According to the WHO, IHD or CHD accounted for 7·4 million deaths in 2012, the leading cause of death worldwide( Reference Mathers, Fat and Inoue 1 , Reference Al-Mawali 2 ). There is growing evidence showing that long-chain n-3 PUFA, particularly EPA (C20 : 5n-3) and DHA (C22 : 6n-3), are beneficial for prevention of CVD( Reference Sala-Vila, Estruch and Ros 3 ). The American Heart Association recommends two servings of fatty fish per week or 500 mg EPA and DHA per day be taken for those without CHD, and 1 g/d of EPA/DHA for those with CHD( Reference Kris-Etherton, Harris and Appel 4 ). The main source of EPA and DHA is fish( Reference Mozaffarian and Wu 5 ). However, the increasing demand on fish consumption may cause problems of sustainability in provision of fish, and pollution – specifically, the accumulation of toxic materials – may neutralise the health benefits of fish, or even have adverse effects( Reference Mozaffarian, Appel and Van Horn 6 ). Also, fish is not applicable for most vegetarians. Therefore, alternative sources of EPA and DHA are needed to maintain the level of intake.
α-Linolenic acids (ALA), an n-3 fatty acid mainly derived from soyabean, flaxseed and walnuts has been proposed as a potential substitution for fish. ALA is converted to long-chain n-3 fatty acids in the body, including EPA and DHA, though the efficiency is low( Reference Rajaram 7 ). A number of studies have been conducted to examine associations between ALA intake and CHD risk( Reference Mozaffarian 8 ), though the results were inconsistent.
A previous meta-analysis of Pan et al.( Reference Pan, Chen and Chowdhury 9 ) indicates that a higher level of ALA intake is associated with reduced risk of fatal CHD, whereas not associated with risk of non-fatal CHD or composite CHD. It also indicates that each additional 1 g/d ALA intake is associated with a 10 % reduction in risk of fatal CHD. Recently, several new studies have examined the associations between ALA intake and risk of CHD( Reference Otto, Wu and Baylin 10 – Reference Bork, Jakobsen and Lundbye-Christensen 14 ), so an updated aggregate association may be informative. Moreover, it will be beneficial to explore the optimal dosing of ALA intake for prevention of CHD – specifically, what threshold of intake is needed for benefit and whether higher doses confer additional benefit. We systematically reviewed the literature and conducted a meta-analysis to investigate the potential dose–response relationship between ALA intake and CHD risk. We hypothesised that higher intake of ALA is linearly associated with lower risk of composite and fatal CHD.
Methods
Search strategy and eligibility criteria
We systematically searched the PubMed, Embase and Web of Science databases for epidemiological studies published in peer-reviewed journals over a 50-year period (from January 1966 to August 2017) that investigated the associations between dietary ALA intake and CHD. Search terms included ‘alpha-linolenic acid’, ‘cardiovascular disease’, ‘coronary heart disease’, ‘coronary artery disease’, ‘ischemic heart disease’ and ‘myocardial infarction’ (see the online Supplementary Appendix). We also searched for studies listed or cited in review papers, in case there were potential studies not captured by the database search strategy. The search was limited to articles written in English. We contacted authors of included articles if important information was missing.
We included original full-text studies that were: (1) cohort studies, (2) included adult participants free from CHD history with assessments of dietary intake of ALA, (3) assessed primary outcomes including fatal and/or non-fatal CHD and (4) had a comparison between the highest and the lowest level of dietary ALA intake. We excluded studies that: (1) included only assessed ALA level in plasma or serum as biomarkers, and (2) included composite outcomes that combined different cardiovascular events and CHD could not be distinguished. If there were several studies based on the same cohort, we retained the studies with the most directly relevant results for particular outcomes.
Study selection was conducted in three steps. First, the titles of studies identified in our literature search were independently reviewed by three reviewers (J. W., R. H., Y. X.). Second, the abstracts of studies that remained after the initial screening were reviewed by three reviewers and disagreements were reconciled. Third, data from studies that met inclusion criteria were extracted, including sample size, study design, age, sex, methods of assessing dietary intake of ALA, amount of ALA intake, outcome of studies and covariates included in the analysis.
Definitions
The information regarding dietary ALA intake was obtained from validated tools, such as FFQ, 24-h dietary recalls, dietary history and food records. The outcomes of our proposed meta-analysis were CHD events defined as one of the following terms: coronary heart disease, coronary artery disease, ischemic heart disease, or myocardial infarction. Composite CHD was defined as total incident cases of CHD events reported in the studies, including fatal or non-fatal CHD. The definition of fatal CHD included CHD death or CHD mortality mentioned in the articles. If a study reported only fatal CHD or non-fatal CHD, then it would be also counted into composite CHD. All incident cases were ascertained by physician diagnosis, medical records or death certificate.
Quality assessment
Quality assessment was conducted using the Newcastle–Ottawa Scale( Reference Wells, Shea and O’Connell 15 ). The Newcastle–Ottawa Scale evaluates quality of cohort studies in three domains:
-
(1) selection of exposed and non-exposed cohorts (representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration of absence of outcome at the beginning of studies);
-
(2) comparability of exposed and non-exposed cohorts (analysis appropriately adjusted for potential confounding factors, including the most important and additional factors, such as medications, history of other chronic diseases and lifestyle factors); and
-
(3) outcome ascertainment (adequacy of outcome assessment, length of follow-up and adequacy of follow-up).
A study would be awarded a maximum of 1 point for each variable within each assessment domain (selection, comparability, and outcome) for a possible maximum total score of 9. The quality assessment was conducted independently by two reviewers (R. H. and A. K.), and the results were reconciled until a consensus was reached.
Statistical analysis
We obtained fully adjusted risk ratios (RR) and 95 % CI from each study. We pooled data across studies using random-effects meta-analysis models, and weighted these by the inverse of the estimated variance, comparing the highest category of ALA intake with the lowest as indicated in each study. We considered the pooled RR to be statistically significant if 95 % CI did not contain 1. We generated forest plots to illustrate individual and pooled risk estimates, and funnel plots to examine potential publication bias. We calculated the I 2 statistic to quantify the proportion of between-study heterogeneity attributable to variability in the association rather than sampling variation. We also conducted subgroup analyses to examine for heterogeneity of associations by regions where studies were conducted (North America, Europe, Asia), mean age of study participants (≥60 years or not), and percentage of female subjects (≥50 % or not).
To examine impacts of the amount of ALA intake and risk of CHD, we conducted a dose–response meta-analysis for each outcome. For each study, we assigned the median or mean grams of ALA for each exposure level to the corresponding RR. To explore potential linear and non-linear relationships between ALA intake and CHD risk, we performed random-effects dose–response meta-analyses. We estimated the predicted relative risk by comparing specific levels of ALA intake with no ALA intake based on the linear model or spline transformation, as appropriate. The linear or non-linear relationship between dietary ALA intake and CHD risk was considered significant if the p value of the chi-square statistic was smaller than 0·05.
All analyses were conducted using Stata 14 (StataCorp).
Results
Study characteristics
The database search started with a total of 1853 citations (Fig. 1). We identified an additional twelve studies from the bibliographies of relevant reports and reviews. After eliminating duplicates, 1422 remained. Of these, we excluded 1368 studies due to irrelevant topics, non-prospective designs, or assessments of exposures and outcomes not meeting the inclusion criteria. We retrieved full-texts of the fifty-four remaining articles to be examined in more detail. Of these, fourteen prospective studies eventually met all inclusion criteria and were included in the systematic review and meta-analysis.
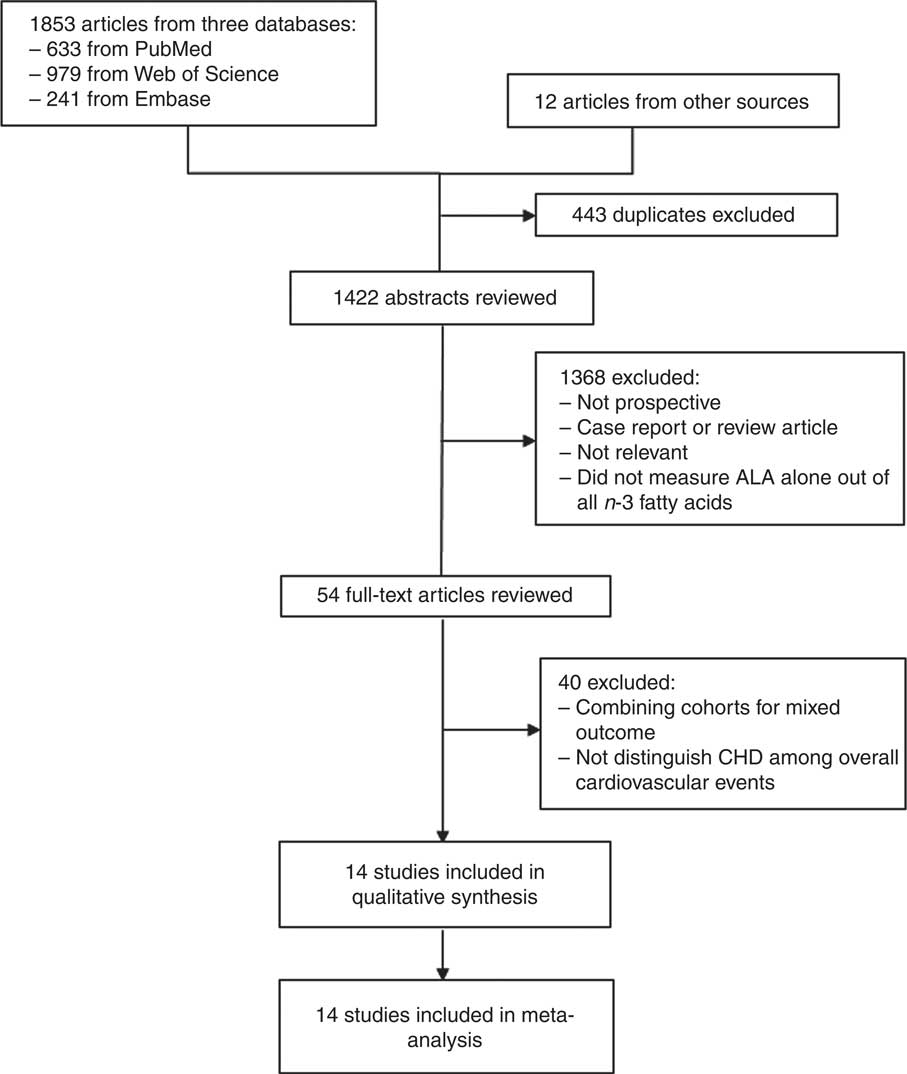
Fig. 1 Flow chart showing selection of study reports for the meta-analysis. ALA, α-linolenic acid.
The online Supplementary Table S1 showed characteristics of included studies. The pooled analysis included data from 345 202 individuals, with a mean length of follow-up between 4 and 22 years. The mean (median) ages of participants ranged from 41·5 to 73·0 years. Included studies were conducted in the USA, the Netherlands, Finland, Denmark, Spain and Singapore. Two studies stratified the analysis into male and female participants separately( Reference Bork, Jakobsen and Lundbye-Christensen 14 , Reference Vedtofte, Jakobsen and Lauritzen 16 ). Two studies were based on the same cohort (one is used for meta-analysis, and one for dose–response analysis). Nine studies examined associations between dietary ALA intake and risk of composite CHD( Reference Otto, Wu and Baylin 10 , Reference Fretts, Mozaffarian and Siscovick 11 , Reference Bork, Jakobsen and Lundbye-Christensen 14 , Reference Vedtofte, Jakobsen and Lauritzen 16 – Reference Rhee, Kim and Buring 22 ). Eight studies examined fatal CHD as outcomes( Reference Fretts, Mozaffarian and Siscovick 11 – Reference Sala-Vila, Guasch-Ferre and Hu 13 , Reference Ascherio, Rimm and Giovannucci 17 – Reference Oomen, Ocke and Feskens 19 , Reference Dolecek 23 , Reference Hu, Stampfer and Manson 24 ).
Pooled analyses
The meta-analysis of fourteen groups of analysis across twelve studies showed that higher intake of ALA was moderately associated with reduced risk of composite CHD (Fig. 2(a), pooled (RR=0·91; 95 % CI 0·85, 0·97). The analysis of nine studies indicated that a higher dietary ALA intake was associated with a lower risk of fatal CHD (Fig. 2(b), pooled RR=0·85; 95 % CI 0·75, 0·96). Heterogeneity was not detected in the associations with ALA intake with risk of composite CHD (I 2=7·9 %, P=0·36), or fatal CHD (I 2=15·8 %, P=0·30). There was no detected publication bias across studies, as the funnel plots showed good symmetry (online Supplementary Fig. S1 and S2).
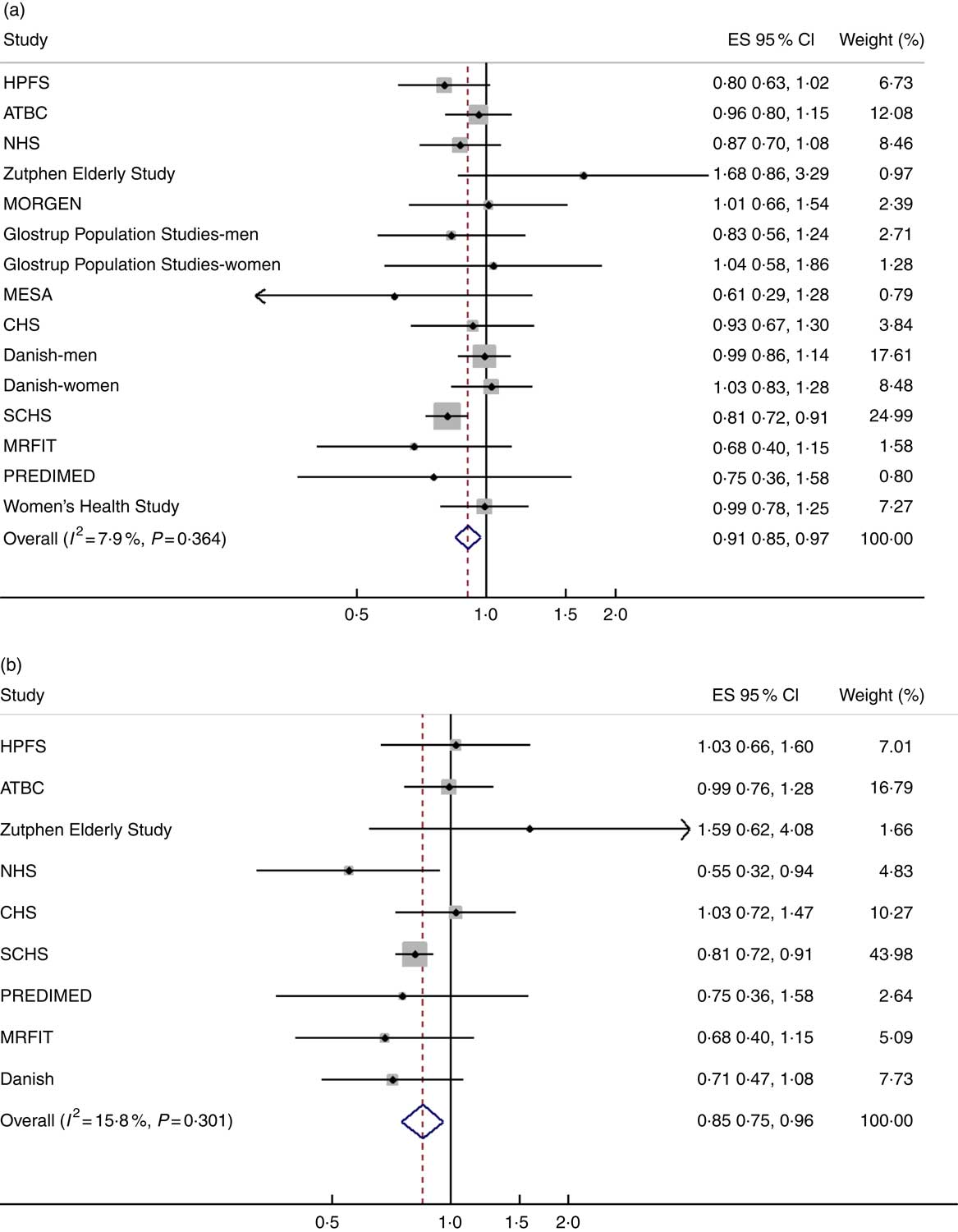
Fig. 2 Pooled association between dietary α-linolenic acid (ALA) intake and risk of (a) composite CHD and (b) fatal CHD. Weights are from random-effects analysis. ES, effect size; HPFS, Health Professionals Follow-Up Study; ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; NHS, Nurses' Health Study; MORGEN, Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands; MESA, Multi-Ethnic Study of Atherosclerosis; CHS, Cardiovascular Health Study; SCHS, Singapore Chinese Health Study; MRFIT, Multiple Risk Factor Intervention Trial; PREDIMED, Prevención con Dieta Mediterránea.
Results of subgroup analyses showed that a higher ALA intake was associated with risk reductions of composite CHD in studies conducted in North America and Asia, studies with a lower percentage of female participants (<50 %) and studies with lower mean age (<60 years). For fatal CHD, subgroup analyses showed risk reductions with ALA intake in studies conducted in Asia, studies with more female (≥50 %) and younger (<60 years) participants (Table 1).
Table 1 Subgroup meta-analysis of the pooled analysis between dietary α-linolenic acid (ALA) intake and risk of CHD (Pooled risk ratios (RR) and 95 % confidence intervals)
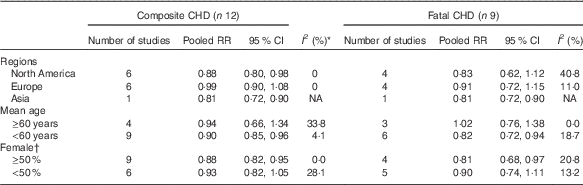
RR, risk ratio; NA, not applicable.
* P>0·05 for all I 2.
† For composite CHD, two studies were analysed by sex, so they were included in both categories.
The two-stage random-effects dose–response analysis did not show a linear relationship between dietary ALA intake and relative risk of composite CHD (χ 2=1·13, P=0·29), but rather a J-shaped curve between ALA intake and relative risk of composite CHD (χ 2=21·95, P<0·001). ALA intake<1·4 g/d showed reduced risk of composite CHD, compared with people without ALA intake. A quantity of 1 g of ALA intake per day was associated with the lowest risk of CHD (Fig. 3(a)). There was a linear relationship between ALA intake and risk of fatal CHD. ALA intake, 1 g/d, was associated with 12 % decrease in risk of fatal CHD (β=−0·12; 95 % CI −0·21, −0·04) (Fig. 3(b)).
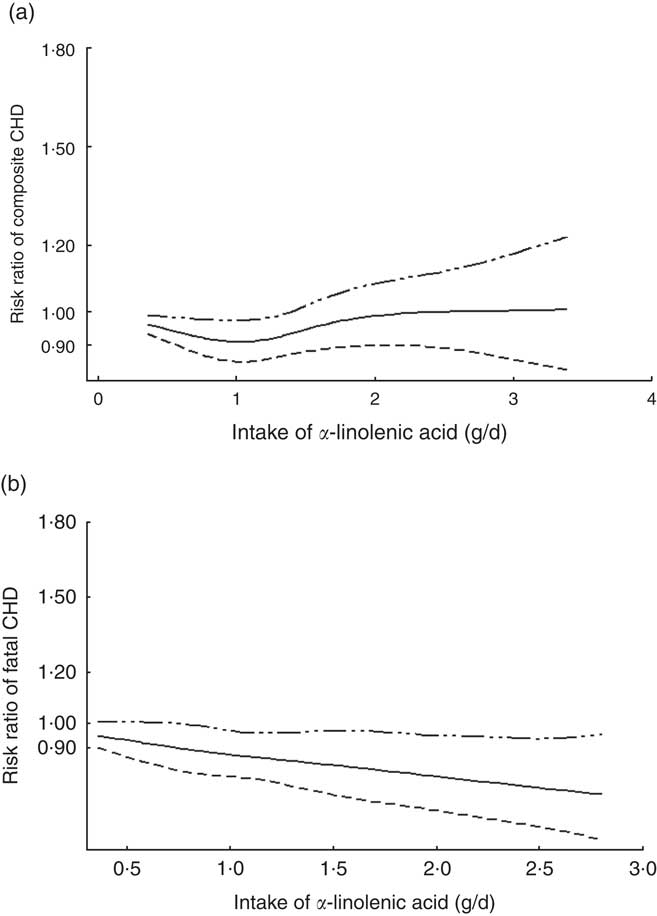
Fig. 3 Dose–response relationship between α-linolenic acid (ALA) intake and relative risk of (a) composite CHD and (b) fatal CHD. Weights are from random-effects analysis.![]() , Lower limit of 95 % CI;
, Lower limit of 95 % CI;![]() , upper limit of 95 % CI;
, upper limit of 95 % CI; ![]() , risk ratio.
, risk ratio.
Quality assessment
The quality of the fourteen studies included in the metaanalysis, as scored with the Newcastle–Ottawa scale, is presented in the online Supplementary Table S2. The mean total quality score was 7·4 out of a maximum score of 9 (range 7–9) indicating that, overall, the methodological quality among cohort studies was moderately good.
Discussion
The present systematic review and meta-analysis showed significant associations between a higher ALA intake with reduced risk of composite CHD and fatal CHD. Our dose–response analysis showed that compared with people without intake of ALA, only a certain range of ALA intake (<1·4 g/d) was associated with reduced risk of CHD. In contrast, a higher intake of ALA was associated with reduced risk of fatal CHD. To our knowledge, this is the largest meta-analysis of dietary ALA intake and CHD risk based on prospective studies. However, given the present publication bias and heterogeneity among studies, the results should be interpreted with caution.
Our results were similar with previous studies to some extent. Pan et al.( Reference Pan, Chen and Chowdhury 9 ) showed a significant inverse association and a linear dose–response relationship between ALA intake and risk of fatal CHD, though it did not show an overall CHD outcome, but a composite of all cardiovascular events, including CHD, stroke and other CVD events with an association of similar magnitude. Vedtofte et al.( Reference Vedtofte, Jakobsen and Lauritzen 25 ) previously found that 1 g/d of ALA consumed was associated with a 15 % lower risk of CHD events and a 23 % lower risk of CHD deaths in males. Although it did not reach statistical significance, a potential non-linear relationship was not tested. Our dose–response analysis indicated a non-linear relationship between ALA intake and risk of composite CHD, which suggested a beneficial level of dietary ALA intake for prevention of CHD. The National Academy of Medicine recommended that men should consume 1·6 g of ALA per day and women should consume 1·1 g/d( Reference Zatonski, Campos and Willett 26 ). This is similar to our present result of optimal level of ALA (1 g/d) in the dose–response meta-analysis.
Although ALA shows promising benefits in CHD prevention, the effect seems to be smaller than expected, particularly our dose–response analysis showed a non-linear relationship. This may be explained by residual or unknown confounding effects, such as intake of other fatty acids or dietary components related to risk of CHD. Furthermore, as Oomen et al. ( Reference Oomen, Ocke and Feskens 19 ) point out, that intake of ALA may be associated with intake of trans-fatty acids, which is associated with an increased risk of CHD( Reference Oomen, Ocke and Feskens 19 ). ALA and trans-fatty acids are also found correlated in biomarkers( Reference Pedersen, Ringstad and Almendingen 27 ). According to our present results, it is likely that under a certain amount of ALA intake, ALA has an effect over trans-fatty acids, whereas trans-fatty acids may play a more important role in association with CHD. However, this does not affect an expected trend of risk in fatal CHD.
The mechanisms of association between dietary ALA intake and CHD are not fully understood yet. The conversion from ALA to EPA and DHA may partially account for the association, whereas previous studies have found that only very limited amounts of ALA were actually converted( Reference Barcelo-Coblijn and Murphy 28 ), so the efficiency is not high. Currently, there is no solid evidence showing a direct causal relationship between dietary ALA intake and CHD risk, whereas previous epidemiological studies showed some clues, for example, Lemaitre et al.( Reference Lemaitre, King and Sotoodehnia 29 ) found an association between ALA in erythrocytes and risks of sudden cardiac death, which is independent of erythrocyte levels of EPA and DHA, linoleic acid and trans-fatty acids. There are more plausible mechanisms to account for the association between ALA intake and CHD, such as reduced inflammation, which is found to be associated with higher intake of nuts( Reference Yu, Malik and Keum 30 ). Potential biological research is needed to further elucidate the mechanisms involved.
Our findings have significant relevance for public health and medicine. A number of studies also examined the association of biomarkers of ALA and CHD risk, whereas studies may have a better significance in guidance of human diet with dietary ALA intake as an exposure Diet has been recognised as an essential aspect in preventing CHD. In commonly applied dietary patterns for prevention of CVD, such as the Mediterranean dietary pattern( Reference Sanchez-Villegas, Martinez and De Irala 31 ) and Dietary Approaches to Stop Hypertension( Reference Sacks, Obarzanek and Windhauser 32 ), both emphasize intake of fish, which is the main source of EPA and DHA. As an alternative source of EPA and DHA, ALA should be considered as an important element in CHD prevention, as the potential for supplying ALA is great( Reference Mozaffarian and Wu 5 ). It is expected that a guideline for specific groups of foods and amount be made to the public for self-administered prevention of CHD. Furthermore, ALA could potentially lead to pharmacological applications. Randomised controlled trials on CHD patients have shown that ALA intake may reduce risk of CHD events, including recurrence of CHD and CHD death( Reference de Lorgeril, Renaud and Mamelle 33 , Reference Kromhout, Giltay and Geleijnse 34 ). Future randomised controlled trials are needed to examine the effects of ALA on reducing primary CHD risk.
Our present study had some noteworthy limitations. First, all the included studies did not report uniform levels of dietary ALA intake and did not report the same outcomes, although the results were consistent in general. Second, our present meta-analysis of prospective cohort studies only included two randomised controlled trials. More randomised controlled trials of the effect of ALA intake on reduction of CHD are needed to remove all unmeasured confounding that may still plague prospective observational studies.
In conclusion, existing prospective studies suggest that higher ALA intake is associated with reduced risk of composite CHD and fatal CHD. Though a higher dietary ALA intake was associated with reduced risk of fatal CHD, the excess composite CHD risk at higher ALA intakes warrants further investigation, especially through randomised controlled trials.
Acknowledgements
The authors thank authors of the included articles for additional information relevant to the analysis.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
J. W. designed the study. J. W., R. H. and Y. X. conducted the literature search and data extraction. R. H. and A. K. performed quality assessment. J. W. conducted data analysis and drafted the manuscript. All authors contributed to and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003294






