Nutritional interventions are central to the treatment of type 2 diabetes. The 2008 Canadian Clinical Practice Guidelines for diabetes recommend increasing the intake of non-oil-seed pulses, including dried peas and beans, chickpeas and lentils(1). A recent meta-analysis of forty-one human randomised controlled trials concluded that pulse consumption predicts improved long-term glycaemic control(Reference Sievenpiper, Kendall and Esfahani2). Despite this conclusion, the heterogeneity among published studies precludes strong statements about the type, dose, duration of intake or mechanism of action. However, the effects of pulses are strongly correlated with the overall glycaemic index of the diet(Reference Sievenpiper, Kendall and Esfahani2), suggesting that the fibre and resistant starch components are important bioactive components.
Pulse seeds consist of an embryo surrounded by a seed coat. The embryo consists of cotyledons and an embryo axis, with the cotyledons making up about 98 % of the embryo by weight in the pea (Pisum sativum L.)(Reference Ayele, Ozga and Reinecke3). The embryo is mainly starch and protein with smaller quantities of soluble and insoluble fibre(Reference Guillon and Champ4, Reference Daveby, Abrahamsson and Aman5). Uncooked field pea starch includes 2·4 % resistant starch(Reference Rochfort and Panozzo6), which can be increased by cooking(Reference Goodlad and Mathers7), although this has not been reported in all studies even though digestibility decreased(Reference Rochfort and Panozzo6). High fibre consumption (50 g/d, with 25 g soluble fibre/d) is associated with reduced fasting plasma glucose, and reduced excursions of glucose and insulin postprandially(Reference Chandalia, Garg and Lutjohann8). Soluble fibre and resistant starch are fermented in the large intestine, producing SCFA, which may have multiple beneficial effects on metabolism. These are hypothesised to include modulation of hepatic glucose output(Reference Higgins9), induction of incretin secretion, and increasing insulin sensitivity(Reference Weickert and Pfeiffer10). However, there is conflicting evidence for these mechanisms in man and identification of mechanisms is limited by the methodology.
The pea seed coats consist mainly of insoluble fibre, and cultivars with clear seed coats have minimal flavonoid content (non-coloured seed coats; NSC), while other cultivars contain polyphenolic flavonoid compounds such as proanthocyanidins, which upon oxidation appear brown (coloured seed coats; CSC)(Reference Xu, Yuan and Chang11). Flavonoids are abundant in many fruits, legumes and nuts but not grains, and are of interest nutritionally for their antioxidant properties(Reference Duenas, Estrella and Hernandez12). For example, grapes are rich in flavonoids and their consumption is associated with reduced risk of cardiovascular and other chronic diseases(Reference Serrano, Puupponen-Pimiä and Dauer13). Less is known about proanthocyanidins and their effects on type 2 diabetes. Most, but not all, studies of consumption of proanthocyanidins derived from grapes suggest that glycaemic control is improved(Reference Vislocky and Fernandez14). One study of db/db mice found that persimmon peel-derived proanthocyanidins given orally had both hypoglycaemic and hypolipidaemic effects(Reference Lee, Cho and Yokozawa15). Proanthocyanidins may also delay glucose absorption from the intestine, improve β-cell function and mimic the effects of insulin on target tissues(Reference Duenas, Estrella and Hernandez12).
Differences in composition suggest that consumption of different pea fractions may elicit different metabolic effects. In a type 2 diabetes population, a mixed meal including whole dried peas results in smaller postprandial increases in glucose and insulin compared with potatoes(Reference Schafer, Schenk and Ritzel16). Muffins made from whole pea flour reduced fasting insulin and improved insulin sensitivity but did not reduce the postprandial glycaemic response of overweight subjects compared with wheat flour(Reference Marinangeli and Jones17). However, diets containing pea seed coats may elicit different effects from embryos or whole peas. Pure pea starch elicited lower post-meal glucose and insulin excursions than maize starch(Reference Seewi, Gnauck and Stute18). Isolated pea fibre added to a meal produced a lower postprandial insulin response when compared with low-fibre test meals in young, healthy subjects(Reference Sandstrom, Hansen and Sorensen19). Fasting insulin but not the postprandial glucose excursion was reduced when ground pea fibre was substituted for wheat flour (22 %, w/w) in muffins(Reference Marinangeli and Jones17).
To a large extent, the experimental designs of these studies does not allow for elucidation of mechanisms and it is thus not understood where pulses, such as peas, predominantly exert their effects on the gastrointestinal tract, on insulin-sensitive tissues such as muscle or on insulin-secreting β-cells. Furthermore, it is unclear which of the pulse components (seed coat v. embryo) has the most beneficial effects, and whether pulses with high levels of antioxidants such as proanthocyanidins exert different effects than those without. The overall goal of the present study is to understand the metabolic and molecular basis for the beneficial effects of peas in the diet on glucose homeostasis.
Methods and materials
Pea seed analysis
The pea seed cultivars ‘Courier’ and ‘Canstar’ used in the present study were grown in western Canada in 2007. Total phenolic content and antioxidant activities of legumes are correlated with the colour of the seed coat(Reference Xu, Yuan and Chang11). The seed coats of ‘Courier’ are brownish in colour (CSC). For protein analysis, 5–10 g of mature seeds, seed coats or embryos were ground to a powder and protein was determined from subsamples of this tissue according to the AOAC method 972.43(20) using 6·25 as the conversion factor for calculating protein content (%). For fibre analysis, 5–10 g of mature seeds, seed coats or embryos were ground to a powder and subsamples of these tissues were used to determine the acid-detergent fibre component according to the ANKOM Technology Method 6(21) and the neutral-detergent fibre component according to the ANKOM Technology Method 5(22). Total proanthocyanidin content values were based on characterisation of proanthocyanidin subunits following phloroglucinolysis and reverse phase–high pressure liquid chromatography–diode array detector analysis as described by Kennedy & Jones(Reference Kennedy and Jones23).
The pea seeds were mechanically dehulled using a dehuller (Forsberg model 2; Forsberg) and separated into embryo and seed coat fractions using an aspirator (Carter Duo). The embryos (‘Courier’ only) and seed coats were then ground to a fine consistency for inclusion in the diets. Fine seed coats were obtained via sieving with a no. 16 Canadian standard sieve.
Experimental animals and diets
All animal protocols followed the guidelines of the Canadian Council on Animal Care and were approved by the Health Sciences Animal Care and Use Committee at the University of Alberta. Sprague–Dawley rats were obtained from Charles River Canada at 8 weeks of age. After 1-week acclimatisation with access to standard chow and water ad libitum, all rats were introduced to a high-fat diet (HFD, 40 % of energy from fat, 20 % of energy from saturated fat) composed as shown in Table 1. At 3 weeks later, diets with uncooked pea fractions added to the base diet were introduced and fed for 4 weeks. Embryos of pea varieties have similar protein composition but can vary widely (up to 7 % in one study) within cultivars over several growing years, depending on agronomic and environmental conditions(Reference Nikolopoulou, Grigorakis and Stasini24). Thus, only embryos from ‘Courier’ peas were used in the present study and total protein was adjusted to be equal in both diets. Composition of the diets containing pea embryos or pea seed coats is shown in Table 1. Diets were formulated to ensure equal total fat (20 %), protein (27 %), carbohydrate (38 %) and fibre (8 %) and thus were equal in energy density.
Table 1 Diet composition (g/kg)*

HFD, high-fat diet; EMB, diet supplemented with pea embryos from ‘Courier’ peas; NSC, diet supplemented with non-coloured seed coats; CSC, diet supplemented with coloured seed coats; AIN, American Institute of Nutrition.
* See Table 2 for protein and fibre analysis of the pea seed fractions. The pea embryo was added in an amount calculated to represent 10 % of total carbohydrate. The pea seed coats were added in an amount to provide approximately half the total fibre in the diet.
† Harlan.
‡ Canada Safeway.
§ Richardson Oilseed.
‖ Shoppers Drug Mart.
¶ MP Biomedicals.
Glucose tolerance tests
Glucose tolerance tests were conducted at baseline, and at 3 and 7 weeks (i.e. before HFD, after 3 weeks of HFD and after 4 weeks of HFD plus pea fractions). At the same time, rats were weighed and food and water intake measured over a 24 h period. Rats were acclimatised to individual metabolism cages for 24 h before food and water intakes were determined. Oral glucose tolerance tests were performed after an overnight fast essentially as described(Reference Chan, Pederson and Buchan25) except that glucose was measured in whole blood with a glucometer (Accu-Check Compact Plus; Roche Diagnostics) over a 2 h period. Additional 50 μl blood samples were centrifuged and the serum collected and stored at − 80°C until assayed for insulin using an ELISA, according to the manufacturer's instructions (Alpco).
Tissue collection
At 5 d following the glucose tolerance test, rats were fasted overnight and then euthanised under anaesthesia (ketamine 100 mg/kg and xylazine 1 mg/kg, intraperitoneally) by exsanguination. Half the rats received insulin (35 pmol/kg intraperitoneally) 15 min before death while the other half received an equivalent volume of saline. Blood (5–10 ml) was obtained from the abdominal aorta, and divided for preparation of plasma and serum, which were frozen at − 80°C. Samples of liver, jejunum, ascending colon, soleus and epitrochearis muscle were snap-frozen in liquid N2 and stored at − 80°C. An additional pancreas sample just adjacent to the spleen was fixed in formalin overnight, then transferred to 70 % ethanol in preparation for embedding in paraffin by standard techniques. Jejunal and colonic samples were similarly fixed and prepared for histology.
Analysis of serum and plasma samples
Fasting serum TAG (Trinder, Sigma), total cholesterol and NEFA were obtained from the baseline sample using kits (Wako Chemicals USA). Haptoglobin in serum was measured using a kit from Tridelta.
Immunoblotting
Frozen samples were thawed on ice and homogenised in LIPA buffer as described(Reference Saleh, Fatehi-Hassanabad and Wang26). Total protein was measured using a Lowry protein assay (Sigma). Samples (20 μg protein) were subjected to SDS-PAGE followed by blotting onto nitrocellulose membranes. The membranes were blocked in 5 % defatted skimmed milk–1 % Tween–PBS for 60 min followed by overnight incubation at 4°C with primary antibodies diluted in Tris-buffered saline–1 % Tween–5 % bovine serum albumin. Antibodies from Cellular Signaling Technology included phospho-Thr172-AMP-dependent kinase (AMPK) α-subunit (1:1000), AMPK α-subunit (1:1000), phospho-Ser473-akt (1:1500), akt (1:1500) and GLUT4 (1:2000). Anti-phosphoenol pyruvate carboxykinase (PEPCK; 1:1000) was from Cayman Chemicals. Membranes were subsequently incubated with the appropriate peroxidase-conjugated secondary antibodies (Sigma) for 1–2 h at room temperature. Membranes were developed using ECL Plus (GE Biosciences) and digital images captured on a Typhoon Trio imaging station (GE Healthcare). Labelling of specific proteins was compared with β-actin (liver; Sigma) or β-tubulin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (muscle; Abcam) used as loading controls using ImageJ (http:///rsb.info.nih.gov/ij).
Histology, immunohistochemistry and morphometry
Before embedding, gut sections were cut into five or six rings and oriented in the paraffin so as to obtain cross-sections. Paraffin-embedded pancreatic or gut tissue was sectioned (5 μm) and serial sections mounted on glass slides. After dewaxing in xylene and graded alcohol, slides were rinsed with distilled water and PBS. For characterisation of gut morphology, the tissues were stained with haematoxylin and eosin and digital photomicrographs acquired using an Axiovert microscope and Axiovision 4.7 software (Carl Zeiss Canada Ltd). Villus height or mucosal thickness was quantified using Image J software, by drawing a line at right angles to the serosa. Only images where good cross-sectional orientation had been achieved were used and at least ten well-oriented samples were taken for each slide. Immunohistochemistry was performed to detect GLUT2, GLUT5 and Na-glucose linked transporter-1 (SGLT1). Sections were blocked in appropriate normal serum (4 %, 1 h, room temperature), then primary antibodies (all 1:000; all from Millipore) for 1 h at room temperature. Appropriate fluorescent (AlexaFluor 488)-labelled secondary antibodies (Invitrogen) were applied for 2–4 h at 1:2000 (against GLUT5) or 1:4000 (against GLUT2 and SGLT1).
For determination of α- and β-cell areas, endogenous peroxidases were blocked with 3 % H2O2 for 30 min. After washing in PBS, the mounted tissue was incubated in 5 % normal sheep serum for 20 min at room temperature, then blotted, and the primary antibody applied. Primary antibodies were mouse anti-glucagon (1:500; Abcam) and mouse anti-insulin (1:100; Dako). Slides were incubated overnight at 4°C in a humidified chamber, then washed and incubated with peroxidase-conjugated sheep anti-mouse antibody (1:200) for 1 h at room temperature. Positive immunoreactivity was visualised by incubating the slides in diaminobenzidine plus H2O2 for 10 min. Slides were then dehydrated in graded alcohol, cleared in xylene and cover-slipped. The entire section on each slide was photographed using an Axiovert microscope equipped with Axiovision 4.7 software (Zeiss). The total pancreatic area (excluding large ducts and veins), and the insulin- and glucagon-positive areas were quantified using ImageJ as described(Reference Saleh, Fatehi-Hassanabad and Wang26).
Oxidative stress was assessed in pancreatic islets by staining for either malondialdehyde (MDA) or nitrotyrosine (NT). To determine if the HFD increased MDA and NT staining, samples obtained from age-matched chow-fed Sprague–Dawley rats were obtained from a slide bank for comparison. For MDA, slides were blocked with 5 % normal rabbit serum, then incubated overnight at 4°C with anti-MDA antiserum (1:100; Ray Biotech). For NT, slides were blocked with 5 % normal goat serum followed by anti-NT antiserum (1:100; Millipore). Appropriate secondary antibodies conjugated to Alexa-546 were used (1:200, room temperature, 1 h; Invitrogen) to visualise antigen localisation. Dual or serial-section labelling was accomplished by applying the antibody against insulin (1:1000; Dako) followed by the appropriate secondary antibody conjugated with Alexa-488.
Statistical analysis
Data were expressed as mean values with their standard errors. Differences between groups were assessed by one-way or two-way ANOVA, as appropriate, and P < 0·05 was considered significant. Specific differences were identified by the Newman–Keuls post hoc test.
Results
Pea embryo and seed coat fraction analysis
The seed coat component of the pea seeds was high in fibre (approximately 50 to 70 % by weight), with ‘Canstar’ seed coats (NSC) having a higher fibre content than that of ‘Courier’ (CSC) (Table 2). Approximately 10 % of the seed coat fibre component was estimated to be hemicellosic in both cultivars (the difference between acid-detergent fibre and neutral-detergent fibre values; Table 2). The protein in the seed coats ranged from 5 to 8 % by weight. The proanthocyanidin content was approximately 4 % of fresh weight of CSC but not detectable in NSC fractions. In the final diet preparation, the proanthocyanidin content was 0·3 %. The protein levels in the embryos of both cultivars ranged from 23 to 26 % by weight (Table 2). Although protein content was higher in the ‘Courier’ peas, the difference fell inside the range of variability expected within a cultivar. Therefore, the rat feeding trials were conducted using only ‘Courier’ pea embryos.
Table 2 Protein and fibre analysis of whole seeds, seed coats and embryos of pea cultivars ‘Courier’ and ‘Canstar’
(Mean values with their standard errors of three replicates)
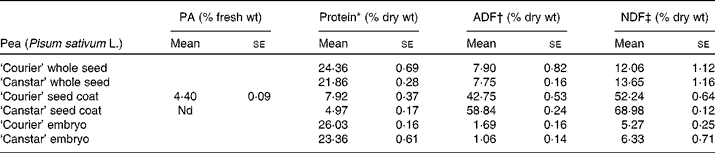
PA, proanthocyanidins (flavonoids); ADF, acid-detergent fibre; NDF, neutral-detergent fibre; nd, not detected.
* Determined using AOAC method 972.43(20).
† ADF is the residue remaining after digesting with H2SO4 and cetyltrimethylammonium bromide (CTAB). The fibre residues are predominantly cellulose and lignin. ANKOM Technology Method 5(22).
‡ NDF is the residue remaining after digesting in a detergent solution. The fibre residues are predominantly hemicelluloses, cellulose and lignin. ANKOM Technology Method 6(21).
Food intake
Dietary consumption was measured at 7 weeks over 24 h while the rats were singly housed in a metabolism chamber. No differences in food intake were observed in the rats fed the HFD (26·0 (se 1·6) g) compared with rats fed the HFD+embryos (27·0 (se 1·9) g), HFD+NSC (28·6 (se 1·4) g) or HFD+CSC (26·9 (se 1·7) g). This suggested that palatability of the diets or altered satiation did not affect the outcome.
Metabolic profile
The metabolic profile of the rats is shown in Table 3. Body weight was compared at baseline and at study completion. The increase in body weight over the 7-week study was approximately 160 g and did not differ between groups (P>0·05). Fasting blood glucose of both NSC and CSC groups was lower than in the embryo group, and that in the NSC group was also lower than in the HFD group (P < 0·05). Fasting serum insulin concentration was lower in the NSC group than in any other group (P < 0·01). Blood lipids, including TAG, NEFA and total cholesterol did not differ among diet treatments. Serum haptoglobin, a marker of inflammation, was similar in all groups.
Table 3 Metabolic profile of rats fed diets containing pea components
(Mean values with their standard errors of eight replicates)
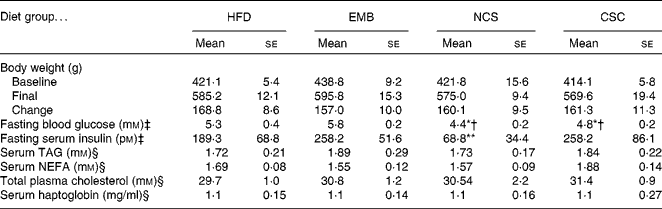
HFD, high-fat diet; EMB, diet supplemented with pea embryos; NSC, diet supplemented with non-coloured seed coats; CSC, diet supplemented with coloured seed coats.
Mean value was significantly different from that of the EMB group: *P < 0·05, **P < 0·01.
† Mean value was significantly different from that of the HFD group (P < 0·05).
‡ Blood sampling was done at completion of the diet feeding period during the oral glucose tolerance test.
§ Serum for lipids and haptoglobin was harvested from blood collected from anaesthetised animals just before euthanasia.
Oral glucose tolerance
To quantify the degree of glucose intolerance induced by feeding the HFD, oral glucose tolerance was measured at baseline, and at 3 and 7 weeks. As shown in Fig. 1(a), the glucose excursion increased with the diet duration (F(2,5) = 27·57; P < 0·0001), with significantly more glucose in the blood at 10 min in the animals fed the HFD for 3 weeks and at 0, 10 and 20 min in the animals fed the HFD for 7 weeks (P < 0·05). The insulin response to glucose after the HFD was also higher than at baseline (F(2,5) = 4·203; P < 0·02) (Fig. 1(b)).

Fig. 1 Effects of feeding pea fractions on oral glucose tolerance in rats. Effect of 3 weeks (![]() ) and 7 weeks (
) and 7 weeks (![]() ) of feeding a 40 % (w/w) fat diet (high-fat diet; HFD) on oral glucose tolerance (a) and insulin secretion (b) in response to administration of 1 g/kg oral glucose. (
) of feeding a 40 % (w/w) fat diet (high-fat diet; HFD) on oral glucose tolerance (a) and insulin secretion (b) in response to administration of 1 g/kg oral glucose. (![]() ), Baseline. Values are means, with standard errors represented by vertical bars. *Mean value was significantly different from that at baseline (P < 0·05). (c) Blood glucose and (d) plasma insulin responses to 1 g/kg oral glucose in rats on the HFD for 3 weeks, then continued on the HFD (
), Baseline. Values are means, with standard errors represented by vertical bars. *Mean value was significantly different from that at baseline (P < 0·05). (c) Blood glucose and (d) plasma insulin responses to 1 g/kg oral glucose in rats on the HFD for 3 weeks, then continued on the HFD (![]() ; n 16) or switched to the HFD plus coloured seed coats (CSC;
; n 16) or switched to the HFD plus coloured seed coats (CSC; ![]() ; n 16), non-coloured seed coats (NSC;
; n 16), non-coloured seed coats (NSC; ![]() ; n 16) or embryos (EMB;
; n 16) or embryos (EMB; ![]() ; n 8) for 4 weeks. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the HFD group: *P < 0·05, **P < 0·01. (e, f) Incremental areas under the curve (AUC) for the glucose tolerance and insulin secretion data shown in (c) and (d). Values are means, with standard errors represented by vertical bars. *Mean value was significantly different from that of the HFD group (P < 0·05). (g) Calculation of insulin sensitivity using the homeostasis model of insulin resistance (HOMA-IR). Values are means, with standard errors represented by vertical bars. *Mean value was significantly different from that of the HFD group (P < 0·05). †Mean value was significantly different from that of the CSC group (P < 0·05).
; n 8) for 4 weeks. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the HFD group: *P < 0·05, **P < 0·01. (e, f) Incremental areas under the curve (AUC) for the glucose tolerance and insulin secretion data shown in (c) and (d). Values are means, with standard errors represented by vertical bars. *Mean value was significantly different from that of the HFD group (P < 0·05). (g) Calculation of insulin sensitivity using the homeostasis model of insulin resistance (HOMA-IR). Values are means, with standard errors represented by vertical bars. *Mean value was significantly different from that of the HFD group (P < 0·05). †Mean value was significantly different from that of the CSC group (P < 0·05).
After 7 weeks (4 weeks fed pea fractions), the effects of the four diets on oral glucose tolerance were compared. Data are shown as the responses at each time point (Fig. 1(c)) and as incremental areas under the curve (Fig. 1(e)). The diet had a significant effect on oral glucose tolerance (F(3,5) = 11·93; P < 0·001) and the embryo group had an overall higher glucose excursion (Fig. 1(e)). Both the CSC and NSC groups had lower blood glucose than controls at 10 and 20 min. Insulin responses also differed as a result of the diets (F(3,5) = 11·01; P < 0·001; Fig. 1(d)), with the total response of the NSC group lower than any other group, and the embryo group higher than controls (Fig. 1(f)). The homeostasis model of insulin resistance (HOMA-IR) is a measure of insulin resistance based on fasting insulin and glucose concentrations. NSC-treated rats were less insulin resistant than HFD control or CSC rats using this calculation (P < 0·05).
Insulin signalling pathways in skeletal muscle and liver
Improved oral glucose tolerance could result from enhanced insulin sensitivity in peripheral tissues. We measured total GLUT4 protein levels in skeletal muscle from HFD, NSC and CSC rat groups. No differences (P>0·05) between the groups were detected in either highly oxidative (soleus) muscle (Fig. 2(a)) or epitrochlearis muscle, which is mainly glycolytic (Fig. 2(b)).

Fig. 2 Effects of feeding pea seed coats on GLUT4 levels in muscle measured by immunoblotting. GLUT4 protein relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in rats fed a high-fat diet alone (control; n 8) or supplemented with coloured seed coats (n 8) or non-coloured seed coats (n 8) in (a) soleus and (b) epitrochlearis muscle lysates. Values are means, with standard errors represented by vertical bars. Insets show representative blots.
To determine if up-regulation of the insulin-signalling pathway regulating glucose transport had occurred, phospho-akt and total akt were measured. Total akt protein expression was increased by 80 % in epitrochlearis muscle of the CSC group (P < 0·05; Table 4). The relative phosphorylation was similar in all groups. No differences in akt or phospho-akt were detected in the soleus muscle or liver.
Table 4 Summary of immunoblotting results in skeletal muscle and liver after insulin stimulation
(Mean values with their standard errors of eight replicates)

HFD, high-fat diet; CSC, diet supplemented with coloured seed coats; NSC, diet supplemented with non-coloured seed coats; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AMPK, AMP-dependent kinase; NA, not available; PEPCK, phosphoenol pyruvate carboxykinase.
* Mean value was significantly different from that of the HFD group (P < 0·05).
† The EMB group was omitted from immunoblotting analyses because there was no beneficial effect of this diet on glucose homeostasis.
AMPK-regulated glucose uptake might also contribute to altered fasting glucose or glucose tolerance. In the soleus muscle of NSC rats, total AMPK protein expression was 2·1-fold greater than in HFD rats (P < 0·05). There were no differences in the relative phospho-AMPK levels. In the liver of NSC-fed rats, total AMPK expression was reduced by about 60 % (P < 0·05). The amount of PEPCK was also analysed in the liver but no differences were observed between the groups. Data are summarised in Table 4.
Pancreatic β-cells and oxidative stress
Lower fasting insulin and a lower insulin response to glucose in the NSC group suggested that the number of β-cells contributing to secretion might be reduced. The area positively immunostained for insulin was quantified in pancreas sections from all four groups and compared with the total pancreatic area. As shown in Fig. 3(a), the β-cell area in the NCS group was decreased by about 70 % compared with HFD controls (F(3,21) = 5·167; P < 0·01). α-Cell area (as assessed by glucagon staining) did not differ between the groups (Fig. 3(b)).

Fig. 3 Morphology of pancreatic islets. The percentage of total pancreatic area occupied by cells immunostaining for (a) insulin and (b) glucagon was quantified. Values are means (n 8 for all groups), with standard errors represented by vertical bars. *Mean value was significantly different from that of the control group fed the high-fat diet (P < 0·05). (c) Representative photomicrographs. Insulin immunostaining is highlighted by the circles around each islet. CSC, coloured seed coat diet; NSC, non-coloured seed coat diet; EMB, embryo diet.
To evaluate whether islets from the pea-fed groups exhibited different patterns of oxidative stress, pancreatic sections were stained for MDA or NT and compared with insulin-immunoreactive cells. MDA was predominantly detected in cells co-staining for insulin, including a chow-fed (low-fat diet) control (Fig. 4(a)). In β-cells, staining was least intense in the NSC and embryo tissue when all slides were photographed under identical parameters. NT was detected in non-β-cells of all groups (Fig. 4(b)) but diet-related changes in staining intensity were not as pronounced as for MDA.

Fig. 4 Assessment of oxidative stress in the islets by dual immunostaining for insulin and malondialdehyde (MDA) (a) and insulin and nitrotyrosine (NT) (b). MDA co-localised with cells labelled for insulin in all groups, and with cells not labelled for insulin only in the non-coloured seed coat (NSC) group ( → ). NT staining was limited to cells not staining for insulin in all groups. LFD, low-fat diet; HFD, high-fat diet; CSC, coloured seed coat diet; EMB, embryo diet.
Gut morphology
No differences were detected in the height of villi in the jejunum (Fig. 5(a)) but the thickness of the colonic mucosa was significantly reduced in NSC-fed rats (Fig. 5(b)). In all groups, GLUT2, GLUT5 and SGLT1 were detected by immunostaining in both the jejunum and ileum but overt differences in staining intensity were not observed (not shown).

Fig. 5 Effects of feeding pea fractions on gut morphology. (a) Thickness of the colonic mucosa. (b) Villus height in the jejunum. Values are means (n 8 for all groups), with standard errors represented by vertical bars. *Mean value was significantly reduced compared with the other groups (P < 0·05). No effects of pea fractions on villus height in the jejunum were detected. CSC, coloured seed coat diet; NSC, non-coloured seed coat diet; EMB, embryo diet.
Discussion
Uncooked CSC and NSC from dried peas alleviated glucose intolerance in glucose-intolerant rats whereas embryos did not have beneficial effects. Lack of effect of the embryos may be attributed to the method of preparation. Uncooked peas have a lower content of amylase-resistant starch and higher digestibility than cooked peas, along with lower SCFA concentration and higher pH(Reference Goodlad and Mathers7). The beneficial effects of pea starch found in human studies(Reference Chandalia, Garg and Lutjohann8–Reference Weickert and Pfeiffer10) are probably because cooked preparations are used. The benefits of pea seed coats may be attributed to their main nutrient component, acid- and neutral-detergent fibres. In the present study, both the NSC and CSC treatments produced a similar improvement in glucose tolerance but had distinct effects on insulin secretion. More beneficial effects on diverse tissues were detected after feeding NSC. However, the concentration of flavonoids in the CSC diet preparations was quite low.
Both NSC and CSC groups had lower fasting blood glucose after 3 weeks of pea diets and peak glucose excursions in the oral glucose tolerance test were significantly reduced. However, the mechanisms by which NSC and CSC feeding led to improved glucose homeostasis were clearly different, because NSC reduced both fasting insulin and the insulin response to oral glucose, whereas CSC had no effect on insulin. Derivation of HOMA-IR from fasting glucose and insulin values suggests that rats fed NSC had improved insulin sensitivity compared with the HFD or CSC groups. The idea that insulin signalling was improved in NSC rats was consistent with the reduction in insulin secretion and in reduced area of β-cells in pancreatic tissue. In response to a HFD, β-cells undergo a rapid hyperplastic response within 10 d in order to adapt to developing insulin resistance(Reference Smith, Zhang and Clermont27); thus, the NSC diet appeared to have reduced the need for this adaptive response. Although these results were obtained from uncooked seed coat preparations, cooking does not greatly affect either insoluble or soluble fibre components(Reference de Almeida Costa, da Silva Queiroz-Monici and Machado Reis28). Therefore, fibre-dependent effects would probably be retained in cooked preparations more relevant to human consumption. Conversely, cooking alters the chemical structure of polyphenols, which could alter their bioavailability and antioxidant properties(Reference Serrano, Puupponen-Pimiä and Dauer13).
Lower circulating glucose in an oral glucose tolerance test may be observed for several reasons: (a) increased uptake of glucose into peripheral, insulin-sensitive tissues; (b) enhanced suppression of liver glucose output by insulin; or (c) reduced glucose absorption from the gastrointestinal tract. In the present study, differences consistent with an increased uptake of glucose were observed in insulin-signalling pathways in skeletal muscle. Skeletal muscle uptake accounts for approximately 75 % of insulin-stimulated glucose disposition. In glycolytic (fast-twitch) muscle of CSC rats (represented by epitrochlearis muscle), total akt protein amount was increased 1·8-fold which, combined with similar relative phosphorylation, could double glucose transport. Akt is one of the critical molecules downstream of phosphatidyl inositol 3-kinase in skeletal muscle that signals GLUT4 translocation and its function is reduced in HFD-induced insulin resistance(Reference Tremblay, Lavigne and Jacques29). Dietary manipulation, such as feeding fish protein, has previously been shown to increase akt phosphorylation and improve glucose disposal(Reference Tremblay, Lavigne and Jacques30). In the present study, the effects of pea seed coat feeding on akt protein expression were restricted to the CSC, which suggests that the effects were not due to fibre or protein. The CSC contains proanthocyanidins, a class of polyphenols. Other polyphenols such as curcumin and epigallocatechin-3-galate (enriched in green tea) inhibit akt phosphorylation and are touted for anti-cancer effects(Reference Lamoral-Theys, Pottier and Dufrasne31). Anthocyanidins (which are chemically related to proanthocyanidins) from black soyabeans also inhibited akt phosphorylation when applied directly to UVB-irradiated cells(Reference Tsoyi, Park and Kim32). However, in neurons, flavonoids (a class of polyphenols that includes proanthocyanidins) are reported to increase akt-related signalling, albeit mainly by increasing phosphorylation(Reference Spencer33). One mechanism by which a flavonoid may regulate total akt protein expression is by interactions with heat-shock proteins (Hsp). Hsp70 attenuates akt expression; its inhibition by the flavonoid myricetin led to an increase in akt protein in a breast cancer cell line(Reference Koren, Jinwal and Jin34). Whether this is the mechanism by which CSC regulate akt activity requires further experimentation.
Adaptations in akt protein expression levels or phosphorylation were not seen in the liver or soleus (oxidative) muscle. However, in the soleus muscle of NSC rats, total AMPK protein expression was elevated, which could contribute to non-insulin-regulated glucose uptake and the lower fasting glucose concentrations. Previously, Liu et al. (Reference Liu, Wan and Guan35) reported that HFD feeding reduced levels of both total and phosphorylated AMPK. Thus, the increase after NSC feeding could represent normalisation of AMPK levels, although not phosphorylation. Unexpectedly, in the liver, total AMPK protein expression was reduced by the NSC diet but, combined with a non-significant increase in phospho-AMPK, the impact on overall metabolism is predicted to be small. To address the possibility that lower blood glucose was secondary to reduced hepatic glucose output the amount of PEPCK, the major regulatory enzyme of gluconeogenesis, was examined but no differences were detected between the groups.
Improved glucose tolerance and lower insulin secretion in the NSC group could also be explained by slower or reduced glucose absorption from the gastrointestinal tract. Assessment of the absorptive area of the jejunum and colon was conducted. The height of the villi was taken as an index of absorptive area in the jejunum and was not different between the groups. In the colon, the thickness of the mucosa was decreased in the NSC group. In rats, carrier-mediated glucose absorption may occur in the colon although with slower kinetics than in the jejunum(Reference Tomei, Torimoto and Hayashi36). GLUT2, GLUT5 and the Na-dependent GLUT SGLT1 were all detectable by immunostaining in the colonic mucosa of all rat groups, concentrated in the epithelial cells at the luminal boundary (data not shown). However, other mechanisms of glucose uptake involving hydrogen ion movement may also be active(Reference Yamamoto, Nakada and Zhao37). Furthermore, soluble fibre from other legume seeds (fenugreek) inhibited intestinal disaccharidase activity and increased gastrointestinal motility, which could also contribute to a reduced absorption of glucose(Reference Hannan, Ali and Rokeya38). Of interest is a study showing that SGLT1 can transport the flavonoid quercetin(Reference Wolffram, Block and Ader39) and inhibit glucose transport in a swine model(Reference Cermak, Landgraf and Wolffram40). These potential mechanisms were not investigated in the present study.
A benefit of feeding pea NSC was reduced stress on pancreatic islet cells. Feeding a HFD is well known to induce β-cell proliferation, with an overall expansion of β-cell mass(Reference Smith, Zhang and Clermont27). The degree of proliferation may depend upon the severity of hyperglycaemia because glucose stimulates β-cell mitosis(Reference Bonner-Weir, Deery and Leahy41). Conversely, a HFD also induce apoptosis and the degree to which islets enlarge is determined by cell proliferation minus cell death(Reference Bonner-Weir42). NSC-fed rats had smaller islets than rats in any other group. We conclude that reduced islet size is not due to increased apoptosis because the structure of the islets was otherwise normal, and both β- and α-cells stained less intensely for MDA, which is formed as a result of lipid peroxidation and a marker of oxidative stress(Reference Trevisan, Browne and Ram43). We also assessed NT staining in islets. Nitration of tyrosine in proteins occurs when NO and superoxide react together(Reference Schopfer, Baker and Freeman44). The present results suggest the effects of feeding NSC involve a reduction of lipid peroxidation but not production of NT. Although flavonoids from various sources and of several chemical classes have previously been shown to have both beneficial (for example, increased response to glucose) or detrimental (for example, increased apoptosis) effects(Reference Pinent, Castell and Baiges45), the present results suggest that flavonoids in the CSC group did not have any effect on β-cells.
Conclusions
Diets containing uncooked pea seed coats but not the embryos had beneficial effects on glucose tolerance in rats fed a HFD. NSC also reduced insulin secretion and this was associated with reduced expansion of the β-cell mass and MDA production, indicating reduced glycaemic stress on the islets. This outcome is potentially important because type 2 diabetes is caused by both insulin resistance and β-cell dysfunction, with the failure of the β-cells to adapt ultimately precipitating hyperglycaemia(Reference LeRoith46). The mechanisms by which pea seed coats exert their effects on blood glucose were not definitely identified but appeared to involve insulin signalling in skeletal muscle.
Acknowledgements
The present study was funded by grants from Alberta Innovates BioSolutions and the Alberta Pulse Growers Commission. Neither funder placed any restrictions on publication of the present study. K. A. W. and L. K. received stipend support from Alberta Innovates Health Solutions. The authors are grateful to D. O'Neill and Z. Fatehi-Hassanabad for technical support. C. B. C., C. J. F., R. C. B. and J. A. O. conceived of and designed the experiments. K. A. W., L. K., H. Y., C. H. and J. M. conducted the experiments and statistical analysis. A. J. prepared the pea fractions and performed seed fraction analysis. All authors participated in data interpretation. C. B. C. wrote the manuscript with input from K. A. W., C. H. and J. M., and C. J. F., R. C. B. and J. A. O. edited it. None of the authors reports any conflicts of interest.











