Introduction
The impacts of physical and chemical processing on the structure of food proteins are many and varied, each adding an extra level of complexity to the already complex relationship between protein structure and function. Food proteins are often denatured during processing, so the properties of both the native and denatured state need to be understood. In turn, any chemical derivatisation and the consequent changes in physical structure associated with protein aggregation (itself a complex phenomenon) may impact on the nutritional quality of the food. These complex relationships are illustrated schematically in Fig. 1. In this paper, we consider case studies to illustrate various aspects of these complex relationships. There are three themes, each of which is briefly introduced here, and will then be illustrated by published studies on wheat and soya proteins, together with new work on egg and dairy proteins.

Fig. 1 Possible interrelationships between food protein structure, protein chemical derivatisation and nutritional value in the final food.
The impact of the Maillard reaction on food proteins
The Maillard reaction is a complex cascade of chemical reactions, initiated by the deceptively simple condensation of an amine with a carbonyl group, often within a reducing sugar or fat breakdown product(Reference Fayle and Gerrard1). Of the many reaction products produced, some remain nutritionally available, but many do not, with the amino acid residue lysine being especially vulnerable(Reference Friedman2). Protein crosslinks form an interesting subset of the Maillard reaction products since, in addition to the loss of essential amino acids such as lysine, the covalently bound aggregate is thought to be less accessible to digestive enzymes(Reference Friedman3) reducing the digestibility of the protein as a whole. While the precise chemical structures of these crosslinks in food is not completely understood, their presence is well established and we, and others, have studied this phenomenon in wheat and soya foods(Reference Cottam and Gerrard4). Herein, we extend these studies to egg and dairy products.
The potential of enzymatic protein-protein crosslinking
Several enzymes have potential as crosslinking agents for use in food processing(Reference Cottam and Gerrard4). Transglutaminase, which catalyses the acyl-transfer reaction between the γ-carboxyamide group of peptide-bound glutamine residues and various primary amines(Reference Zhu, Bol and Rinema5), is of particular interest as the formation of this crosslink does not reduce the nutritional quality of the food(Reference Seguro, Kumazawa and Kuraishi6). The use of this enzyme has been the subject of a series of reviews, covering both the scientific and patent literature(Reference Friedman2, Reference Jaros, Partschefeld and Henle7–Reference Nielsen9) and is widely considered a versatile food processing aid. We have raised concerns about the use of this enzyme in foods containing wheat proteins, since its side deamidase activity may encourage generation of the epitope responsible for the celiac response upon digestion(Reference Gerrard and Sutton10). However, the risk is specific to wheat proteins and does not apply in other food systems.
Aggregation of proteins within a food: chemical and physical modification and the overall nutritional quality of the food
The complex pathways of protein misfolding and aggregation are increasingly well understood for individual proteins(Reference Luheshi, Crowther and Dobson11) and much of this knowledge can be interpreted in the context of a food matrix. In particular, the complex relationship between chemical derivatisation, protein aggregation and protein digestibility opens up new understandings about how food processing might impact on the fate of food in the gut. Early studies on Maillard protein crosslinking examined the digestibility of a model protein following glycation, observing up to a 30 % reduction in the digestibility of lysozyme by a pepsin-pancreatin solution after glycation with crosslinking agents(Reference Kato, Chuyen and Utsunomiya12); the digestion process can also be inhibited by the Maillard reaction(Reference Friedman3). However, the molecular details of these processes need to be accurately understood to establish the role of chemical derivitisation per se and the subsequent impact on aggregation pathways, some of which may, in themselves, reduce accessibility of digestive enzymes. Herein we present preliminary data which begin to probe these relationships for a model egg white system.
Experimental methods
Methods for much of the work described in this review have been previously published. Only unpublished methods are included in this section.
Materials
Unless otherwise stated, all chemicals, reagents and solvents were obtained from Sigma–Aldrich Ltd. (Auckland, New Zealand) or BDH Chemicals Ltd. (Palmerston North, New Zealand) and were of analytical grade. Whey protein isolate (WPI) was WPI895 obtained from Fonterra Research Centre (Palmerston North, New Zealand). Ovalbumin was purified from day-fresh hen egg white by anion exchange chromatography(Reference de Groot and de Jongh15).
In vitro incubations
The methods employed for incubations were based on previously described methods(Reference Fayle, Gerrard and Simmons16–Reference Yasir, Bin and Sutton18). For egg white samples, fresh raw hen egg white was homogenised and centrifuged. The supernatant was used as a stock solution for all experiments. Incubations of egg white protein were carried out at varying concentrations, at 80–90°C, with 200 mM of the following Maillard reaction partners: glucose, fructose, lactose, glutaraldehyde, methylglyoxal. Egg white samples incubated in dH2O or in 200 mM NaCl served as controls. Whey protein isolate – WPI895 (10 mg/mL) was incubated at 50°C with varying concentrations of glutaraldehyde. Each of the samples was made up to a volume of 1 mL to allow excess sampling. The samples were removed from incubation at specified times frozen in liquid nitrogen and stored at − 80°C. The zero-time sample and a whey protein frozen control were first dipped in liquid nitrogen before being stored at − 80°C. A whey protein control (i.e. no crosslinking agent added) was also incubated for the duration of the experiment.
Model food systems
The standard procedure for tofu(Reference Yasir, Bin and Sutton14) and croissant(Reference Gerrard, Newberry and Ross19, Reference Gerrard, Fayle and Brown20) manufacture was as previously described. For the model dairy food, an acid-gel system was used. Two protein solutions were made up, the first a 20 % (w/w) skim milk solution and the second a 2·22 % total solids solution of WPI. Equal amounts of each solution (20 g) weighed out into separate containers plus sufficient glutaraldehyde to reach the required concentration (0·0125 mM–5 mM) were then added to either one or both of the solutions as required. The samples were then stirred for 1 minute, transferred to glass vials, and heated in a water bath at 50°C for 30 minutes. Fifteen g of each of the two protein solutions were then combined and stirred for 1 minute and heated for 30 minutes at 80°C in an oil bath. Twenty g of this heated sample were then weighed into a container and acidified with GDL (glucono-δ-lactone) to simulate yoghurt manufacture. The rheological properties of the acid-gel samples were determined using an Anton Paar MCR301 Rheometer (Anton Paar, Graz, Austria) and in-house methods. The milk samples were monitored for approximately 4 hours, using a 4°, 4 cm diameter cone and plate geometry for all experiments. The required level of GDL (glucono-δ-lactone) was added to the sample, the sample was then stirred for 30 seconds and 1·2 mL was transferred to the rheometer plate and the cone lowered to give the required gap between the cone and the plate. Three sequential tests were performed in each experiment, where the initial measurements involved monitoring gelation as the milk samples were acidified. Firstly a dynamic time sweep was employed, where a strain of 0·1 % at an oscillation frequency of 0·1 Hz was used. The temperature of the sample was maintained at a constant 30°C with measurements taken every 60 seconds for the next 3 hours to determine G’ (storage modulus). These initial measurements involved monitoring gelation as the milk was acidified. After the time sweep was complete, a temperature sweep of the set gel was performed where the temperature was reduced from 30°C to 5°C at a rate of 1°C/15 sec while monitoring the rheological properties at 1°C intervals. After the frequency sweep was complete, the deformation rheological properties of the set acid gels at 5°C were monitored by progressively increasing the strain at a constant shear rate of 0·005 s− 1. The stress was monitored until the gel structure yielded, with measurements taken every second for 5 minutes. In this manner, the fracture stress and fracture strain of the set gel were obtained.
Analytical methods
The OPA (o-phthaldialdehyde) method was used to measure the lysine availability in water-soluble proteins based on a method from Bertrand-Harb(Reference Bertrand-Harb, Nicolas and Dalgalarrondo21). Polyacrylamide Gel Electrophoresis (PAGE) was carried out as previously described(Reference Gerrard, Fayle and Wilson13, Reference Yasir, Bin and Sutton14). Scanning Electron Microscopy (SEM) was carried out as previously described(Reference Kang22, Reference Goldstein, Newbury and Echlin23). The egg white samples were prepared by adding 200 μL of buffer containing 500 mM cross-linker (controls dH2O and 500 mM NaCl) to 800 μL of day 0 fresh hen egg white. The samples were mixed and then incubated at different temperatures. The samples were then analysed in the same way as the tofu samples, with up to five replications for each treatment.
Differential scanning fluorimetry (DSF)
DSF was used to analyze ovalbumin stability by measuring the melting temperatures of the purified ovalbumin. One μL of the fluorescent SYPROR Orange Gel Stain (Sigma-Aldrich) (250 × concentrated) was used with 4 μL of protein sample (1 mg/mL) and 20 μL of buffer. Effects on melting point changes upon incubation of buffered ovalbumin with glucose, fructose, lactose, glutaraldehyde, methylglyoxal were assessed, with NaCl and dH2O as controls. Melting points were measured at ten different protein concentrations (0·2–200 mM final concentration), referenced to a solution containing appropriate amounts of buffer, dye and water. All measurements were made in triplicate and the readings were averaged. Data were recorded on a BioRad IQ5 Multicolor Real-Time PCR Detection System with iCycler (BioRad). For fluorescence measurements, 96 flat well optical plates (Sarstedt) were used with 236707 sealing tape (Nunc™). The plate was sealed and heated from 20°C to 95°C in increments of 0·2°C, with a 10 second dwell time at each temperature. Fluorescence changes were monitored simultaneously with a charge-coupled device camera. The wavelengths for excitation and emission were 490 and 575 nm, respectively and melting temperatures were determined as the maximum point of inflection using dRFU/dt(Reference Ericsson, Hallberg and DeTitta24).
Small Angle X-ray Scattering (SAXS)
Scattering experiments of treated ovalbumin samples were carried out at the Australian Synchrotron in Melbourne. For the beamline setup, a camera length of 3334·83 mm was chosen and the experiments were carried out at a wavelength of 1·03 320Å. A Pilatus M1 detector (981 × 1043 pixels) was used. The measured q-range (q = 4*π*sin( ∇ )/λ)) was 0·005–0·287Å− 1. Ovalbumin samples of different concentrations were loaded into a glass capillary and blanked against the respective buffers containing no protein. An exposure time of 20 × 1 second was chosen.
Digestibility assays
An in vitro model of human digestion was used to study protein digestibility, based on previous studies(Reference Fu, Abbott and Hatzos25–Reference Pharmacopeia27). Firstly, egg white samples were subjected to simulated mastication in a glass homogeniser. To 560 μL of dH2O, 50 mg of homogenised sample were added and mixed. Subsequently amylase digestion was carried out to mimic polysaccharide breakdown in the mouth by adding 2 μL of a 10 % amylase stock (Sigma No.: A3176) and incubation for 5 minutes at 37°C. The pH was then adjusted to 1·5 by adding 20 μL 1 M HCl to simulate the acidic stomach environment. Following acidification, 20 μL of 10 % porcine pepsin (Sigma No.: P7000) stock solution was added to the sample and the sample was incubated for 30 minutes at 37°C. Samples were then neutralised using 40 μL of NaHCO3 to stop any further pepsin digestion. To simulate small intestinal digestion, porcine pancreatin (Sigma No.: P1750) was chosen. The pH of the sample was adjusted to 7·5 using 200 μL of 167 mM KH2PO4 pH 7·5. Subsequently 100 μL of 2·5 % pancreatin stock solution was added and samples were digested for 2 hours at 37°C. Aliquots were taken after each digestion step and analysed using PAGE to analyse the amount of protein digested.
Results and Discussion
The impact of the Maillard reaction on food proteins
We have previously published research on the impact of the Maillard reaction, and particularly the resulting protein crosslinking, on food texture in wheat and soya foods. Introduction of protein crosslinks into baked products has been shown to improve a number of properties that are valued by the consumer(Reference Gerrard, Fayle and Wilson13, Reference Gerrard, Newberry and Ross19). In situ studies revealed that following addition of glutaraldehyde to dough, the albumin and globulin fractions of the extracted wheat proteins were crosslinked(Reference Gerrard, Brown and Fayle28). Inclusion of glutaraldehyde during bread preparation resulted in the formation of a dough with an increased dough relaxation time, relative to the commonly used flour improver ascorbic acid(Reference Gerrard, Brown and Fayle29). These results confirmed that chemical crosslinks are important in the process of dough development, and suggest that they can be introduced via Maillard type chemistry.
In tofu, Kaye et al. (Reference Kaye, Easa and Ismail30) reported that following incubation with glucose, a Maillard network formed within the internal structure of tofu, resulting in a loss in tofu solubility and a reduction in tofu weight loss. Furthermore, Kwan and Easa(Reference Kwan and Easa31) employed low levels of glucose for the preparation of retort tofu, which resulted in the production of firmer tofu product. Changes in tofu structure have also been observed in our laboratory when including glutaraldehyde, glyceraldehyde or formaldehyde in tofu preparation(Reference Yasir, Bin and Sutton14, Reference Yasir, Bin and Sutton18). However, these results suggested that protein crosslinking agents may change the functional properties of tofu via non-crosslinking modifications of the sidechains of the amino acid residues, perhaps by changing their isoelectric point and gelation properties(Reference Fayle, Healy and Brown32). This illustrates the challenge of attributing specific impacts on the quality of food with particular introduced changes, especially when using chemistry as complicated as Maillard chemistry, which results in many different products. Nevertheless, the impact of the Maillard reaction on both the ultrastructure of the tofu and the functional properties, as monitored by fracture force measurements, was clearly evident (Fig. 2). The extent to which these changes impact on the nutritional qualities of the tofu or wheat based products has yet to be investigated.

Fig. 2 Treatment of soya milk with glutaraldehyde during the preparation of tofu changes the fracture force and the ultrastructure of the final product. (a) and (b) represent micrographs of 1 and 2 mM glutaraldehyde added before soyamilk boiling. (c) represents the control. (d) and (e) represent micrographs of 1 and 2 mM glutaraldehyde added after soymilk boiling. The images are representative of five replicate experiment. The scale bar represents 10 μm. For more detail, refer to Yasir et al. (Reference Yasir, Bin and Sutton14, Reference Yasir, Bin and Sutton18).
The covalent polymerisation of milk during food processing has also been reported(Reference Singh and Latham33). This phenomenon was shown to be sugar dependent, as determined in model studies with β-casein. Further, pentosidine formation paralleled protein aggregation over time at 70°C(Reference Pellegrino, van Boekel and Gruppen34), suggesting that Maillard chemistry plays an active role. Recent results in our laboratory are illustrated in Fig. 3, in which whey protein isolate was incubated with various concentrations of glutaraldehyde and the resulting conjugates analysed by PAGE. Loss of lysine residues was assessed using the OPA method. Results indicated that 1 % whey protein isolate incubated with 2 mM glutaraldehyde at 50°C, pH 7 sustained a loss of 20 % of the available lysine residues during the course of the incubation, which is consistent with the crosslinking pattern observed (Fig. 3). When this Maillard chemistry takes place in a model food system, such as an acid gel (Fig. 4), then there is a clear change in the functional properties of the proteins in solution. In particular, the storage modulus is seen to decrease with an increased concentration of glutaraldehyde. This pattern could either be attributed to the crosslinking pattern observed in Fig. 3 or, analogous to the tofu case, may correspond to changes in physicochemical properties resulting from non-crosslinking protein glycation. Further work is underway to investigate these possibilities.

Fig. 3 SDS PAGE analysis of crosslinking of milk proteins. Lanes 1/2, WPI controls containing no glutaraldehyde, non heated (lane 1), heated for the duration of the experiment (lane 2). Lane 3 – WPI+50 mM glutaraldehyde; lane 4 – WPI+20 mM glutaraldehyde; lane 5 – WPI+10 mM glutaraldehyde; lane 6 – WPI+6 mM glutaraldehyde; lane 7 – WPI+5 mM glutaraldehyde; lane 8 – WPI+4 mM glutaraldehyde; lane 9 – WPI+2 mM glutaraldehyde; lane 10 – WPI+1 mM glutaraldehyde.

Fig. 4 Plot of gelation curves obtained with the whey protein solution (WPI) pre-crosslinked with glutaraldehyde in acid-gel experiments. Storage modulus (G′) as a function of time (hrs). ![]() WPI Control (no glutaraldehyde),
WPI Control (no glutaraldehyde), ![]() WPI (0·125 mM glutaraldehyde),
WPI (0·125 mM glutaraldehyde), ![]() WPI (0·25 mM glutaraldehyde),
WPI (0·25 mM glutaraldehyde), ![]() WPI (0·5 mM glutaraldehyde),
WPI (0·5 mM glutaraldehyde), ![]() WPI (1 mM glutaraldehyde),
WPI (1 mM glutaraldehyde), ![]() WPI (2 mM glutaraldehyde).
WPI (2 mM glutaraldehyde).
The potential of enzymatic protein-protein crosslinking
The enzyme transglutaminase has been explored in many different foods. We have investigated its potential as an additive in wheat-based foods, and found a particularly dramatic improvement in the quality of croissant pastry was afforded by the addition of a commercial preparation of the enzyme (Fig. 5). An analysis of the proteins extracted from the pastry of croissants treated with transglutaminase showed an average of 5 % lysine residues lost, with a small degree of crosslinking observed in the albumin and globulin and insoluble glutenin fractions (Fig. 5). From this and other studies(35), it was concluded that only a few crosslinks in specific protein fractions were sufficient to confer the improvement in pastry quality(Reference Gerrard, Newberry and Ross19).

Fig. 5 Transglutaminase has a dramatic impact on croissant pastry. In this image, the control croissant is made to a standard recipe whilst the croissant on the left is made to the same recipe, with the addition of transglutaminase. Unfortunately, doubts have been cast on the safety of transglutaminase for the use in wheat-based products. For more details refer previous paper(Reference Gerrard, Newberry and Ross19, Reference Gerrard and Sutton10).
Later work by our group and others has demonstrated that soya products may also benefit from the introduction of transglutaminase. The enzyme is reported to enhance the quality of tofu made from old soya crops, giving a product with increased water holding capacity, a good consistency, a silky and firmer texture and one that is more robust in the face of temperature change(Reference Kuraishi, Yamazaki and Susa36, Reference Soeda37). Transglutaminase has also been used to incorporate soya protein into new products, such as chicken sausages(Reference Muguruma, Tsuruoka and Katayama38). In our work, treatment with transglutaminase produced a firmer tofu, with a significantly increased fracture force. SEM revealed that the microstructures of the samples were consistent with the changes in fracture force, in an analogous manner to the glutaraldehyde treated tofu, shown in Fig. 2(Reference Yasir, Bin and Sutton14, Reference Yasir, Bin and Sutton18). The firmness varied according to both the concentration of enzyme added and the point of addition in the manufacturing process, suggesting opportunities for customising tofu by simple alterations in the timing of transglutaminase addition. However, when proteins were extracted from the tofu matrix after treatment with transglutaminase in situ, little, if any, cross-linking occurred as assessed by PAGE (Fig. 6). This suggested that the change in functional properties afforded by addition of transglutaminase to tofu is perhaps due to a side reaction of the enzyme, hydrolysis of glutamine residues to glutamate, as previously postulated by Babiker(Reference Babiker39). Since water is abundant during tofu manufacture, it is likely that transglutaminase-promoted hydrolysis of glutamine residues of the protein to glutamate residues could occur. The resulting change in isoelectric point of the soya proteins would be predicted to change the gelation and microstructure of the proteins(Reference Dickinson40). It would also suggest that in food systems where the side reaction out-competes the crosslinking reaction, the nutritional quality of the food is likely to be diminished, in contrast to the work of Seguro et al. (Reference Seguro, Kumazawa and Kuraishi6), where the lysine residue was protected from Maillard chemistry by the crosslinking process, and later released as a bioavailable residue. Further work is needed to test this hypothesis in this, and other, systems in which side reactions of transglutaminase are implicated as important for changing the properties of food(Reference Gerrard and Sutton10).

Fig. 6 SDS-PAGE analysis of proteins extracted from croissant pastry. Lane 1, molecular weight markers. Lanes 2/3, albumin and globulin fraction with/without transglutaminase; lanes 4/5, gliadin fraction with/without transglutaminase; lanes 6/7 soluble glutenin fraction with/without transglutaminase; lanes 8/9 insolble glutenin fraction with/without transglutaminase. Arrows highlight crosslinked proteins that correspond to an increase in product quality. For more details see Rasiah et al. (Reference Rasiah, Sutton and Low35).
Aggregation of proteins within a food: chemical and physical modification and the overall nutritional quality of the food
The results thus far presented in sections 1 and 2 hint at a relationship between chemical modification of protein side chains and the physical process of protein aggregation. Either or both of these may impact on both food structure and the nutritional quality of the food in question. To examine these relationships (Fig. 1), we are exploring model egg white proteins. Specifically, we aim to unravel the relationship between chemical modification, aggregation and nutritional quality. Some preliminary data from early experiments are presented here. In Fig. 7, SEM micrographs of egg white treated with various reaction partners are presented. Some marked differences were observed from the control protein, especially upon treatment with glutaraldehyde which produces an open mesh-like structure compared to the control (Fig. 6D).

Fig. 7 SEM micrographs of egg white in the presence of different glycation partners and controls. From top left to bottom: Egg white incubated for 5 h in the presence of (a) 100 mM glucose, (b) 100 mM fructose, (c) 100 mM lactose, (d) 100 mM glutaraldehyde, (e) 100 mM methylglyoxal, (f) 100 mM NaCl, (g) water. The scale bar is 10 μm.
This change in structure is concomitant with a change in the melting temperatures of the derivatised protein, as seen in Table 1. It is interesting to note that the two most effective crosslinking agents(Reference Meade, Miller and Gerrard41) also produce the lowest melting temperature for the proteins.
Table 1 Melting temperature (TM) for ovalbumin incubated with a variety of crosslinking agents during the melting experiment from 20°C to 95°C. Measurements were made via differential scanning fluorimetry according to the method of Ericsson et al. 2006
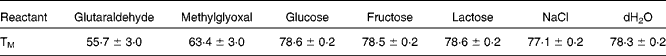
To probe the impact of chemical modification on physical aggregation, we have initiated a small angle x-ray scattering study on the early stages of ovalbumin aggregation, with and without modification by a Maillard reactive molecule. Figure 8 shows the results of these early experiments, illustrating that modification by methyl glyoxal alters the scattering data, indicative of an altered aggregation pathway and validating the technique as a method to explore these differences. Further analysis is underway to inform future experiments to reveal specific changes to particle size as a function of modification and time.

Fig. 8 SAXS experiments. Scattering intensity plotted against q at five different protein concentrations ranging from 0·8 mg/mL to 45·5 mg/mL. Ligands are held constant at 100 mM. (a) water, (b) methylglyoxal, (c) glucose, (d) NaCl. The predominant distances in the ovalbumin samples in the presence of water or glucose decrease significantly in the NaCl and methylglyoxal samples. This suggests different molecule behaviour in the presence of different possible ligands.
In concert with these measurements, a parallel study of digestibility of ovalbumin is being undertaken, some results from which are illustrated in Fig. 9. In the first instance, different cooking methods were employed and the digestibility of the samples compared. In agreement with literature data(Reference Evenepoel, Geypens and Luypaerts42–Reference Van der Plancken, Delattre and Indrawati45), all cooking methods rendered the egg white protein more digestible than the raw protein (data not shown). Repeating these experiments with Maillard reactive reagents reveals some greater variation, and in particular the crosslinked samples are harder to digest, although quite harsh conditions are required before these differences are manifest. These observations are consistent with previous reports(Reference Valle-Riestra and Barnes46) and future work aims to unravel whether these differences are due to the chemical or physical modifications to the protein.

Fig. 9 Egg white + crosslinkers heated at 90°C for (a) 0 hours, (b) 5 hours, (c) 24 hours and then subjected to an in vitro model of human digestion. 1–8: 1) protein marker, 2) egg white +200 mM glucose, 3) egg white +200 mM fructose, 4) egg white +200 mM lactose, 5) egg white +200 mM glutaraldehyde, 6) egg white +200 mM methylglyoxal, 7) egg white + dH2O, 8) egg white +200 mM NaCl. The protein smear in the lanes corresponds to crosslinked, non digested high molecular weight protein. The visible distinct bands are enzymes from the pancreatin mixture and correspond to amylase, trypsin, chymotrypsin.
Concluding comments and future directions
The various case studies presented here illustrate the complexities in the relationship between chemical and physical modification of food proteins, and their potential effects on digestion. Protein crosslinking, whether chemically or enzymatically induced, is likely to reduce digestibility, although in the particular case of transglutaminase-catalysed crosslinking the reverse situation may be true in some food systems. In this case, transglutaminase treatment is likely to improve nutritional quality, especially in cases where high reducing sugar content may otherwise lead to loss of lysine residues through Maillard chemistry.
The Maillard reaction of food proteins, or protein glycation, involves a large number of non-crosslinking products, many of which may have an impact on both the nutritional and functional properties of food.
In general, the interplay between chemical modification of proteins and their physical aggregation properties and how this might impact on food quality, in terms of both sensory qualities and nutritional value, warrants further investigation. Methods such as mass spectrometry and SAXS, in tandem with in vitro and in vivo assays of protein digestibility may prove very valuable in untangling these relationships.
Acknowledgements
This work has been funded from a variety of sources over several years, including the following contracts: Dairy Ingredient Solutions for Nutritional Vitality (DRIX0801); Bakery Products & Beverages (C02408); Advanced Biological Materials (C02X0001, C02X0204); “Cereal Based Food” (C02803); scholarship to SY from the Malaysian government and the Riddet Institute, Palmerston North, New Zealand. JAG: project leader, wrote manuscript; NGL: co-project leader; ML: carried out egg work and SAXS experiements; JC: carried out acid gel experiments on milk proteins; JPH: assisted with electrophoretic analysis and lab management; SEF: completed early work on wheat proteins, including croissant experiment; IR: completed later work on wheat proteins using enzymatic crosslinking; PKB: completed later work on wheat proteins using Maillard chemistry; SY: completed work on soy proteins; KHS contributed to supervision of several students and provided expertise in protein analysis. The authors state that there are no conflicts of interest.












