Psychiatric disorders affect millions of people around the world( Reference Murphy, Horton and Laird 1 ). Depression and anxiety are the most commonly diagnosed psychiatric conditions, influencing global health, quality of life, life expectancy and economy( Reference Mojtabai 2 – Reference Sobocki, Jönsson and Angst 4 ). It is estimated that 4·7 and 7·3 % of population, around the world, suffer from depression and anxiety, respectively( Reference Baxter, Scott and Vos 5 , Reference Ferrari, Somerville and Baxter 6 ). In Iran, according to national statistics, about 20·8 and 20 % of Iranian adults are affected, respectively( Reference Noorbala, Bagheri Yazdi and Yasamy 7 ).
In the aetiology of depressive disorders, the simultaneous occurrence of various factors including personal, genetic and environmental factors seem to be important( Reference Saveanu and Nemeroff 8 ). Much evidence is available on the role of diet in the development of depression and anxiety( Reference Murakami and Sasaki 9 ). For instance, consumption of green leafy vegetables, legumes, nuts, seeds and whole grains was associated with lower odds of depression( Reference Quirk, Williams and O’Neil 10 – Reference Opie, O’Neil and Itsiopoulos 14 ). Although the exact component of these foods affecting depression and anxiety is unknown, all these foods and food groups are rich sources of Mg, which plays an important role in the nervous system via its actions on the release and metabolism of neurotransmitters and other mechanisms( Reference Mohamed Salih, Nallasamy and Muniyandi 15 ). Several clinical trials had assessed the effect of high doses of Mg supplementation on depression in a short time period( Reference Oliveira, de Jesus and Freire 16 , Reference Messina 17 ). Findings from these investigations are not easily generalisable to routine lifestyle. Little attention has been paid on usual intakes of Mg. In a Finnish cohort study, an inverse relationship between dietary Mg intake and depression has been demonstrated( Reference Yary, Lehto and Tolmunen 18 ), whereas a prospective study failed to find any conclusive evidence on this association( Reference Martínez-González and Sánchez-Villegas 19 ). Moreover, a meta-analysis in this regard has indicated that moderate Mg intake may be inversely associated with the risk of depression( Reference Barra, Camardese and Tonioni 20 ). Furthermore, several studies have shown a significant higher risk of depression in hypomagnesaemic individuals( Reference Barra, Camardese and Tonioni 20 ), whereas others have not reached such findings( Reference Camardese, De Risio and Pizi 21 , Reference Cheungpasitporn, Thongprayoon and Mao 22 ). Therefore, data in this regard are conflicting. Overall, it seems that further studies are required to shed light on this issue.
Prior investigations on the association between dietary Mg intake and mental disorders have mostly been performed in Western nations, and limited studies have been carried out in this regard in Middle East countries. Examining the association between dietary Mg intake and mental disorders is particularly relevant for the Middle Eastern region where the prevalence of mental disorders is alarmingly high and the consumption of legumes, nuts and vegetables as the main dietary sources of Mg is low( Reference Ferrari, Somerville and Baxter 6 , Reference Noorbala, Bagheri Yazdi and Yasamy 7 ). Furthermore, previous studies on the association between dietary Mg intake and mental disorders were conducted without controlling for potential confounding variables. With controlling for confounders, this study aimed to examine the association between dietary Mg intake and mental disorders among a large population of Iranian adults.
Methods
Participants
This cross-sectional study was carried out within the framework of Studying the Epidemiology of Psycho-Alimentary Health and Nutrition (SEPAHAN) project, which was performed on a large population of Iranian adults working in fifty different health centres in Isfahan, Iran. Detailed information about SEPAHAN project has been published elsewhere( Reference Adibi, Keshteli and Esmaillzadeh 23 ). Briefly, collecting data at two separate main phases in this project leads to higher accuracy of collected data and participation rate. At the first phase, data on demographic variables along with dietary intakes were collected for 8691 people. At the second phase, data regarding psychological health were collected. By merging data from both phases, we had complete information for 4763 people. In the current analysis, we excluded participants who did not have total energetic intakes in the range of 3347–17 573 kJ/d (800–4200 kcal/d) as under-reporters and over-reporters of energy intake (n 787). In addition, individuals with missing data on psychological, demographic, anthropometric and dietary information were excluded (n 130). After these exclusions, a data set of 3172 participants including 1398 men and 1774 women was supplied for this study. All participants provided signed informed written consent forms. The whole project of SEPAHAN was ethically approved by the Bioethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran( Reference Adibi, Keshteli and Esmaillzadeh 23 ).
Dietary intake assessment
The usual dietary intakes of participants were assessed by a validated Willett-format dish-based 106-item semi-quantitative FFQ (DS-FFQ), which was designed particularly for Iranian adults( Reference Willett 24 ). Details on design, food items and validity of this FFQ have been reported previously( Reference Keshteli, Esmaillzadeh and Rajaie 25 ). In brief, first we prepared a comprehensive list of foods and dishes commonly consumed by Iranian adults. Then, we chose those that were nutrient-rich, consumed reasonably often or that contributed to between-person variations from this list. Selection of a food as a usual food item was done according to dietary records and recalls that had been collected in our prior investigations. Finally, 106 food items in five different categories existed in this questionnaire: (1) mixed dishes (cooked or canned, twenty-nine items); (2) carbohydrate-based foods (different types of bread, cakes, biscuits and potato, ten items); (3) dairy products (dairies, butter and cream, nine items); (4) fruit and vegetables (twenty-two items); and (5) miscellaneous food items and beverages (including sweets, fast foods, nuts, desserts and beverages, thirty-six items).
We asked individuals to report their dietary intakes of foods and mixed dishes based on nine multiple-choice frequency response categories varying from ‘never or less than once a month’ to ‘twelve or more times per day.’ The frequency response categories for all the food items were not constant and varied from six to nine choices. We omitted the high-frequency categories for foods consumed infrequently, as well as increasing the number of multiple-choice categories for common foods with a high consumption. Furthermore, in order to increase the accuracy of the responses, we used the most popular serving sizes familiar to Iranian adults. Finally, we calculated the daily intakes of all foods and dishes and converted them to grams per day using household measures( Reference Ghaffarpour, Houshiar-Rad and Kianfar 26 ). Next, in order to compute the daily energy and nutrient intakes (particularly Mg) of each participant, we summed up the energy and nutrient contents of all foods and dishes. Energy and nutrients contents of each food were obtained using the US Department of Agriculture’s national nutrient databank( 27 ). Earlier studies have indicated that data on food groups’ intake, as well as nutrient intake, from this questionnaire provide reasonably valid data of dietary intakes( Reference Esmaillzadeh, Keshteli and Feizi 28 – Reference Zaribaf, Falahi and Barak 31 ). Although the FFQ was fulfilled based on last year’s dietary intakes of participants, this questionnaire provided data about usual and long-term (more than 1 year) dietary intakes.
The validity of the DS-FFQ was evaluated in a subgroup of 200 randomly selected participants of the SEPAHAN project( Reference Keshteli, Esmaillzadeh and Rajaie 25 , Reference Salehi-Abargouei, Esmaillzadeh and Azadbakht 32 ). All participants in the validation study completed the DS-FFQ at study baseline and 6 months later. During this validation study, 3-d detailed dietary records, which were used as gold standard, were reported by individuals. According to findings from this study, the DS-FFQ could constitute reasonably valid and reliable measures of long-term dietary intakes in Iranian population; for instance, dietary carbohydrate intake estimated from DS-FFQ was significantly correlated with value obtained from the average of 3-d dietary records (r 0·81). Such correlation coefficients were also seen for other food groups and nutrients including Mg (r 0·61), proteins (r 0·72) and legume and nut consumption (r 0·69).
Psychological profile assessment
Anxiety and depression were assessed by the Iranian validated version of the Hospital Anxiety and Depression Scale, which provided valid measures of mental health on the basis of a previous study( Reference Montazeri, Vahdaninia and Ebrahimi 33 ). This scale is a brief and useful questionnaire to measure psychiatric disorders in addition to symptom and severity of anxiety disorders and depression( Reference Montazeri, Vahdaninia and Ebrahimi 33 ). It contains fourteen items with a four-point scale for each item and consists of two subscales: anxiety and depression; higher scores indicate the greater degree of anxiety and depression. The possible score range is from 0 to 21 for each subscales. Scores of 8 or more on either subscale were considered to indicate the presence of psychiatric disorders and scores of 0–7 were defined as ‘normal’ in the current study( Reference Montazeri, Vahdaninia and Ebrahimi 33 ). Overall, our previous investigations revealed that the questionnaire provides relatively valid measures of mental health( Reference Montazeri, Vahdaninia and Ebrahimi 33 ).
To assess psychological distress, we used the Iranian validated version of the General Health Questionnaire (GHQ), which contained twelve items( Reference Schmitz, Kruse and Heckrath 34 ). Each item constitutes a four-point rating scale (less than usual, no more than usual, rather more than usual or much more than usual). We used the bimodal scoring method (0–0 to 1–1) in order to calculate the total score of psychological distress for each participant. The total scores in this method range from 0 to 12; higher scores indicate a greater degree of psychological distress( Reference Montazeri, Harirchi and Shariati 35 ). In our study, we considered the score of 4 or more as having psychological distress. A validation study on 748 Iranian adults showed a significant inverse correlation between the GHQ-12 and global quality-of-life scores (r −0·56, P<0·001)( Reference Montazeri, Harirchi and Shariati 35 ).
Assessment of covariates
We used a self-reported questionnaire in order to obtain data on age, sex, marital status (single/married), education (high school diploma or below/above high school diploma), smoking status (non-smoker/former smoker/current smoker), family size (≤4/>4 members), home ownership (owner/non-owner), gestational and lactating status, disease history (diabetes, asthma, colitis, stroke, myocardial infarction, heart failure and cancers), current use of anti-psychotic medications (including nortriptyline, amitriptyline or imipramine, fluoxetine, citalopram, fluvoxamine and sertraline) and dietary supplements (including intake of Fe, Ca, vitamins and other dietary supplements). Assessing physical activity of study participants was carried out via a General Practice Physical Activity Questionnaire (GPPAQ), which is a simple validated screening tool for grading the physical activity of adults by focusing on current general activities. On the basis of the type and intensity of individual’s physical activity in work hours and during the weekends, they were categorised into four groups: active (>3 h/week), moderately active (1–3 h/week), moderately inactive (<1 h/week) and inactive (no physical activity). The validity of the GPPAQ for assessment of habitual physical activity levels has been valuated elsewhere( Reference Adibi, Keshteli and Esmaillzadeh 23 ). To gather information on anthropometric measures including weight and height, we used a self-reported questionnaire. BMI was calculated as weight in kg divided by the height in m2. The validity of self-reported weight and height was examined in a pilot study on 200 participants from the same population, which showed that correlation coefficients for self-reported weight and height v. technician-measured values were 0·95 (P <0·001) and 0·83 (P <0·001), respectively. In addition, the correlation coefficient for computed BMI from self-reported values and the one from measured values was 0·70 (P <0·001). On the basis of these results, the self-reported values of anthropometric indices supply reasonably valid measures in this study.
Statistical analysis
All statistical analyses were separately performed for men and women. First we obtained energy-adjusted intake of dietary Mg using residual method( Reference Willett 24 ), and then we categorised men and women by sex-specific quintiles of energy-adjusted Mg intake (men: Q1: <277 mg/d, Q2: 277–<301 mg/d, Q3: 301–<326 mg/d, Q4: 326–<358 mg/d, Q5: ≥358 mg/d; women: Q1: <281 mg/d, Q2: 281–<303 mg/d, Q3: 303–<325 mg/d, Q4: 325–<356 mg/d, Q5: ≥356 mg/d). The analyses were also performed using cut-off points of estimated average requirement (EAR) for Mg in men and women ((men: 320 mg/d, women: 265 mg/d))( Reference Kathleen Mahan, Escott-Stump and Raymond 36 ). On the basis of the Kolmogorov–Smirnov test, all variables had normal distribution. To assess differences in continuous variables (including demographic and dietary variables) across quintiles of Mg intake, one-way ANOVA was used, followed by pairwise post hoc tests with Bonferroni correction. The distribution of men and women in terms of categorical variables across quintiles of dietary Mg intake was evaluated using the χ 2 test. Binary logistic regression in different models was applied to examine the association between dietary Mg intake and psychiatric disorders including depression, anxiety and psychological distress. First we included all confounders including age (continuous), marital status (single/married), education (under university/university graduated), physical activity (<1/≥1 h/week), smoking status (non-smoker/former smoker/current smoker), family size (≤4/>4 members), home ownership (owner/non-owner), diabetes mellitus (yes/no), dietary supplement use (yes/no), anti-psychotic medications (yes/no) and dietary intake of different micronutrients into models and then in the final model, which was presented, and we retained those variables that had a significant contribution. Therefore, different adjusted models for different outcomes in men and women were retained as follows: in men and for depression, model 1 included education, smoking status and anti-psychotic medications, and model 2 included model 1 plus dietary intake of energy, fat, carbohydrate and vitamin B2; for anxiety, model 1 included smoking status, anti-psychotic medications and supplement use, and model 2 included model 1 plus dietary intake of energy, fibre, vitamin B1 and B2; and for psychological distress, model 1 included anti-psychotic medications and model 2 included model 1 plus dietary intake of energy, fibre and vitamin B6. In women and for depression, model 1 included age, marital status, education, family size, smoking status and anti-psychotic medications, and model 2 included model 1 plus dietary intake of energy and vitamin B5; for anxiety, model 1 included marital status, education, smoking status, home ownership and anti-psychotic medications, and model 2 included model 1 plus dietary intake of energy, fibre, vitamin B1 and B2; and for psychological distress, model 1 included education and anti-psychotic medications, and model 2 included model 1 plus energy intake and dietary intake of vitamin B3 and Fe. In the analyses, the first quintile of Mg intake was considered as the reference category. In the analysis based on EAR, participants with adequate intake of Mg were considered as the reference category. To determine the overall trend of OR across increasing quintiles of dietary Mg intake, we considered the quintiles as an ordinal variable in the logistic regression models. BMI-stratified analysis (normal weight (BMI<25 kg/m2) and overweight (BMI≥25 kg/m2)) was also performed. All statistical analyses were conducted using SPSS software (version 19.0; SPSS Inc.). P values were considered significant at <0·05.
Results
Mean age of men and women was 38·4 (sd 8·2) and 35·1 (sd 7·4) years, respectively. Prevalence of depression, anxiety and psychological distress was 20·4, 8·4 and 16·6 % among men and 33·7, 17·3 and 27·1 % among women, respectively.
General characteristics of men and women across quintiles of energy-adjusted Mg intake are provided in Table 1. Men in the highest quintile of dietary Mg intake were older and more likely to be obese, married, physically active, diabetic and less likely to be depressed, anxious and psychologically distressed compared with those in the lowest quintile. Compared with women in the bottom quintile, those in the top quintile of Mg intake were older and more likely to be overweight or obese, and less likely to be depressed, anxious and psychologically distressed.
Table 1 General characteristics of men and women across quintiles (Q) of energy-adjusted magnesium intakeFootnote * (Mean values and standard deviations; percentages)
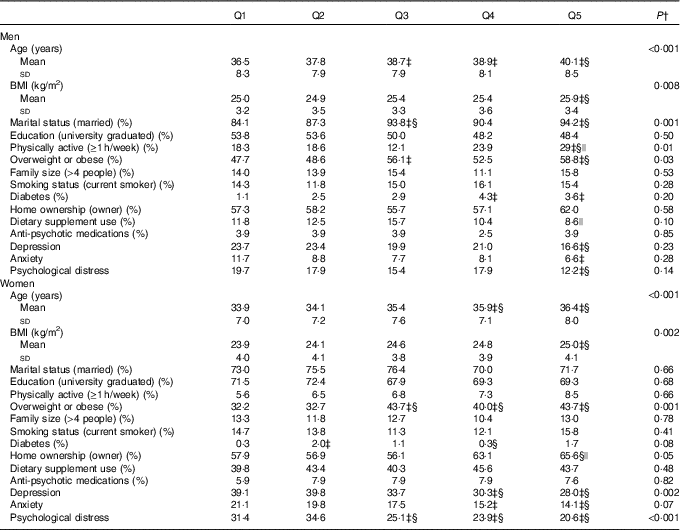
* Men: Q1: <277 mg/d, Q2: 277–<301 mg/d, Q3: 301–<326 mg/d, Q4: 326–<358 mg/d, Q5: ≥358 mg/d; women: Q1: <281 mg/d, Q2: 281–<303 mg/d, Q3: 303–<325 mg/d, Q4: 325–<356 mg/d, Q5: ≥356 mg/d.
† Obtained from ANOVA with Bonferroni correction or χ 2 test, where appropriate.
‡ Significant compared with Q1.
§ Significant compared with Q2.
|| Significant compared with Q3.
Selected food groups and nutrient intakes of men and women across quintiles of energy-adjusted Mg intake are shown in Table 2. Men and women in the top quintile of Mg intake had greater intake of fruit, vegetables, legumes and nuts, whole grains, refined grains, dairy products, tea and coffee, protein, fibre, vitamins B2, B3, B5, B6 and B12 compared with those in the bottom quintile. Among men, dietary intake of red meat, energy and vitamin B1 was different across quintiles of Mg intake. In addition, women were different in terms of dietary intake of red meat, energy, fat and carbohydrate across quintiles of dietary Mg intake.
Table 2 Selected food groups and nutrient intakes of men and women across quintiles (Q) of energy-adjusted magnesium intakeFootnote * (Mean values with their standard errors)

* All food groups and nutrients are energy adjusted. Mg quintiles for men: Q1: <277 mg/d, Q2: 277–<301 mg/d, Q3: 301–<326 mg/d, Q4: 326–<358 mg/d, Q5: ≥358 mg/d; Mg quintiles for women: Q1: <281 mg/d, Q2: 281–<303 mg/d, Q3: 303–<325 mg/d, Q4: 325–<356 mg/d, Q5: ≥356 mg/d.
† Obtained from ANOVA with Bonferroni correction.
‡ Significant compared with Q1.
§ Significant compared with Q2.
|| Significant compared with Q3.
¶ Significant compared with Q4
Multivariable-adjusted OR for depression, anxiety and psychological distress across quintiles of dietary Mg intake in men are shown in Table 3. Compared with those in the bottom quintile, men in the top quintile of dietary Mg intake were less likely to be depressed (OR 0·64; 95 % CI 0·41, 0·97), anxious (OR 0·53; 95 % CI 0·29, 0·98) and psychologically distressed (OR 0·56; 95 % CI 0·35, 0·89). These associations were significant even after controlling for demographic characteristics; however, taking dietary intakes of energy and relevant nutrients into account made these associations non-significant (depression; OR 0·77; 95 % CI 0·47, 1·25, anxiety; OR 1·23; 95 % CI 0·51, 3·00, psychological distress; OR 1·07; 95 % CI 0·59, 1·94)). When we performed BMI-stratified analysis in men, a significant inverse association was found between dietary Mg intake and depression among normal-weight men (OR 0·41; 95 % CI 0·21, 0·80), such that after adjusting for potential confounders men in the fifth quintile of Mg intake had a 55 % lower risk of depression compared with those in the first quintile (OR 0·45; 95 % CI 0·20, 0·99). Among overweight men, those in the top quintile of dietary Mg intake had lower odds of psychological distress compared with those in the lowest quintile (OR 0·44; 95 % CI 0·22, 0·88); however, after controlling for dietary confounders, this association became non-significant (OR 1·15; 95 % CI 0·48, 2·73). No other significant association was found between Mg intake and psychiatric disorders either in normal-weight or overweight men. When men were categorised on the basis of EAR of Mg, no significant association was found between deficient Mg intake and mental disorders either in the whole population or in BMI-stratified analysis.
Table 3 Psychiatric disorders based on quintiles (Q) of energy-adjusted magnesium intake and estimate average requirement (EAR) among men (Odds ratios and 95 % confidence intervals)

* All men: Q1: <277 mg/d, Q2: 277–<301 mg/d, Q3: 301–<326 mg/d, Q4: 326–<358 mg/d, Q5: ≥358 mg/d; BMI<25 kg/m2: Q1: <274 mg/d, Q2: 274–<297 mg/d, Q3: 297–<319 mg/d, Q4: 319–<349 mg/d, Q5: ≥349 mg/d; BMI≥25 kg/m2: Q1: <279 mg/d, Q2: 279–<304 mg/d, Q3: 304–<328 mg/d, Q4: 328–<363 mg/d, Q5: ≥363 mg/d.
† EAR; men: 350 mg/d.
‡ Depression: model 1: adjusted for education, smoking status and anti-psychotic medications, and model 2: additionally adjusted for dietary intake of energy, fat, carbohydrate and vitamin B2.
§ Anxiety: model 1: adjusted for smoking status, anti-psychotic medications and supplements use, and model 2: additionally adjusted for dietary intake of energy, fibre, vitamin B1 and vitamin B2.
|| Psychological distress: model 1: adjusted for anti-psychotic medications, and model 2: additionally adjusted for dietary intake of energy, fibre and vitamin B6.
Multivariable-adjusted OR for psychiatric disorders across quintiles of dietary Mg intake in women are shown in Table 4. Compared with those in the bottom quintile, women in the top quintile of dietary Mg intake were less likely to be depressed (OR 0·60; 95 % CI 0·43, 0·82), anxious (OR 0·61; 95 % CI 0·41, 0·90) and distressed (OR 0·56; 95 % CI 0·40, 0·79). Such significant associations were also seen after controlling for potential confounders; however, when dietary intakes of energy and relevant nutrients were taken into account, these associations became non-significant (depression; OR 0·72; 95 % CI 0·49, 1·06 and psychological distress; OR 0·74; 95 % CI 0·49, 1·11), except for anxiety, such that women in the fifth quintile of Mg intake were 39 % less likely to be anxious compared with those in the first quintile (OR 0·61; 95 % CI 0·40, 0·93).
Table 4 Psychiatric disorders based on quintiles (Q) of energy-adjusted magnesium intake and estimated average requirement (EAR) among women (Odds ratios and 95 % confidence intervals)
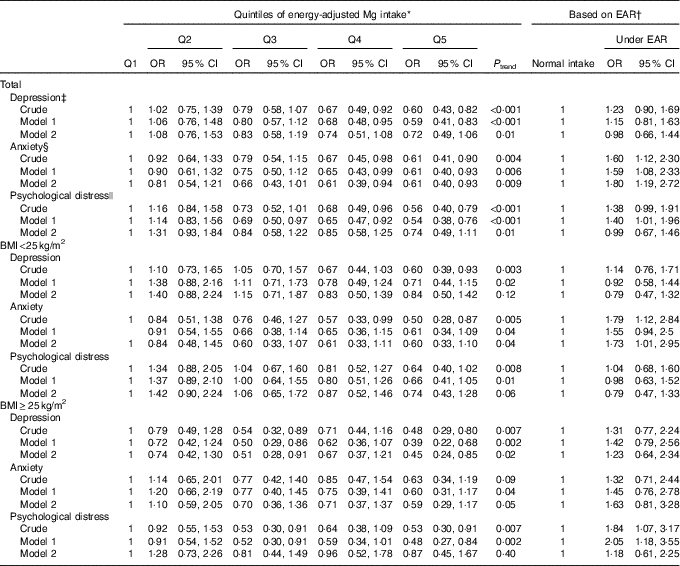
* All women: Q1: <281 mg/d, Q2: 281–<303 mg/d, Q3: 303 to <325 mg/d, Q4: 325–<356 mg/d, Q5: ≥356 mg/d; BMI<25 kg/m2: Q1: <278 mg/d, Q2: 278–<299 mg/d, Q3: 299–<321 mg/d, Q4: 321–<353 mg/d, Q5: ≥353 mg/d; BMI≥25 kg/m2: Q1: <286 mg/d, Q2: 286–<310 mg/d, Q3: 310–<330 mg/d, Q4: 330–<330 mg/d, Q5: ≥359 mg/d.
† EAR; women: 265 mg/d.
‡ Depression: model 1: adjusted for adjusted for age, marital status, education, family size, smoking status and anti-psychotic medications, and model 2: additionally adjusted for dietary intake of energy and vitamin B5.
§ Anxiety: model 1: adjusted for marital status, education, smoking status, home ownership and anti-psychotic medications, and model 2: additionally adjusted for dietary intake of energy, fibre, vitamin B1 and vitamin B2.
|| Psychological distress: model 1: adjusted for education and anti-psychotic medications, and model 2: additionally adjusted for energy intake and dietary intake of vitamin B3 and Fe.
BMI-stratified analysis in women revealed no significant association between Mg intake and psychiatric disorders among normal-weight women. However, in overweight women, a significant inverse association was seen between dietary Mg intake and depression (OR 0·48; 95 % CI 0·29, 0·80), such that in fully adjusted model women in the highest quintile of Mg intake had 55 % lower odds of depression compared with those in the lowest quintile (OR 0·45; 95 % CI 0·24, 0·85). No other significant association was found between dietary intake of Mg and anxiety and psychological disorders in overweight women.
When we performed the analysis based on the cut-off points of EAR of Mg among women, we found a significant positive association between deficient Mg intake and anxiety either in crude (OR 1·60; 95 % CI 1·12, 2·30) or adjusted models (OR 1·80; 95 % CI 1·19, 2·72). Such a finding was also seen among normal-weight women (OR 1·73; 95 % CI 1·01, 2·95). In addition, a significant positive association was found between deficient Mg intake and psychological distress among overweight women (OR 2·05; 95 % CI 1·18, 3·55); however, this association was non-significant when dietary variables were adjusted for.
Discussion
In this cross-sectional study, we found that higher dietary Mg intake was associated with lower odds of anxiety among women. Moreover, deficient Mg intake was positively associated with anxiety among all women and also normal-weight women. In addition, a significant inverse association was found between dietary Mg intake and depression among normal-weight men and overweight women. To our knowledge, this is the first observational study assessing the association between dietary Mg intake and psychiatric disorders in the Middle East.
Depression and anxiety are among highly prevalent psychiatric disorders in the world( Reference Murphy, Horton and Laird 1 , Reference Mojtabai 2 ), which are associated with CVD, diabetes and cancers( Reference Markowitz, Friedman and Arent 37 – Reference Penninx, Guralnik and Pahor 40 ). Although not life-threatening, they adversely affect the quality of life and life expectancy( Reference Olesen, Gustavsson and Svensson 3 , Reference Sobocki, Jönsson and Angst 4 ). In the present study, we observed that higher Mg intake was related to a lower risk of depression among normal-weight men and overweight women. These findings were in line with a Spanish cross-sectional study, in which an inverse association between Mg intake and depressive symptoms was reported among schoolchildren( Reference Rubio-López and Morales-Suárez-Varela 41 ). Furthermore, data from a 20-year prospective study suggested similar associations in middle-aged Finnish men( Reference Yary, Lehto and Tolmunen 18 ). In a meta-analysis, dietary intake of Mg was inversely associated with risk of depression( Reference Li, Lv, Wang and Zhang 42 ). Nevertheless, findings from a Spanish prospective study revealed no significant association between dietary Mg intake and risk of depression( Reference Martínez-González and Sánchez-Villegas 19 ). Such findings were also reported in Spanish university graduates( Reference Derom, Martínez-González and del Carmen Sayón-Orea 43 ). In all mentioned studies, findings were not reported stratified by sex or BMI. Discrepant findings might be explained by various reasons. For instance, some confounding variables related to psychological characteristics, family factors and lifestyle have not been controlled for in other studies( Reference Derom, Martínez-González and del Carmen Sayón-Orea 43 , Reference Hunang, Lu and Cheng 44 ).
In the present study, a significant inverse association was seen between dietary Mg intake and anxiety among women. Moreover, deficient Mg intake was positively associated with anxiety in all women and normal-weight women. In line with our findings, in a cross-sectional study, Sadeghi et al.(45) reported that whole-grain consumption, known as a source of Mg, was inversely associated with anxiety in women. In another similar study, adherence to dietary pattern rich in Mg was associated with decreased odds of anxiety in females( Reference Valipour, Esmaillzadeh and Azadbakht 46 ). Boyle et al. (47) reported that Mg supplementation among anxious individuals had beneficial effects on symptoms. In addition, findings from a systematic review introduced Mg supplementation as an effective modality for treating anxiety and anxiety-related conditions( Reference Lakhan and Vieira 48 ). However, some studies did not reach a significant correlation( Reference Messina 17 , Reference Barra, Camardese and Tonioni 20 ). For instance, a clinical trial concluded that Mg and zinc supplementation did not reduce postpartum anxiety and depressive symptoms among women( Reference Messina 17 ). Furthermore, an Italian study failed to find any association between total plasma Mg levels and anxiety among patients with major depressive disorders( Reference Barra, Camardese and Tonioni 20 ). Different findings on the association between Mg intake and anxiety might be explained by adjusting for confounders, particularly dietary intakes. As seen in the current study, some associations between Mg intake and psychiatric disorders became non-significant after considering other dietary intakes as covariates. However, in some previous studies, potential confounders such as dietary variables were not considered on the association between Mg intake and psychiatric disorders( Reference Messina 17 , Reference Barra, Camardese and Tonioni 20 ).
The sex disparity on the association between dietary Mg intake and psychiatric disorders could be explained by the differential influence of gonadal steroids on mood( Reference Laurin, Lavoie and Bacon 49 , Reference Nolen-Hoeksema 50 ). In addition, the accuracy of dietary assessment might be different between men and women. Previous studies have indicated that actual food choices( Reference Beer-Borst, Hercberg and Morabia 51 ), self-reported preferences for foods( Reference O’Doherty Jensen and Holm 52 ) and accuracy of reported dietary intakes( Reference Marks, Hughes and van der Pols 53 ) are different between men and women.
In the current study, we observed different findings among normal-weight and overweight participants. Different Mg requirement in overweight participants compared with normal-weight ones might be a reason for this. In addition, hormonal imbalance in obese individuals compared with normal-weight ones might also explain the different associations( Reference Mcelroy, Kotwal and Malhotra 54 ). Furthermore, the accuracy of dietary assessment might be different between normal-weight and overweight individuals. As shown in previous studies, under-reporting of dietary intakes is common among individuals with overweight and obesity( Reference Lissner, Troiano and Midthune 55 ). Although we controlled for total energy intake, it must be paid attention that such controlling in the analyses might not entirely exclude the effect of energy intake on the associations.
In this study, we found no significant association between dietary Mg intake and psychological distress in the fully adjusted model. To our knowledge, earlier studies that assessed the contribution of diet to psychological distress have mostly focused on dietary patterns rather than on individual foods and nutrients. For instance, in an Australian cross-sectional study, adherence to a Mediterranean-style diet (rich in Mg) was not associated with psychological distress( Reference Crichton, Bryan and Hodgson 56 ). Conversely, Hodge et al. (57) reported that adherence to a dietary pattern rich in legumes and nuts was inversely associated with psychological distress. Furthermore, in another cross-sectional study, an inverse association was reported between a dietary pattern rich in Mg and psychological distress among Indian individuals( Reference Bhattacharyya, Marston and Walters 58 ). The beneficial effects of these dietary patterns on psychological distress might be mediated through their Mg content. Conflicting findings about Mg intake in relation to psychological distress might be because of the lack of taking potential confounders into account along with the use of unacceptable tools for assessment of diet or psychiatric disorders in previous studies( Reference Crichton, Bryan and Hodgson 56 , Reference Bhattacharyya, Marston and Walters 58 ). Therefore, further studies are needed to shed light facts in this regard.
The inverse association between Mg intake and depression plus anxiety might be explained by several mechanisms. Mg as an essential trace element might have a role in different pathways( Reference Mohamed Salih, Nallasamy and Muniyandi 15 ). It acts as a cofactor for the synthesis and release of numerous enzymes, neurotransmitters and hormones required for normal neuronal functioning( Reference Mohamed Salih, Nallasamy and Muniyandi 15 , Reference Barra, Camardese and Tonioni 20 ). Mg has an important role in stability of neurons, such as membrane stability( Reference Nechifor 59 , Reference Barra, Camardese and Tonioni 20 ). Neuron membrane is involved in releasing neurotransmitters affecting intracellular messaging. Therefore, Mg contributes indirectly to intracellular messaging( Reference de Baaij, Hoenderop and Bindels 60 ). In addition, owing to a significant association between inflammatory markers and psychiatric disorders, the inhibitory effects of Mg on secretion of inflammatory markers might be another reason explaining the inverse relationship between Mg intake and mental disorders( Reference Mohamed Salih, Nallasamy and Muniyandi 15 ). The inverse association between Mg intake and inflammation has been shown in both animal and human studies( Reference Mohamed Salih, Nallasamy and Muniyandi 15 ).
This study has several strengths. As far as we know, this is the first study examining the association between dietary Mg intake and psychiatric disorders in the Middle East. Furthermore, the large sample size of the study, including either sex, should also be considered. However, during the interpretation of our findings, some limitations should be also noticed. The main limitation is the cross-sectional design of our study, which prohibit us inferring causality. Therefore, further prospective studies are needed to confirm our findings. In addition, measurement error is another potential limitation, as is in all dietary assessment methods. Because of the use of FFQ to assess usual dietary intakes, misclassification of study individuals is another concern. However, we used a validated FFQ for assessment of dietary intakes. Furthermore, we cannot exclude residual confounders despite adjusting for a wide range of potential confounders.
In conclusion, we found that dietary Mg intake was associated with lower odds of depression and anxiety among Iranian adults. No significant association was seen between dietary Mg intake and psychological distress among men and women. Our findings should be confirmed by future prospective studies.
Acknowledgements
The study was financially supported by Isfahan University of Medical Sciences in collaboration with Tehran University of Medical Sciences. Professor A. E. was supported by a grant from Iran National Science Foundation as well.
J. A.-S., O. S. and A. E. contributed to the conception and design of the study, data collection and statistical analysis and drafting of the manuscript; A. H. K., H. A. and P. A. contributed in data collection and manuscript drafting. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.







