weber, Stuetz and Bernhard33). If plasma volume expansion is obstructed in pregnancy, there will be low placental blood flow, which leads to high maternal vitamin A concentration but low blood delivery of vitamin A to fetus. Therefore, low plasma volume expansion is associated with poor fetal growth(Reference Salas, Rosso and Espinoza46). As for macrosomia, our study reported a negative association between vitamin A and macrosomia in the second and the third trimester. It has been proved in an animal study that diabetic rats delivering macrosomia had lower vitamin A concentrations(Reference Yessoufou, Soulaimann and Merzouk47). This relation can also be explained by the low blood expansion(Reference Salas, Rosso and Espinoza46). It appears that the elevated vitamin A concentrations relate with decreasing birth weight overall. Further study to clarify the mechanism of the influence of maternal vitamin A status on infant birth weight is warranted. Nevertheless, based on the results of our study, maintaining a moderate concentration of vitamin A during pregnancy might contribute to normal birth weight.
Among samples with adequate concentration of vitamin E in each trimester, we observed a significantly positive association between vitamin E in all trimesters and macrosomia (Table 7). In the stratified analysis, among women who had full-term delivery, those who gave birth to macrosomia have higher vitamin E concentrations, in comparison with those who gave birth to normal weight infants especially in the non-preterm group (Table 8). In a study, plasma concentrations of vitamin E at entry and at week 28 were positively related to increased fetal growth (birth weight), a decreased risk of SGA births and an increased risk of large-for-gestational-age(Reference Scholl, Chen and Sims14), which had similarities with our study. Therefore, maintaining a high concentration of vitamin E during pregnancy may not be good for fetal growth.
Our study had some strengths. First, it is the first study exploring the associations between maternal fat-soluble vitamin status and birth weight in Beijing, a highly urbanised and well-developed city in China. Second, we examined the vitamin status at every stage of pregnancy rather than at delivery and tested the maternal serum vitamin concentrations directly, which represented the accumulation of fat-soluble vitamins in recent time. Third, sample size of this study is relatively large. Finally, nearly all previous studies classified vitamin status as categorical variables (‘deficient’, ‘insufficient’, ‘sufficient’, ‘excessive’ or ‘quantiles’); however, we further analysed Z score in consideration of the low prevalence of vitamin A and E deficiency and severe vitamin D deficiency in Tongzhou hospital.
Limitation of our study should be acknowledged. Our study was a retrospective study; some variables that might be adjusted as potential confounders were not collected, especially the prevalence of chronic diseases such as diabetes and high blood pressure, which are crucial causes of preterm birth and LBW. The vitamin concentrations of pregnant women in Tongzhou may be different from that of other areas in China. Our results might not be generalised to population living outside Beijing. There are a large number of missing measurements of vitamin tests, as participants were not compulsory to do tests in all trimesters at the time of antenatal care, and we have performed multiple imputation to solve the problem.
Conclusion
In conclusion, this study implies a need to be aware of excess vitamin E and vitamin D deficiency during pregnancy and relatively increasing proportion of macrosomia in China. Excessive concentration of vitamin E in the second trimester was found to be associated with macrosomia. Women are thus recommended to avoid having high concentration of vitamin E during pregnancy. Our results that higher maternal vitamin A concentrations in all three trimesters being associated with higher risk of LBW and lower risk of macrosomia in the second and third trimesters suggest maintaining the moderate concentration of vitamin A during pregnancy. Nonetheless, more prospective studies and experimental studies are warranted to illustrate such associations in different population and the mechanism between vitamins A and E in pregnancy and fetal growth.
Acknowledgements
The authors sincerely thank the staff in Maternal and Child Health Care Hospital of Tongzhou District for data collection.
This study was funded by the Peking University Research Initiation Fund (BMU2018YJ005) and National Natural Science Foundation of China (81973053, 81703240). Both funding bodies had no role in the design, analysis or writing of this article.
Q. Z. and W. Y. conceptualised the study. M. J., L. X. and N. H. collected the data. W. Y. analysed the data and drafted the manuscript. Q. Z. supervised in drafting the manuscript and provided critical comments on this paper. H. W. provided critical comments on this paper. S. L. was responsible for data management, and X. X. was responsible for project management. All authors have read and approved the final manuscript.
All authors declare that they have no competing interests.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003347











