Dietary habits developed in childhood(Reference Movassagh, Baxter-Jones and Kontulainen1,Reference Mikkilä, Räsänen and Raitakari2) and adolescence(Reference Cruz, Ramos and Lopes3,Reference Lake, Mathers and Rugg-Gunn4) may sustain into adulthood to some extent(Reference Movassagh, Baxter-Jones and Kontulainen1,Reference Mikkilä, Räsänen and Raitakari2) , and dietary intake during the early stages of life potentially influences the development of obesity(Reference te Velde, Twisk and Brug5) and CVD(Reference Kaikkonen, Mikkilä and Raitakari6) in the later stages of life. Therefore, establishment of healthy dietary habits during childhood and adolescence is essential for improving the health of children and adolescents on a life-long basis.
The WHO defines free sugars as monosaccharides and disaccharides added to foods and drinks by the manufacturer, cook or consumer, and sugars naturally present in honey, syrups, fruit juices and fruit juice concentrates, and it advocates for strong (<10 % of total energy intake (%E)) and conditional (<5 %E) recommendations on free sugar intake for the prevention of unhealthy weight gain and dental caries in children and adolescents(7). The nature of high energy and low nutrient density of free sugars can lead to poor micronutrient intakes (i.e. nutrient dilution) in consumers of high free sugars(7,8) . Previous studies in Western children and adolescents support this nutrient dilution hypothesis(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10) . For instance, a national survey of British children found the association between moderate free sugar intake (>13 %E) and trend reductions in micronutrient intakes(Reference Gibson, Francis and Newens9); similarly, lower micronutrient intakes were found to be associated with high free sugar intake (≥20 %E) in children and adolescents in an Australia national surveys(Reference Wong, Mok and Ahmad10). These findings indicate that the thresholds for preventing nutrient dilution may differ among countries, and the WHO recommendations for free sugar intake(7) may be insufficient to prevent nutrient dilution.
Japanese children and adolescents have relatively lower mean free sugar intake (5·8 %E in boys aged 8–14 years to 7·7 %E in girls aged 3–6 years)(Reference Fujiwara, Murakami and Asakura11) than Western children and adolescents (11·5 %E in Australian children aged 2–3 years(Reference Lei, Rangan and Flood12) to 15·4 %E in British adolescents(Reference Gibson, Francis and Newens9)), probably due to lower intakes of soft drinks and confectioneries among Japanese children and adolescents than among Western counterparts(Reference Fujiwara, Murakami and Asakura11). The Japanese diet is lower in whole grains, processed meat, nuts and higher in refined grains (mainly white rice), vegetables, seaweeds, fish, soya foods and green tea than the Western diet(Reference Micha, Khatibzadeh and Shi13,14) . Thus, the effects of free sugars on micronutrient intakes may differ between Japanese children and adolescents and Western counterparts. Indeed, previous studies found that Japanese adults(Reference Fujiwara, Okada and Okada15) had a lower threshold (≥5 %E) for nutrient dilution than Australian adults(Reference Mok, Ahmad and Rangan16) (≥25 %E). Moreover, because of the sparseness of epidemiological studies on free sugar intake and health issues, there is no recommendation on free sugar intake for Japanese children and adolescents(17). Notably, it is essential to confirm the association between free sugar intake and micronutrient intake before making any recommendations on free sugar intake for Japanese children and adolescents. However, to our knowledge, no study has investigated the association of free sugars with nutrient dilution among Japanese children and adolescents.
This cross-sectional study aimed to clarify the relationship between free sugars and selected nutrients intakes among Japanese children and adolescents using 1-d dietary data from the 2016 National Health and Nutrition Survey, Japan (NHNS). The thresholds of free sugar intake for nutrient dilution were set at 2·5, 5 and 10 %E based on a previous study in Japanese adults(Reference Fujiwara, Okada and Okada15) and the WHO guidelines(7).
Methods
Data source and sample selection
The NHNS is a national nutrition survey conducted annually since 1945 by the local public health centres under the supervision of the Ministry of Health, Labour and Welfare based on the Health Promotion Law. This cross-sectional analysis was performed using data from the 2016 NHNS and was conducted with the permission of the Ministry of Health, Labour and Welfare. Data from the 2016 survey were used for the present analysis because of the much larger sample size of the survey than that of more recent studies. Details of the survey have been described elsewhere(14,Reference Fujiwara, Okada and Okada15,Reference Ikeda, Takimoto and Imai18) . Briefly, 462 census units were sampled as survey areas on the basis of the 2010 national census. All non-institutionalised Japanese aged ≥1 year (as of 1 November 2016) living in the survey areas were asked to participate. Households in which the heads were foreign citizens, individuals without a self-selected diet and individuals on a special diet (mainly because of disease) were excluded from the survey. The survey was conducted between October and November 2016. A total of 10 745 of 24 187 eligible households (44·4 %) participated in the final survey.
The number of participants aged 1–19 years who completed the dietary survey and underwent anthropometric measurements was 4282 and 3264, respectively. The final sample used in this analysis comprised 1505 male and 1414 non-lactating and non-pregnant female participants aged 1–19 years with complete information on the variables of interest (2919 in total) (Fig. 1).
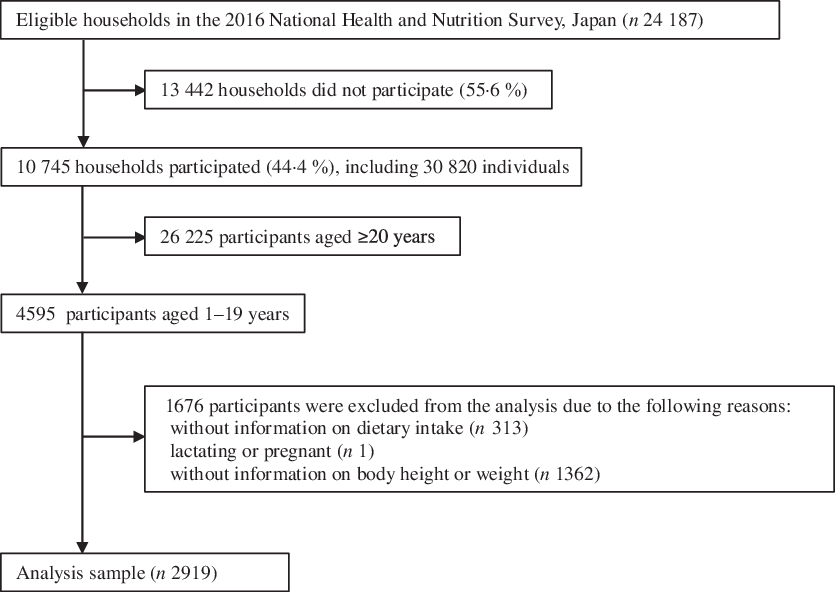
Fig. 1. Flow chart of participants included in the present analysis.
This survey was conducted according to the guidelines laid down in the Declaration of Helsinki, and verbal informed consent was obtained from all individual participants. Under Article 33 of the Statistics Act, the Ministry of Health, Labour and Welfare anonymised individual-level NHNS data and provided the first author with the data sets for this study. This analysis was exempt from the need for ethical and institutional review board approval according to the Ethical Guidelines of Epidemiological Research established by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare(19) because only anonymised data were used.
Dietary assessment
Dietary intake was assessed by a 1-d weighed household dietary record, as described previously(14,Reference Fujiwara, Okada and Okada15,Reference Ikeda, Takimoto and Imai18) , and data on all members of each household were included in the analysis. Briefly, a primary meal preparer in the household (referred to as a record keeper) was provided with a diary and asked to weigh and record all food and beverages (except for drinking water) consumed by household members on the recording day other than Sundays, national holidays and days with special events (e.g. wedding party or funeral). If household members shared food items from a single dish, the record keeper was asked to record the approximate proportions of the food consumed by each member, as well as the amount of leftovers. In the cases that the record keeper encountered any difficulty with weighing in some occasions, such as eating out or having school-prepared lunch, he/she was asked to document as much information as possible, including the estimated portion sizes and details of leftovers. The recorded diary was collected during the next few days and checked by a trained fieldworker. If there was any missing or unclear information in the diary, the trained fieldworker collected additional information from the record keeper. The weights of foods and beverages consumed were estimated according to the portion sizes recorded using household measures. When participants ate school-prepared lunch, the trained fieldworker was asked to obtain the menu of lunch, including information on names and weights of the ingredients, from each school. Thereafter, food codes were assigned to all items (which were classified mainly based on the Standard Tables of Food Composition in Japan (STFCJ)(20)) by trained fieldworkers, based on the NHNS study manual. The dietary intake data were inputted using the software developed especially for the NHNS by trained fieldworkers at the local centre and then compiled to create an overall dietary data set by trained investigators at the central office.
The energy and selected nutrient intakes of each participant were estimated from the household food consumption record (for shared dishes/foods, approximate proportions consumed by each household member were calculated) according to the STFCJ(21,22) . Free sugar intake(7) was estimated using a recently developed comprehensive food composition database(Reference Fujiwara, Murakami and Asakura11) for common Japanese food items included in the STFCJ(21,22) . In the development steps of the free sugar database(Reference Fujiwara, Murakami and Asakura11), the free sugar content of all food items was determined based on total sugar and saccharide contents using a published stepwise method(Reference Mok, Ahmad and Rangan16,Reference Louie, Moshtaghian and Boylan23) (online Supplementary Document 1). Only nutrient intake from foods and beverages was assessed in the present analysis; thus, dietary supplements were not included in the nutrient intake calculation. Food grouping was performed based on similarity in nutrient profiles and culinary usage according to the STFCJ(21,22) . Given the differences in dietary intakes due to varying body sizes and energy requirements, nutrient and food group intake was expressed as nutrient density, that is, %E for macronutrients (except for protein), an amount per 1000 kcal (4184 kJ) for other nutrients and food groups, and both for protein. The absolute intake was also presented because the use of nutrient density could lead to the report of lower intakes of nutrients and food groups in the case of higher free sugar intake (%E) due to mathematical effects.
The utility of the household dietary record was assessed previously on the basis of individual-level dietary intake in the Japanese population(Reference Iwaoka, Yoshiike and Date24). Briefly, the dietary intake of young women (aged about 20 years) was assessed by comparing a 1-d household dietary record by the women themselves with that by their mothers (mean age 49 years) (n 32). Mean differences between intakes estimated by the two dietary records for energy, protein, fat and carbohydrate were 6·2, 5·7, 6·7 and 6·3 % with Pearson correlation coefficients of 0·90, 0·89, 0·91 and 0·90, respectively(Reference Iwaoka, Yoshiike and Date24).
Assessment of characteristics
Information on sex and age was collected by a self-administered questionnaire. Anthropometric measurements were conducted by trained fieldworkers for 54·9 % of the participants using the following standardised procedures. Body height (nearest to 0·1 cm) and weight (nearest to 0·1 kg) were measured in participants while wearing light clothes and being barefoot. For the remaining 45·1 % of participants, heights and weights were measured by other household members at home or were self-reported. The weight status was defined based on the BMI (calculated as weight (kg) divided by height squared (m2)) according to the age- and sex-specific cut-offs of the International Obesity Task Force(Reference Cole and Lobstein25) for participants aged 1–17 years or the WHO recommendations(26) for those aged 18–19 years.
Statistical analysis
We performed statistical analyses using the SAS statistical software, version 9.4 (SAS Institute Inc.). Free sugar intake was classified into four categories (<2·5, 2·5 to <5, 5 to <10 and ≥10 %E) according to the WHO guidelines(7) and the previous study in Japanese adults(Reference Fujiwara, Okada and Okada15). The differences in basic characteristics among those in various free sugar intake categories were examined using a linear trend test for continuous variables and a Mantel–Haenszel χ 2 test for categorical variables. The adjusted mean intakes and standard errors (se) of energy, nutrients and food groups were estimated for each free sugar intake category using general linear models with adjustment for sex, age and weight status. The difference in mean intakes across the free sugar intake categories was examined using the Tukey–Kramer test, while the presence of linear associations was tested by linear regression analysis with the median value of each free sugar intake category as a continuous variable. For the assessment of the adequacy of nutrient intake across the free sugar intake categories, the prevalence of inadequacy was determined. On the basis of nutrient density, the prevalence of inadequacy was determined as the percentage of participants with a lower intake than the estimated average requirement of the Dietary Reference Intakes for Japanese, 2020(17) (online Supplementary Table S1), except for n-6 PUFA, n-3 PUFA, vitamins D, E, and K, pantothenic acid, P and Mn (determined as the percentage of participants with lower intake than the adequate intake); dietary fibre and K (determined as the percentage of participants with lower intake than the tentative dietary goal for preventing lifestyle-related diseases (DG)); SFA and Na (determined as the percentage of those with higher intake than the DG); and protein (%E), fat and carbohydrates (determined as the percentage of those with intake above the range of the DG). For SFA, dietary fibre and K, the prevalence of inadequacy was estimated for participants aged 3–19 years because the reference values of these nutrients for children aged 1–2 years are not advocated by the Dietary Reference Intakes for Japanese, 2020(17). All reported P values were two-tailed. The significance level for the Mantel–Haenszel χ 2 test was set at P < 0·05, while that for the linear trend and Tukey–Kramer test was set at P < 0·001 to minimise type I error, as demonstrated in previous studies(Reference Wong, Mok and Ahmad10,Reference Fujiwara, Okada and Okada15,Reference Mok, Ahmad and Rangan16) .
Sensitivity analysis
Sensitivity analysis was conducted by excluding misreporters for energy intake (n 70, 2·4 %) because selective under-reporting of confectioneries and soft drinks is common in participants with under-reporting energy intake(Reference Murakami, Sasaki and Okubo27,Reference Murakami, Miyake and Sasaki28) ; and under-reporting is more frequently observed in participants with the lowest free sugar intake(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10,Reference Mok, Ahmad and Rangan16) . As described previously(Reference Fujiwara, Okada and Okada15), misreporting of energy intake was defined as the ratio of reported energy intake:the BMR, i.e., the Goldberg cut-off for the 1-d dietary data and the physical activity level for a sedentary lifestyle(Reference Black29). Briefly, based on age and body height and weight, BMR was estimated using Henry’s sex- and age-specific equations(Reference Henry30) or the sex- and age-specific equations developed for the Japanese(Reference Ganpule, Tanaka and Ishikawa-Takata31,Reference Miyake, Tanaka and Ohkawara32) participants aged 1–17 or 18–19 years, respectively. Because exclusions would have introduced selection bias(Reference Livingstone and Black33), the results of all participants were reported as the main analysis. The results of the sensitivity analysis (online Supplementary Tables S2–S4) were not substantially different from those of the primary analysis; thus, the effect of misreporting was not sufficiently significant to change the present findings.
Results
The mean free sugar intake was 6·0 (sd 4·4) %E, and the prevalence of those with excessive free sugar intake according to the WHO conditional recommendation (≥5 %E) was 49·7 %. Participants with high free sugar intake were more likely to be girls and younger and have a lower BMI (Table 1).
Table 1. Basic characteristics of Japanese children and adolescents (n 2919) according to free sugar intake: the 2016 National Health and Nutrition Survey, Japan
(Numbers and percentages; mean values and standard deviations)
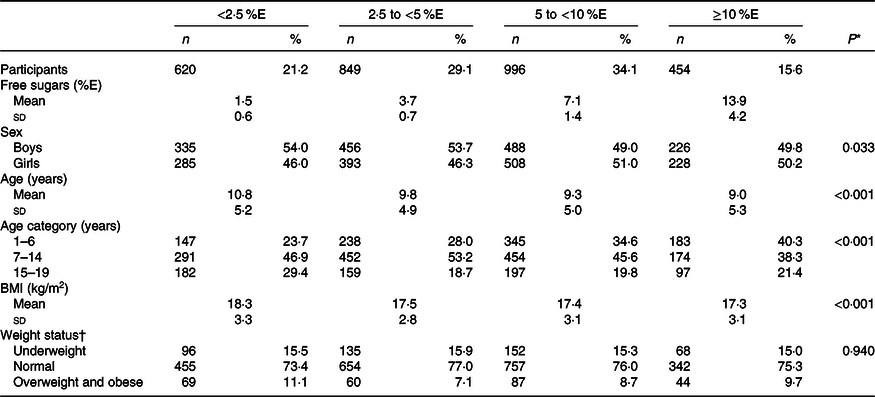
%E, percentage of energy.
* For continuous variables, a linear regression was used with the median value of each category of free sugar intake (1·5, 3·7, 6·8 and 12·7 %E) as a continuous variable; for categorical variables, Mantel–Haenszel χ 2 test was used.
† For subjects aged ≤17 years, weight status was defined according to International Obesity Task Force age- and sex-specific BMI (calculated as kg/m2) cut-offs(Reference Cole and Lobstein25), which correspond to an adult BMI of <18·5 kg/m2 for underweight, ≥18·5 to <25 kg/m2 for normal and ≥25 kg/m2 for overweight and obese subjects; for subjects aged 18–19 years, weight status was defined based on BMI according to WHO recommendations(26): <18·5 kg/m2 for underweight, ≥18·5 to <25 kg/m2 for normal and ≥25 kg/m2 for overweight and obese subjects.
The mean intakes of energy and nutrients according to the free sugar intake category are shown in Table 2. As mentioned above, we mainly described the results of nutrient density in this study. After adjustment for potential confounding factors, free sugar intake was positively associated with energy and carbohydrate intake and negatively associated with protein and fat intake. For all selected micronutrients, except Ca, the peak mean intakes were found in the first two categories of free sugar intake (i.e. <5 %E). Inverse linear associations were observed between free sugar intake and selected micronutrients, except for vitamins A and C, and Ca. Further, for all micronutrients examined, except for vitamins A and C, mean intakes in the ≥10 %E category of free sugars were lower than those in at least one of the other categories, while mean intakes of fourteen micronutrients were lower in the 5 to <10 %E category than in at least one of the lower two categories (<2·5 and 2·5 to <5 %E). The mean micronutrient intakes did not differ between <2·5 and 2·5 to <5 %E categories. Table 3 presents the prevalence of inadequacy according to the free sugar intake category. For almost all micronutrients investigated, the prevalence of inadequacy in the ≥10 and 5 to <10 %E categories tended to be higher than in the 2·5 to <5 %E categories.
Table 2. Intake of energy and nutrients in Japanese children and adolescents (n 2919) according to free sugar intake: the 2016 National Health and Nutrition Survey, Japan
(Numbers and percentages; mean values with their standard errors)
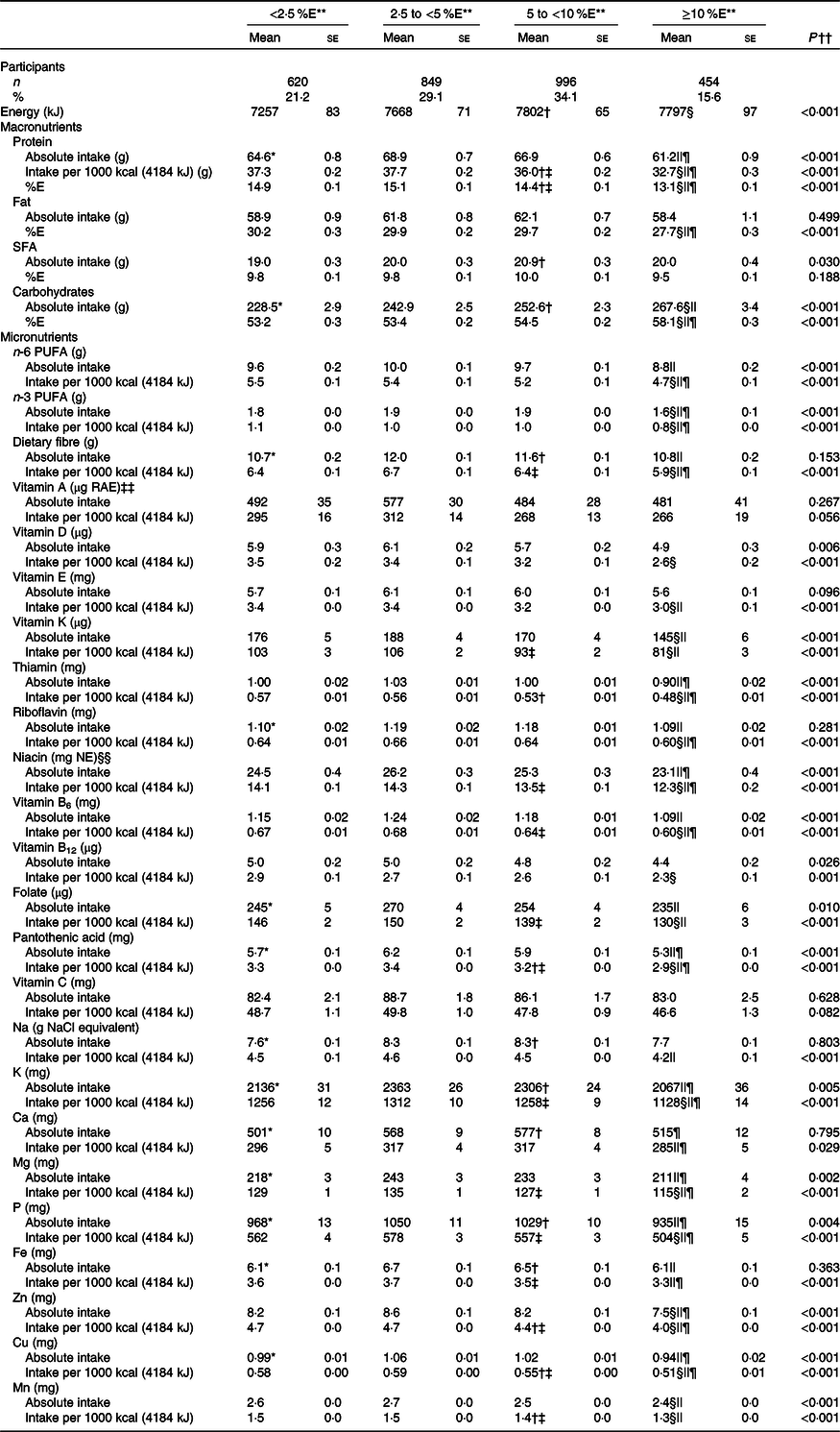
%E, percentage of energy intake; RAE, retinol activity equivalent; NE, niacin equivalent.
** Tukey–Kramer test was conducted with adjustment for sex, age and weight status (underweight; normal; or overweight and obese). P < 0·001 for difference between: <2·5 %E and 2·5 to <5 %E*, 5 to <10 %E and <2·5 %E†, 5 to <10 %E and 2·5 to <5 %E‡, ≥10 %E and <2·5 %E§, ≥10 %E and 2·5 to <5 %E|| and ≥10 %E and 5 to <10 %E¶. There was no difference between <2·5 %E and 2·5 to <5 %E.
†† A linear regression was conducted using the median value of each category of free sugar intake (1·5, 3·7, 6·8 and 12·7 %E) as a continuous variable with adjustment for same variables used in Tukey–Kramer test.
‡‡ 1 µg RAE = sum of retinol (µg) + β-carotene (µg) × 1/12 + α-carotene (µg) × 1/12 + β-cryptoxanthin (µg) × 1/24.
§§ 1 mg NE = niacin (mg) + protein (mg)/6000.
Table 3. Prevalence (%) of inadequacy of nutrient intake in Japanese children and adolescents (n 2919) according to free sugar intake: the 2016 National Health and Nutrition Survey, Japan*
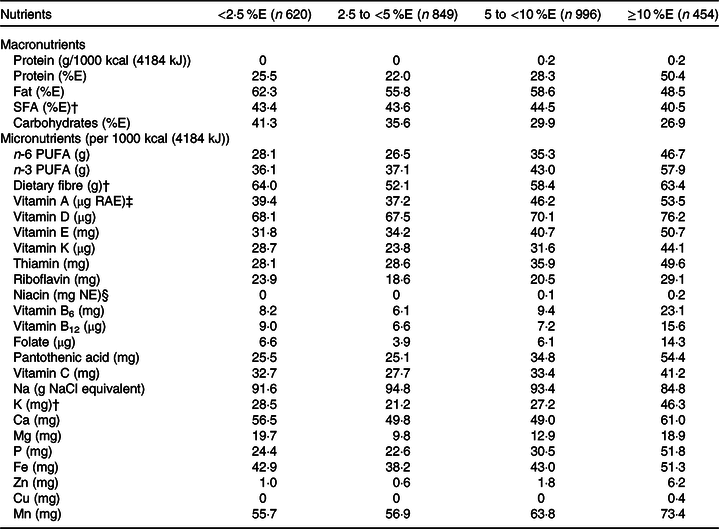
%E, percentage of energy intake; RAE, retinol activity equivalent; NE, niacin equivalent.
* Using nutrient density, prevalence of inadequacy was estimated as the percentage of participants with lower intake than the reference value the Dietary Reference Intakes for Japanese, 2020(17), except for SFA and Na (estimated as the percentage of participants with higher intake than the reference value); and protein (%E), fat, and carbohydrates (estimated as the percentage of participants with intake beyond the range of the reference value).
† Estimated for participants aged 3–19 years (n 569, 773, 912 and 410 for <2·5 %E, 2·5 to <5 %E, 5 to <10 %E and ≥10 %E, respectively) because the Dietary Reference Intakes of SFA, dietary fibre and K for children aged 1–2 years are not advocated.
‡ 1 µg RAE = sum of retinol (µg) + β-carotene (µg) × 1/12 + α-carotene (µg) × 1/12 + β-cryptoxanthin (µg) × 1/24.
§ 1 mg NE = niacin (mg) + protein (mg)/6000.
The mean intake of food groups according to the free sugar intake category is displayed in Table 4. Free sugar intake was positively associated with the intakes of sugars and jams, confectioneries, fruit and vegetable juices, and soft drinks, after adjustment for potential confounding factors. The peak mean intakes for these food groups were observed in the ≥10 %E category of free sugars. In contrast, free sugar intake was inversely associated with the intake of rice and grains, potatoes, pulses and nuts, vegetables, meats, dairy products, and fat and oils. For these food groups, the peak mean intakes were observed in the <5 %E categories.
Table 4. Food group intake in Japanese children and adolescents (n 2919) according to free sugar intake: the 2016 National Health and Nutrition Survey, Japan
(Numbers and percentages; mean values with their standard errors)
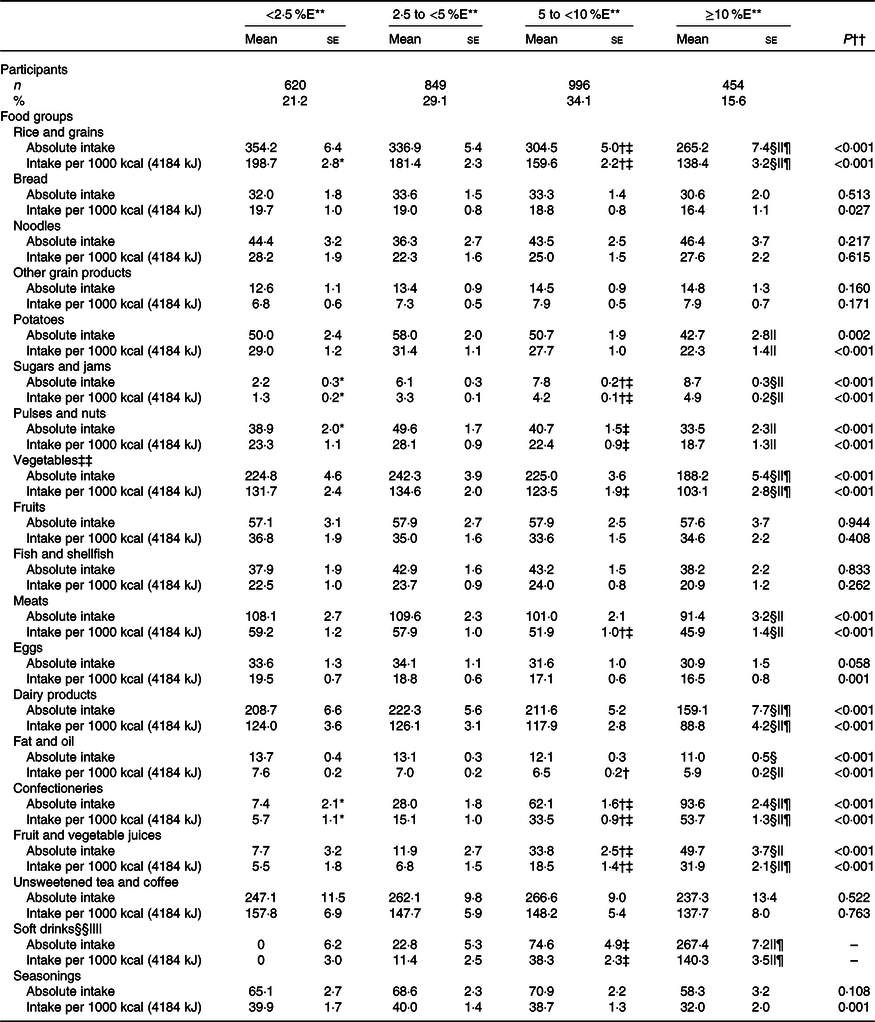
%E, percentage of energy intake.
** Tukey–Kramer test was conducted with adjustment for sex, age and weight status (underweight; normal; or overweight and obese). P < 0·001 for difference between: <2·5 %E and 2·5 to <5 %E*, 5 to <10 %E and <2·5 %E†, 5 to <10 %E and 2·5 to <5 %E‡, ≥10 %E and <2·5 %E§, ≥10 %E and 2·5 to <5 %E|| and ≥10 %E and 5 to <10 %E¶.
†† Linear regression was conducted using the median value of each category of free sugar intake (1·5, 3·7, 6·8 and 12·7 %E) as a continuous variable with adjustment for same variables used in Tukey–Kramer test.
‡‡ Including mushrooms and seaweeds.
§§ Including soda, sports drinks, fruit drinks, milk beverages and pre-sweetened coffee.
|||| Negative adjusted mean is presented as zero.
Discussion
To the best of our knowledge, this study is the first to investigate the association between free sugars and nutrients intakes in Japanese children and adolescents. We found that free sugar intake was inversely associated with almost all twenty-four micronutrients examined and lower mean micronutrient intakes were detected in the ≥10 and 5 to <10 %E categories (twenty-two and fourteen micronutrients, respectively) than in the lower categories. However, these inverse associations were not identified for vitamins D, E and B12 and Na based on the results of the absolute intake. Therefore, the influence of the free sugar intake on these micronutrients may be the result of the mathematical effect of the use of nutrient density.
In line with previous studies in Western children and adolescents(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10) as well as Japanese adults(Reference Fujiwara, Okada and Okada15), lower mean intakes of micronutrients were observed in children and adolescents with higher free sugar intake in the present study, mainly because of higher intakes of sugar-dense and poor-nutrient foods, such as soft drinks, confectioneries, and sugars and jams, and lower intakes of nutrient-dense foods, such as vegetables and pulses and nuts in the children and adolescents with higher free sugar intake. Because the use of the absolute intake did not change the results, the decreased intake of nutrient-dense foods associated with higher free sugar intake could not be due to the mathematical effects of the nutrient density. The sugar-dense and poor-nutrient food groups were the major sources of free sugar intake in Japanese children and adolescents (soft drinks 14·0 %; confectioneries 26·1 %; and sugars and jams 24·7 %) in the present study, and the findings are consistent with the results of previous Japanese(Reference Fujiwara, Murakami and Asakura11,Reference Fujiwara, Okada and Okada15) and Western studies(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10) . However, the mean intake of soft drinks in Japanese children and adolescents (72·7 g/d) in the present study was lower than that in their Western counterparts (66–732 ml/d)(Reference Guelinckx, Iglesia and Bottin34), thereby resulting in the lower mean intake of free sugars in Japanese children and adolescents. In contrast, the positive association of free sugar intake with vitamin C intake observed in British children(Reference Gibson, Francis and Newens9) was not identified in Japanese children and adolescents in the present study. This absence of association may reflect the relatively lower mean intake of fruits and vegetable juices (24·4 g/d, contributing to 3·9 % of total free sugar intake) in Japanese children and adolescents than in Western counterparts (86–629 ml/d)(Reference Guelinckx, Iglesia and Bottin34).
Comparable with Japanese adults(Reference Fujiwara, Okada and Okada15), Japanese children and adolescents in the present study had lower thresholds of free sugar intake associated with inadequate micronutrient intake (i.e. ≥5 %E) than Western children and adolescents (>13–20 %E)(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10) . These lower thresholds were mainly due to the lower mean free sugar intake in Japanese children and adolescents (6·0 %E in the present study and 5·8–7·7 %E in the previous study(Reference Fujiwara, Murakami and Asakura11)) than in Western counterparts (>11·5 %E)(Reference Gibson, Francis and Newens9,Reference Lei, Rangan and Flood12) , accompanied with lower micronutrient intake at higher free sugar intake, although other discrepancies, related to the study design and population characteristics, might have also contributed to the difference. As mentioned above, the lower soft drink intake in Japanese children and adolescents could be one of the possible reasons for such lower mean free sugar intake. Another possible reason could be related to the school system, including the school lunch programme in Japan(Reference Asakura and Sasaki35). Under the Standards for the school lunch programme, 98·6 and 83·7 % of Japanese elementary and junior high schools provide uniformed school lunch, respectively, and the nutrients contents are regulated(Reference Asakura and Sasaki35,36) . In the present study, a dietary assessment was conducted on a week day except Sundays and national holidays, and accordingly, 63·3 % of our children and adolescents had school-prepared lunch. Additionally, tuck shops and vending machines are generally not available in public elementary and junior high schools, and children are not allowed to bring money to public elementary schools, although there is no standard in Japan. The present findings suggest that the WHO guidelines for free sugar intake would be effective to prevent insufficient nutrient intake in children and adolescents, as well as adults(Reference Fujiwara, Okada and Okada15), in Japan. Further, studies are needed to assess the efficacy of these guidelines in other countries with a different dietary habit.
Previous findings suggest that very low free sugar intake may lead to poor micronutrient intake due to over-restriction in the diet(Reference Erickson and Slavin37). Intakes of several micronutrients were lower in the <2·5 %E category of free sugar intake in Japanese children and adolescents in the present study, although the differences did not reach statistical significance; the findings are in consistent with that in Japanese adults(Reference Fujiwara, Okada and Okada15). This discrepancy could be partly explained by the smaller number of children and adolescents (n 2919) in the present study than that of adults (n 16 652) in the previous study(Reference Fujiwara, Okada and Okada15), as well as small statistical power; in addition, differences in dietary habits between generations might also contribute to the discrepancy. Therefore, the public health programme to reduce free sugar intake should be conducted with the promotion of a balanced diet to maintain adequate nutrient intake in children and adolescents with very low free sugar intake. Notably, the risk of nutrient inadequacy may be lower in children and adolescents than in adults.
Fortified sugar-containing food items, such as breakfast cereals, which are frequently consumed in Western countries, can mask the negative effect of sugars on nutrient density(Reference Alexy, Sichert-Hellert and Kersting38). In these countries, food fortification should be considered to assess the influence of free sugar intake on nutrient dilution. However, because only unfortified breakfast cereals (that were included in other grain products in this study) are included in the STFCJ(21,22) , fortification of these food items may not influence the present findings. Moreover, the very low mean intake (i.e. 1·3 g) of breakfast cereals in Japanese children and adolescents makes it unlikely to produce any substantial effect.
The present study has several limitations that should be mentioned. First, while NHNS aims to include a nationally representative sample of the non-institutionalised population of Japan, the participation rate of sampled households was only 44·4 %. This rate is somewhat lower than that of the national nutritional surveys in other countries, such as the USA and the UK (61·3(39) and 53 %(40), respectively). One reason for the lower participation rate in the present analysis may be the higher burden of the dietary assessment method (i.e. a weighed dietary record compared with a 24-h recall or an estimated dietary record)(Reference Ahluwalia, Dwyer and Terry41,Reference Whitton, Nicholson and Roberts42) . Indeed, a change from a weighed dietary record to an estimated dietary record has improved the response rate (from 47 to 55 %(Reference Whitton, Nicholson and Roberts42)). Unfortunately, information on the characteristics of the households that refused to participate was not available, and the exact response rate of individual participants was not determined in the present analysis(14). Previous Japanese surveys(Reference Nishi, Nakade and Sarukura43,Reference Okubo and Yokoyama44) reported that female or older participants had a higher response rate and were associated with higher micronutrient intakes(Reference Murakami, Livingstone and Fujiwara45). These findings suggest that adult participants of the NHNS have higher micronutrient intake than non-participants. However, the influence of an adult household member on the diet of their children is unknown; thus, we could not conclude whether the diet micronutrient intake in the children and adolescents in the present study was higher than that in non-participants. Additional studies are warranted to investigate the association between the response rate and dietary intake in children and adolescents, clarify reasons for non-participation and identify methods to enhance motivation to participate in the future NHNS.
Second, the 1-d weighed household dietary records are insufficient to capture the habitual dietary intake of individual participants because the error introduced by the day-to-day variations in dietary intakes among free-living individuals is too large to be ruled out. This issue is the weakest part of the methodology of the present analysis. A previous study in Japanese adults suggested that the number of days of dietary records required to assess the usual intake was largest for nuts and seeds and smallest for cereals(Reference Ogawa, Tsubono and Nishino46). For nutrients, the largest number of days was required to assess usual intake of retinol(Reference Ogawa, Tsubono and Nishino46), possibly due to the relatively larger number of days required for evaluating usual intake of meats (including offal). These factors could result in the absence of an association between free sugar intake and vitamin A intake in the present study, in contrast to previous studies wherein habitual dietary intakes have been estimated based on multiple-day dietary data(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10) . On the contrary, the number of days required to assess the usual intake of energy and other nutrients was relatively small(Reference Ogawa, Tsubono and Nishino46). Therefore, caution is needed for interpreting the present results because the random error introduced by within-individual variations might attenuate the between-individual variations in nutrient intake in relation to free sugar intake. While the dietary assessment was not conducted on Sundays or national holidays, there was no information on the day selected for recording(14). Furthermore, seasonal variations could affect the dietary intake due to a specific time window of the survey(Reference Sasaki, Takahashi and Iitoi47,Reference Tani, Asakura and Sasaki48) . These limitations might produce bias in the assessment of the average dietary intake and thus hinder the accuracy of the present finding. Ideal solutions to overcome these limitations are a multiple-day dietary assessment covering all seasons and all the days of the week or the use of a validated dietary assessment questionnaire. Unfortunately, no nationally representative dietary survey on the basis of multiple-day dietary assessment methods is available in Japan. The feasibility of these strategies should be considered in future NHNS.
Third, the misreporting of dietary intake commonly occurs in dietary assessments and may influence the accuracy of the relationship between dietary intake and the variables of interest. Consumers with the lowest free sugar intake tend to under-report energy intake(Reference Gibson, Francis and Newens9,Reference Wong, Mok and Ahmad10,Reference Mok, Ahmad and Rangan16) , while those with under-reporting energy intakes selectively under-report confectioneries and soft drinks intakes(Reference Murakami, Sasaki and Okubo27,Reference Murakami, Miyake and Sasaki28) . However, the sensitivity analysis suggested that the influence of misreporting on the present finding was not significant enough to change the conclusion on the association between free sugar and nutrient intake.
Fourth, to assess the occurrence of nutrient dilution based on the WHO guidelines(7), we showed free sugar intake as nutrient density (i.e. %E). Because the use of nutrient density for free sugar intake is affected by energy and other macronutrient intakes, the association of free sugar intake with nutrient intake reported in the present study might be dependent on the intakes of total energy and other macronutrients(Reference Forshee and Storey49). Intake of nutrients and food groups was also shown as nutrient density to consider differences in these intakes derived from varying body sizes and energy requirements. This provides the same problem as in the case of free sugars, although the use of absolute value cannot take into account the difference mentioned above. Further, the primary objective of WHO guidelines is the prevention of dental caries and obesity, and insufficient nutrient intake and energetic control are not included in the guidelines(7). Because both macro- and micronutrient intakes are positively associated with the total energy intake(Reference Forshee and Storey49,Reference Willett50) , it is possible to achieve the recommended nutrient intake if the energy intake is high enough irrespective of free sugar intake. This is another problem of the use of absolute values. Hence, we showed the results of both the absolute values and nutrient densities and discussed the influence of energy adjustment on the present findings. However, since no optimal approach is available to adjust for the variations in the reported energy intake, any finding of nutrient dilution by free sugar intake should be carefully interpreted in accordance with the methodology used(Reference Rennie and Livingstone51,Reference Livingstone and Rennie52) .
Finally, despite a possible association between free sugar intake and social environmental status (SES)(Reference Fujiwara, Murakami and Asakura53), SES was not adjusted as a confounding factor in the present study; thus, the present findings were not independent of the influence of SES. Unfortunately, the NHNS 2016 only collected information on the occupation of adult participants, but not information on the household income as well as occupation and educational levels of parents of children and adolescents. Therefore, the present findings should be confirmed in other Japanese children and adolescents with information on SES variables. Further, the NHNS should consider collecting information on SES in children and adolescents, or information on the relationship between household members (e.g. fathers and their children; this can enable the link between children and adolescent members to the occupational status of adult members) in the future.
Conclusion
This national survey with a cross-sectional design reported lower nutrient intake in participants with higher free sugar intake in Japanese children and adolescents with relatively low free sugar intake. The present results suggested that the WHO guidelines for free sugar intake could help prevent nutrient dilution in Japanese children and adolescents, as demonstrated in Japanese adults. Although these findings may aid the development of guidelines for free sugar intake and public health policy in Japan, the present results should be interpreted with cautions because the association between free sugar intake and nutrient dilution could be attenuated by the random error introduced by day-to-day variations.
Acknowledgements
We thank the participants of the 2016 NHNS and the staff who supported the survey in each local public health centre and the central office for their valuable contribution.
This work was supported by the Health Japan 21 (the second term) Analysis and Assessment Project by the Ministry of Health, Labour and Welfare, Japan. The funding sources had no role in the design, analysis or preparation of this manuscript.
A. F. developed research questions, analysed and interpreted the data, and wrote the first draft of the manuscript. E. O., C. O. and H. T. contributed data collection and assisted in the writing of the manuscript. M. M. assisted in the writing of the manuscript. All authors read and approved the final manuscript.
All authors declare that there was no conflict of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520003657








