Cardiovascular health is influenced by various factors including ageing, sex, genetic factors and lifestyle factors including dietary behaviours. Besides its critical role in skeletal metabolism, the potential effects of Ca on non-skeletal health outcomes, such as CVD, have recently drawn attention( Reference Peterlik and Cross 1 ). Some epidemiological studies have reported inverse associations of Ca intake with cardiovascular risk and mortality( Reference Umesawa, Iso and Ishihara 2 , Reference Kaluza, Orsini and Levitan 3 ). Similarly, vitamin D deficiency has been suggested to be associated with various health disorders including CVD( Reference Anderson, May and Horne 4 ). In contrast, excessive Ca intake via supplementation has recently been suggested to adversely affect vascular events( Reference Bolland, Avenell and Baron 5 ).
Atherosclerotic changes in arteries mainly contribute to the pathogenesis of CVD, and increased arterial stiffness is associated with atherosclerosis. Arterial stiffness can be assessed by measuring pulse wave velocity (PWV); carotid–femoral PWV is an established index for assessing aortic stiffness( Reference Lehmann 6 ). However, measuring carotid–femoral PWV is rather complicated and time consuming. Alternatively, brachial–ankle pulse wave velocity (ba-PWV) measurement is convenient, reproducible and relatively quick. Although ba-PWV reflects the arterial stiffness of both large- and mid-sized arteries( Reference Tomiyama, Yamashina and Arai 7 , Reference Yamashina, Tomiyama and Takeda 8 ), it is well correlated with carotid–femoral PWV as well as aortic PWV assessed using a direct catheter method( Reference Yamashina, Tomiyama and Takeda 8 ). Therefore, ba-PWV measurement has become widespread for screening arterial atherosclerotic changes in Asian countries. Despite epidemiological findings of inverse associations of Ca intake with the risk factors for CVD, little is known about the associations of dietary Ca and vitamin D intakes with arterial stiffness. In the present study, the associations of dietary Ca and vitamin D intakes with arterial stiffness on the basis of ba-PWV were evaluated in Japanese men, whose mean daily Ca intake is considerably lower than that of Westerners. Because dietary Ca and vitamin D may act synergistically, their combined associations with measures of arterial stiffness were also evaluated.
Methods
Study subjects
A total of 574 men aged 35–69 years who participated in the baseline survey of a prospective cohort study in Tokushima Prefecture, Japan, from November 2009 to January 2012 and underwent ba-PWV measurements were included in the present cross-sectional study. The subjects were mostly office workers and not shift workers. The present study was carried out as part of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study, a prospective cohort study( Reference Hamajima and J-MICC Study Group 9 ). Briefly, the J-MICC Study aims to examine the associations of lifestyle and genetic factors, as well as their interactions, with lifestyle-related diseases. All participants of the J-MICC Study provided written informed consent before participation. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and the ethics committees of Nagoya University School of Medicine (with which the former principal investigator, Nobuyuki Hamajima, is affiliated), Aichi Cancer Center (with which the current principal investigator, Hideo Tanaka, is affiliated) and the University of Tokushima Graduate School approved the study protocol.
Questionnaire
Information regarding individual lifestyle characteristics over the past year was obtained through a structured self-administered questionnaire. All the responses were checked by trained staff at the time of the survey. Leisure-time exercise was estimated on the basis of the International Physical Activity Questionnaire( Reference Craig, Marshall and Sjöström 10 ). Exercise was divided into three levels: light exercise (e.g. walking and hiking); moderate exercise (e.g. light jogging and swimming); vigorous exercise (e.g. marathon running and competitive sports). The degrees of leisure-time exercise for the three levels were expressed as metabolic equivalent (MET)-h/week (MET level × hours of activity × events per week) and summed. In this estimation, light, moderate and vigorous exercise levels were assigned to be 3·4, 7·0 and 10·0 MET, respectively.
Dietary intake was evaluated using a validated short FFQ in the baseline survey of the J-MICC Study( Reference Tokudome, Goto and Imaeda 11 – Reference Imaeda, Goto and Tokudome 14 ). This FFQ included questions about the dietary intake of forty-seven varieties of foods and beverages (i.e. green tea, coffee and alcohol) over the previous year. Information regarding the intake frequency and amounts of the three staple foods (i.e. rice, bread and noodles) consumed at breakfast, lunch and dinner was obtained. The intake volume and frequency of alcoholic beverages including sake, beer, Shōchū (a Japanese distilled beverage), Chūhai (a sweetened beverage mixed with Shōchū), whisky and wine were determined. Only intake frequency was determined for the other forty-three foods and beverages. Daily total energy (kJ/d), Ca (mg/d) and vitamin D (μg/d) intakes were calculated using a program developed by the Department of Public Health, Nagoya City University School of Medicine( Reference Tokudome, Goto and Imaeda 11 , Reference Tokudome, Goto and Imaeda 12 ).
Pulse wave velocity measurement
ba-PWV was measured using a waveform analyser (Model BP-203RPE III; Colin Company Limited) as described previously( Reference Tomiyama, Yamashina and Arai 7 ). Briefly, the subjects were examined while resting in the supine position in an air-conditioned room. Extremity blood pressure was measured using an oscillometric method, and the ankle–brachial pressure index (ABI) was automatically calculated. ba-PWV was calculated using time–phase analysis between the right brachial artery pressure and volume waveforms at both ankles. PWV is a measure of the speed of the pulse wave travelling over a specific distance in the artery. This speed of the pulse wave increases as the stiffness of artery wall increases; therefore, a higher PWV value indicates increased arterial stiffness. To reduce inter-observer variation, all ba-PWV measurements were performed by a single researcher throughout the study. Individual ba-PWV and ABI data are expressed as the means of the bilateral ba-PWV and ABI, respectively.
Anthropometric and biochemical measurements
Body height was obtained from the questionnaire, while body weight was measured to the nearest 0·1 kg during the baseline survey. BMI was calculated as weight (kg) divided by height (m). Venous blood was drawn from each subject, and serum was separated within 3 h. Serum lipid concentrations were measured at an external laboratory (BML, Inc.). Total cholesterol and TAG concentrations were determined using an enzyme assay, and HDL-cholesterol concentrations were determined using a direct method.
Statistical analyses
Among the 574 men initially included in the present study, nineteen with a history of IHD or stroke as well as five who had a low right or left ABI ( ≤ 0·9), indicating peripheral arterial occlusive disease, were excluded. Apart from them, five subjects whose daily total energy intake was extremely high (>16 800 kJ/d; this is almost equal to >4000 kcal/d) or low ( < 4200 kJ/d; this is equal to < 1000 kcal/d) or who were taking Ca or vitamin D supplements were excluded. After excluding an additional ten subjects for whom serum lipid concentration data were missing, a total of 535 men were included in the analyses.
Dietary Ca and vitamin D intakes were adjusted for total energy intake after log transformation using the residual method and divided into quartiles in the analyses such that the number of subjects in each category was nearly equal; the highest quartile, which was thought to represent relatively sufficient intake, was used as the reference.
Continuous variables are expressed as means and standard deviations or medians with their 25th and 75th percentiles. Categorical variables are expressed as the proportion (%). ANOVA, the Kruskal–Wallis test and Fisher's exact test were used to compare the baseline characteristics among the quartile categories of dietary Ca or vitamin D intake where appropriate. General linear models were used to evaluate the associations of dietary Ca and vitamin D intakes with ba-PWV after adjusting for the following probable covariates: age (continuous) and systolic blood pressure ( < 120, 120– < 130, 130– < 140, 140– < 160, or ≥ 160 mmHg with no medical treatment or antihypertensive agent use), which are recognised as being very closely associated with arterial stiffness, in model 1 and age, systolic blood pressure, BMI (kg/m2, quartiles), current smoking status (no/yes), current drinking status (no/yes), leisure-time exercise (MET-h/week, quartiles), hypercholesterolaemia ( ≥ 5·7 mmol/l or ≥ 220 mg/dl or receiving medical treatment, no/yes), low HDL-cholesterol concentrations ( < 1·0 mmol/l or < 40 mg/dl, no/yes), elevated TAG concentrations ( ≥ 1·7 mmol/l or ≥ 150 mg/dl, no/yes), diabetes (receiving medical treatment, no/yes), and daily energy intake (continuous, log-transformed) in model 2. Tests for trends were performed by assigning the ordinal categorical variables of 1, 2, 3 and 4 for each quartile of dietary Ca and vitamin D intakes. The association between dietary Ca intake and ba-PWV was further evaluated using similar general linear models stratified by dietary vitamin D intake (median or below/above median) to assess whether dietary vitamin D intake had a modifying effect on the association between dietary Ca intake and arterial stiffness. Interaction terms of two exposure variables (Ca intake, continuous, log-transformed; vitamin D intake, dichotomous) were created and included in the model to assess statistical interactions.
All calculations and statistical tests were performed using SAS (version 8.2; SAS Institute, Inc.). All statistical tests were based on two-sided probabilities, and the level of significance was set at P< 0·05.
Results
The mean age and BMI of the study subjects were 50·0 (sd 8·6) years and 24·4 (sd 3·3) kg/m2, respectively. The mean ba-PWV value was 1436 (sd 237) cm/s.
Baseline characteristics of the subjects according to dietary calcium and vitamin D intakes
The baseline characteristics of the subjects are summarised in Tables 1 and 2 according to their dietary Ca and vitamin D intakes, respectively. Subjects with lower dietary Ca intake were younger, had a higher current smoking status and had a lower level of leisure-time exercise. Subjects with the lowest dietary Ca intake had highest systolic blood pressure and highest ba-PWV. Subjects with lower dietary vitamin D intake were younger and had higher values of low HDL-cholesterol concentrations. ba-PWV was not statistically different among the vitamin D intake quartiles.
Table 1 Baseline characteristics of the subjects according to dietary calcium intake* (Mean values and standard deviations; median values with their 25th and 75th percentiles; number of subjects and percentages)

Q, quartiles; MET, metabolic equivalents; BP, blood pressure; ABI, ankle–brachial pressure index; ba-PWV, brachial–ankle pulse wave velocity.
* Ca intake was adjusted for total energy intake after log transformation using the residual method.
Table 2 Baseline characteristics of the subjects according to dietary vitamin D intake* (Mean values and standard deviations; median values with their 25th and 75th percentiles; number of subjects and percentages)

Q, quartiles; MET, metabolic equivalents, BP, blood pressure; ABI, ankle–brachial pressure index; ba-PWV, brachial–ankle pulse wave velocity.
* Vitamin D intake was adjusted for total energy intake after log-transformation using the residual method.
Associations of dietary calcium and vitamin D intakes with arterial stiffness
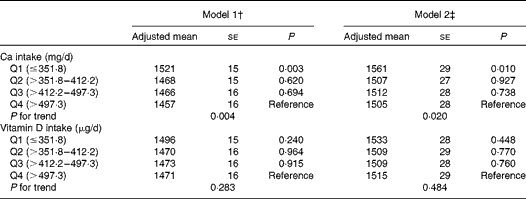
The associations of dietary Ca and vitamin D intakes with ba-PWV in the general linear models are summarised in Table 3. Lower dietary Ca intake was significantly associated with increased ba-PWV after adjusting for the multivariable covariates (P for trend = 0·020 in model 2). No adjusted associations were found between dietary vitamin D intake and ba-PWV.
Table 3 Associations of dietary calcium and vitamin D intakes with brachial–ankle pulse wave velocity (cm/s) (Adjusted mean values with their standard errors)*
Q, quartiles.
* Ca and vitamin D intakes were adjusted for total energy intake after log transformation using the residual method.
† Model 1: adjusted for age and systolic blood pressure.
‡ Model 2: adjusted for age, systolic blood pressure, BMI, current smoking status, current drinking status, leisure-time exercise, hypercholesterolaemia, low HDL-cholesterol concentrations, elevated TAG concentrations, diabetes and daily energy intake.
Association between dietary calcium intake and arterial stiffness stratified according to dietary vitamin D intake
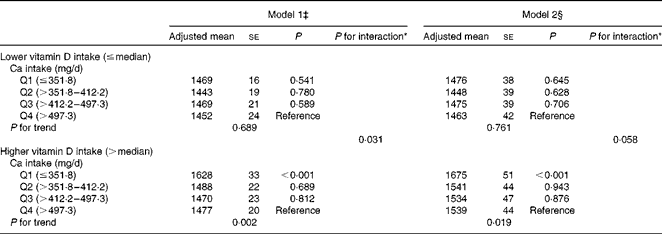
Stratified analyses revealed a strikingly significant inverse association between dietary Ca intake and ba-PWV in subjects whose dietary vitamin D intake was greater than the median (P for trend = 0·019 in model 2; Table 4). In contrast, no such association was observed in subjects whose dietary vitamin D intake was less than or equal to the median (P for trend = 0·761 in model 2; Table 4). The interactions of dietary Ca intake (continuous, log-transformed) and vitamin D intake (dichotomous) with ba-PWV were marginally significant even after adjusting for the multivariable covariates (P for interaction = 0·058 in model 2).
Table 4 Association between dietary calcium intake and brachial–ankle pulse wave velocity (cm/s) stratified by dietary vitamin D intake† (Adjusted mean values with their standard errors)
Q, quartiles.
* P value for interaction of Ca intake (continuous, log-transformed) and vitamin D intake (dichotomous, ≤ median and >median).
† Ca and vitamin D intakes were adjusted for total energy intake after log transformation using the residual method.
‡ Model 1: adjusted for age and systolic blood pressure.
§ Model 2: adjusted for age, systolic blood pressure, BMI, current smoking status, current drinking status, leisure-time exercise, hypercholesterolaemia, low HDL-cholesterol concentrations, elevated TAG concentrations, diabetes and daily energy intake.
Discussion
In the present study, the inverse association between dietary Ca intake and arterial stiffness was investigated after adjusting for the classical atherosclerotic risk factors in Japanese men.
Ca is the most abundant mineral in the human body. Besides its core role in skeletal metabolism, Ca is required for numerous critical biological functions including muscle contraction, vascular tone and various enzyme-mediated processes( Reference Vaskonen 15 ). Some prospective studies have reported inverse associations between Ca intake and the risk of stroke incidence( Reference Umesawa, Iso and Ishihara 2 ) and cardiovascular mortality( Reference Kaluza, Orsini and Levitan 3 ). In the present study, insufficient dietary Ca intake was found to be associated with increased ba-PWV in Japanese men. Because humans cannot produce Ca, sufficient Ca intake is required for Ca homeostasis. However, the mean daily Ca intake of the Japanese population remains below the recommendation levels( 16 ) (650 and 700 mg/d for men aged 30–49 and ≥ 50 years, respectively; 650 and 600 mg/d for women aged 30–69 years and ≥ 70 years, respectively)( 17 ) and is far lower than that of Westerners( Reference Ervin, Wang and Wright 18 ).
Studies on the associations between dietary Ca intake and arterial stiffness are scarce. Kesse-Guyot et al. ( Reference Kesse-Guyot, Vergnaud and Fezeu 19 ) have reported that a nutritionally poor dietary pattern including low Ca intake is related to increased stiffening of large arteries, which is consistent with the present findings. There are several possible explanations for the inverse association between Ca intake and arterial stiffness. The leading explanation is the hypotensive effect of Ca. Dietary Ca intake or supplementation was found to be inversely associated with systolic blood pressure in a cross-sectional study in Japan( Reference Iso, Terao and Kitamura 20 ) and in some meta-analyses of randomised controlled trials( Reference Bucher, Cook and Guyatt 21 , Reference Allender, Cutler and Follmann 22 ). In the present study, subjects with the lowest dietary Ca intake had highest systolic blood pressure; this finding is in agreement with those of other studies( Reference Iso, Terao and Kitamura 20 – Reference Allender, Cutler and Follmann 22 ). Experimental studies suggest that Ca is involved in the regulation of vascular smooth muscle cell contractility( Reference Zemel 23 , Reference Ledoux, Werner and Brayden 24 ) and the down-regulation of the renin–angiotensin system( Reference Resnick, Laragh and Sealey 25 , Reference Maillard, Tedjani and Perregaux 26 ); this system contributes to proper blood pressure regulation. Ca has also been demonstrated to be involved in the reduction of platelet aggregation( Reference Renaud, Morazain and Godsey 27 ) and regulation of lipoprotein metabolism ( Reference Vaskonen, Mervaala and Sumuvuori 28 , Reference van Meijl, Vrolix and Mensink 29 ). Dietary Ca promotes the formation of insoluble complexes with fatty acids, reducing fatty acid absorption( Reference van Meijl, Vrolix and Mensink 29 ). Moreover, Ca increases lipid excretion by binding to bile acids; dietary Ca subsequently lowers LDL-cholesterol synthesis( Reference Lorenzen, Nielsen and Holst 30 ). In the present study, serum LDL-cholesterol concentrations were found to be not associated with dietary Ca intake; this might be due to the low Ca intake in the study subjects. Further studies are required to elucidate the effect of Ca intake on circulating lipid profiles.
Excessive Ca intake via supplementation has recently been suggested to adversely affect vascular events( Reference Bolland, Avenell and Baron 5 ). Taking Ca or vitamin D supplements is not popular among Japanese people, especially men. Furthermore, subjects taking Ca or vitamin D supplements were excluded from the analyses carried out in the present study. Therefore, it is thought that none of the subjects was consuming excessive Ca or vitamin D.
Vitamin D is a fat-soluble vitamin obtained from foods, beverages and supplements and is also generated by cutaneous synthesis resulting from sun exposure. Vitamin D insufficiency has recently drawn attention as a potential risk factor for CVD. Studies on the association between dietary vitamin D intake and arterial stiffness are scarce and their results are inconsistent( Reference Dong, Stallmann-Jorgensen and Pollock 31 , Reference Gepner, Ramamurthy and Krueger 32 ). In the present study, no multivariate-adjusted association was found between dietary vitamin D intake and arterial stiffness. One possible reason for the lack of such an association is that vitamin D intake from the diet is lower than that from supplements. Therefore, the body burden level of vitamin D did not reach the effective level. Another possible serious reason is that we did not have information about cutaneous vitamin D synthesis from sun exposure or data on circulating 25-hydroxyvitamin D concentration, which is an index of systemic vitamin D status, as we focused on the association between dietary factors (foods and beverages) and arterial stiffness. The systemic body burden level of vitamin D cannot be explained by dietary vitamin D intake alone. A recent study( Reference Yu, Kim and Kwon 33 ) in Korean adolescents has demonstrated that seasonal differences in the amount of sunlight exposure affect serum 25-hydroxyvitamin D concentrations and furthermore mean serum 25-hydroxyvitamin D concentrations over the year are associated with the intake frequencies of vitamin D food sources. We evaluated the mean intake frequencies of foods and beverages over the previous year and therefore our estimate of dietary vitamin D intake may be associated to some extent with mean serum 25-hydroxyvitamin D concentrations over the year. Otherwise, there may in fact be no association between vitamin D intake and arterial stiffness. Thus, further studies including circulating 25-hydroxyvitamin D measurement are required to determine the association between systemic vitamin D status and arterial stiffness.
Interestingly, in the present study, a strong inverse association was found between dietary Ca intake and arterial stiffness in subjects with higher dietary vitamin D intake. However, no association was found in subjects with lower dietary vitamin D intake. Among subjects with higher dietary vitamin D intake, an elevation of about 140 cm/s in multivariate-adjusted ba-PWV values was observed in the lowest quartile of Ca intake compared with the highest quartile. In a recent study carried out in Japan, it has been found that an elevation of 100 cm/s in ba-PWV indicates a significant increase in age- and sex-adjusted hazard ratio for future vascular events (hazard ratio 1·06, 95 % CI 1·01, 1·10; P =0·015 per 100 cm/s elevation in ba-PWV)( Reference Nagai, Shibata and Akishita 34 ). Therefore, the difference of 140 cm/s in ba-PWV is considered to have a considerable impact on future vascular events. The human body has difficulty in absorbing Ca. Activated vitamin D enhances the intestinal absorption of Ca( Reference Vaskonen 15 ). Therefore, Ca absorption is more efficient in individuals with higher dietary vitamin D intake, and protective effects of Ca (and vitamin D) against hypertension and atherosclerosis including direct action on the vascular system might be greater in individuals with higher dietary vitamin D intake. However, whether dietary vitamin D supplementation contributes to the reduction of ba-PWV cannot be discussed based on only our data. Dietary supplementation of both Ca and vitamin D is probably effective at reducing ba-PWV.
The present study has several limitations. First, because of the cross-sectional study design, the causal relationship between Ca intake and arterial stiffness should be interpreted with caution. Second, information about dietary and other lifestyle factors was self-reported; therefore, non-differential misclassification may have been inevitable. Similarly, Ca and vitamin D intakes were estimated on the basis of self-reported information from a short FFQ, and their estimations were relatively low. However, validity of this FFQ has been confirmed by comparison with a 3 d diet record, and this FFQ appears to be applicable to categorise individuals according to the intakes of energy and most nutrients( Reference Tokudome, Goto and Imaeda 12 ). Third, circulating 25-hydroxyvitamin D concentrations reflecting actual systemic vitamin D status in these individuals were not determined. Finally, as all subjects were Japanese men, the results of the present study may not be applicable to women or other ethnic populations.
In conclusion, the results of the present study demonstrate that dietary Ca intake is inversely associated with arterial stiffness independent of the classical atherosclerotic risk factors in Japanese men. This inverse association was striking in people with higher dietary vitamin D intake. Adequate dietary Ca and vitamin D intakes may act jointly to protect against the development of arterial stiffness, which eventually can lead to atherosclerosis and life-threatening cardiovascular events. Further larger and prospective or interventional studies including women and determinations of serum 25-hydroxyvitamin D concentrations that reflect systemic vitamin D status are required to confirm these findings.
Acknowledgements
The authors thank the following researchers for providing the FFQ and program to calculate nutrient intake: Shinkan Tokudome from the National Institute of Health and Nutrition (formerly Nagoya City University); Chiho Goto from Nagoya Bunri University; Nahomi Imaeda from Nagoya Women's University; Yuko Tokudome from the Nagoya University of Arts and Sciences; Masato Ikeda from the University of Occupational and Environmental Health; Shinzo Maki from the Aichi Prefectural Dietetic Association.
The present study was supported in part by Grants-in-Aid for Scientific Research on Priority Areas of Cancer (no. 17015018) and on Innovative Areas (no. 221S0001) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
The authors’ contributions are as follows: H. U. and K. A. designed the research; H. U., S. K.-K., M. Y., M. N., M. H. and K. A. collected the data; H. U. and K. A. analysed the data; H. U. wrote the article; K. A. reviewed the article. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.






