Colon cancer
A study of the immune populations involved
Colon cancer inhibition by yoghurt containing live microorganisms was studied in an experimental model using BALB/c miceReference Perdigón, Valdez and Rachid1. Animals were fed with yoghurt for 10 consecutive days. Colon tumours were chemically induced by dimethylhydrazine (DMH) injection and the animals were given yoghurt cyclically again after tumour induction (for ten consecutive days followed by a one week break and then again for ten days) until the end of the experiment (six months). Yoghurt feeding inhibited tumour growth (yoghurt-DMH-yoghurt group). In this experimental model, a large inflammatory immune response was observed during tumour development in the large intestine in the mice treated only with DMH (DMH group). The inflammatory response was observed histologically by identification of infiltrating immune cells in the intestinal wall. The administration of yoghurt to DMH-injected mice resulted in an increase in the number of IgA-secreting cells and CD4+T lymphocytes in the lamina propria of the large intestine together with a decrease in the IgG+ and CD8+ cellsReference Feghali and Wright4. The increase in the number of IgA+ secreting cells but not of IgG+ secreting cells in the large intestine of the mice fed with yoghurt could limit the inflammatory response, since IgA is considered as an important barrier in colonic neoplasiaReference Isaacson2.
Relationship between inflammation and tumour
The association of chronic inflammation with several malignant diseases has been reported for a long timeReference Prescott and Fitzpatrick3. There is also evidence that this relationship is mediated by cytokinesReference Feghali and Wright4 or by reactive oxygen species generated by inflammatory phagocytes that can cause injury to target cells, contributing to cancer developmentReference Weitzman and Gordon5. As regards the relationship between inflammation and tumours, several drugs such as the non-steroidal anti-inflammatory drugs (NSAID), which are inhibitors of the cyclooxygenase enzyme (COX), can delay or prevent the development and metastatic spread of certain cancersReference Fulton6, Reference Lala, Parhar and Singh7, Reference Reddy, Maruyama and Kelloff8. Based on the results obtained with yoghurt in DMH-injected mice, we analysed the effect of an NSAID (indomethacin) on colon cancer in order to compare it with the anti-tumour activity of yoghurt (DMH-indomethacin group). Histological observations showed that, during indomethacin administration, the immune cells that infiltrated the large intestine wall were smallerReference de Moreno de LeBlanc, Valdéz and Perdigón9, and differed from those observed with the yoghurt feeding (yoghurt-DMH-yoghurt group). Cellular infiltration also occurred in the large intestine of long-term yoghurt-fed mice, suggesting that these infiltrative cells could explain part of the anti-tumoural and anti-inflammatory activity of yoghurt.
During the fourth month of the indomethacin treatment, the histology of the large intestine from this group of mice was similar to the non-treatment control group. When indomethacin administration was stopped due to cachexia in the animals, the tumour developed with the same characteristics as in the DMH group in the sixth monthReference Perdigón, de Moreno de LeBlanc, Valdez and Rachid10.
This last observation suggests different mechanisms for indomethacin and yoghurt, since when the yoghurt feeding was stopped at the end of the experiment (six months), the animals from the yoghurt-DMH-yoghurt group, which were observed until the ninth month, did not develop tumours.
Cytokine profiles
The studies concerning the cell population in this model allowed us to suggest the possible anti-inflammatory role of yoghurt to account for its anti-tumour activityReference de Moreno de LeBlanc, Valdéz and Perdigón9 which would be different to indomethacin's mechanism of action. Since cytokines are the biological messengers for the regulation and modulation of the immune response, they are a potential target for the study of the mechanisms induced by LAB or yoghurt to modulate the immune response.
Different cytokines were studied using immunofluorescent methods on large intestine slices from the different groups of mice. Proinflammatory cytokines (TNF-α and IFN-γ) increased in the cells from the large intestines of the tumour control mice (DMH group, Fig. 1) and in the yoghurt-DMH-yoghurt group (Table 2). Yoghurt-fed mice also had high levels of these proinflammatory cytokinesReference de Moreno de LeBlanc, Valdéz and Perdigón9 Mice injected with indomethacin presented fewer infiltrating cells in the large intestine with a low number of positive TNF-α and IFN-γ secreting cells. When the drug treatment was stopped, both cellular and proinflammatory cytokines increased and the tumour grew (Fig. 1).
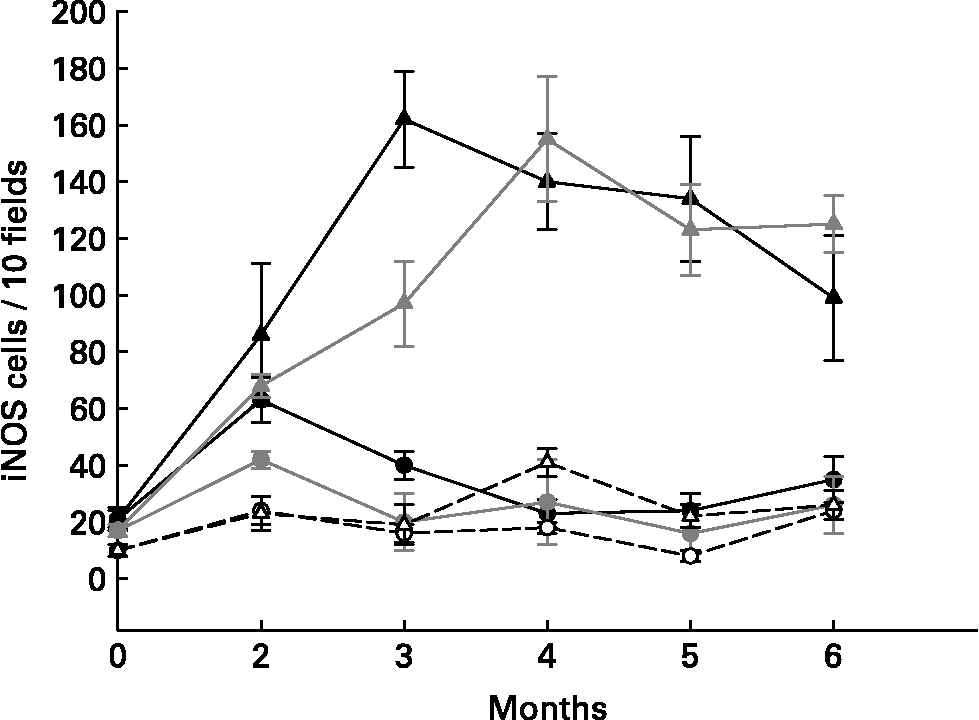
Fig. 1 Comparative study of mice that were injected with DMH and treated (circles) or not (triangles) with indomethacin. TNF-α+ (gray), IFN-γ+ (black) secreting cells and iNOS enzyme (white) were analysed by immunoflourescence in tissues from large intestine.
For the purpose of demonstrating that the proinflammatory cytokine increase in the large intestine from mice fed yoghurt was not related to the development of gut inflammatory responses and that this cytokine response was being regulated, the nitric oxide synthase enzyme was studied. The inducible oxide nitric synthase enzyme (iNOS) was evaluated in slices from the large intestine of different groups of mice (Table 1 and Fig. 1). the iNOS enzyme is induced during the course of the immune response by microbial products and/or cytokines and plays a role in the antimicrobial mechanism of macrophagesReference Bogdan, Rollinghoff and Diefenbach11. One of the Th1 mechanisms mediated by IFN-γ is iNOS induction.
Table 1 Comparative study of the cell populations in mice from colon cancer model

Cells were analysed by indirect immunofluorescence on large intestine tissues. Results are expressed as number of positive cells for the corresponding cytokine or protein in 10 fields of vision as seen at 1000 × magnification using a fluorescence light microscope.
Values for each cytokine, iNOS or Bcl-2 positive cells without a common letter differ significantly (P < 0·05).
Y-DMH-Y = yoghurt-DMH-yoghurt; ND = Not determined.
Tumour bearing mice (DMH group) were shown to present high amounts of iNOS+ cells (Table 1), suggesting an increase in nitric oxide (NO) production by these cells. The iNOS enzyme synthesis could be induced by IFN-γ which was increased in the intestinal tissue from the DMH group. In the DMH group fed with yoghurt, when the inflammation decreased, the iNOS+ cells were also depleted (Table 1). Long-term yoghurt administration showed an iNOS+ cell number similar to that of the non-treatment control group throughout the experiment. The DMH-indomethacin group showed an increased number of iNOS+ cells at the beginning of the anti-inflammatory treatment and at the end of the study, when the tumour grew. iNOS+ cells increased according to the IFN-γ+ secreting cell increase observed in the DMH-indomethacin group (Fig. 1)
The lack of iNOS enzyme induction in the yoghurt-DMH-yoghurt and yoghurt control groups may show the pathway through which yoghurt may regulate the immune system by modulating the inflammatory response. In spite of the increased number of IFN-γ+ secreting cells, these animals did not increase NO production and consequently tumour growth was not observed, only cellular infiltration. Therefore, we suggest that the large number of positive cells in mice fed yoghurt is related to the increased number of immune cells number observed in the intestine; IFN-γ being regulated by other cytokines.
We also analysed a regulatory cytokine (IL-10) that could be involved in the immune response of the mice fed with yoghurt since, unlike the DMH group, the increased pro-inflammatory cytokine positive cells were not related to the inflammation and tumour development observed in those animals.
In our model, the number of IL-10+ secreting cells increased significantly in all the samples from the three experimental groups (DMH, yoghurt-DMH-yoghurt and yoghurt) (Table 1) It is important to remark that there were more IL-10+ secreting cells in the yoghurt-DMH-yoghurt group than in the DMH groupReference de Moreno de LeBlanc, Valdéz and Perdigón9. In view of these results we suggest that the immune mechanisms through which yoghurt may act may be different from those induced by the anti-inflammatory drug (indomethacin), which did not lead to an increased activity of the infiltrating immune cells in the large intestine, where cytokine levels were lower than in the other groups and iNOS diminution was only evident during treatment. Yoghurt exerted its anti-tumour activity by an anti-inflammatory activity. It appears that yoghurt could modulate the immune response by 1) stimulating cytokine production when this is required, or 2) inducing down-regulation of the immune cells to avoid an exacerbated immune response. This effect would occur mainly through IL-10, which was increased in the tissue during all the periods tested.
Breast cancer
Considerable advances have been made in recent years in understanding the molecular factors involved in breast cancer development. There are genetic and environmental factors that increase the chances of breast cancer and the most common breast cancer types are oestrogen-dependent. Some factors, such as diets rich in cultured dairy products, may inhibit the growth of many types of cancer, including breast tumours.
In addition to LAB, fermented milks can possess non-bacterial components produced during fermentation that may contribute to their immunogenicity and to properties such as their anti-tumour activities. Matar et al. Reference Matar, LeBlanc, Martin, Perdigón and Farnworth12, reported different roles and functions of biologically active peptides released from fermented milks. Peptide fractions liberated during milk fermentation with Lactobacillus (L.) helveticus R389 stimulated the immune system and inhibited the growth of an immuno-dependent fibrosarcoma in a mouse modelReference LeBlanc, Matar, Valdez, LeBlanc and Perdigón13. The peptide profiles of milk proteins were significantly different after fermentation by LAB, suggesting that microbial proteolysis could be a potential source of bioactive peptidesReference Matar, Amiot, Savoie and Goulet14. Milk fermented by L. helveticus R389, a bacterium with high protease and peptidase activity, exerted an anti-mutagenic effect while a mutant strain (L89), deficient in proteolytic activity, did notReference Matar, Nadathur, Bakalinsky and Goulet15. Likewise, milk fermented with the proteolytic strain increased the number of IgA+ secreting cells in the small intestine as well as in the bronchus of mice, but fermented milk obtained with the proteolytic deficient mutant strain did not show the same in vivo resultsReference Matar, Valdez, Medina, Rachid and Perdigón16.
The aim of this work was to study the effects of the consumption of milk fermented by L. helveticus R389 or its proteolytic deficient variant, L. helveticus L89, on a murine hormone-dependent breast cancer model, studying the systemic and local immune responses in the mammary glands and tumours.
Mice were fed with milk fermented by L. helveticus R389 (P+) or L89 (P − ), for 2 or 7 d. The ATCC tumoural cell line 4T1 was used to induce breast tumour growth. Each mouse was challenged by a single subcutaneous injection (0·5 mL) of tumour cells (1·4 × 104 cells/mL) in the upper right mammary gland. The experimental groups: 1) P(+)2d, 2) P( − )2d, 3) P(+)7d and 4) P( − )7d, were fed a diet supplemented with milk fermented by L. helveticus R389 or L89, for 2 or 7 consecutive days, respectively. At the end of each feeding period mice were injected with the tumour cells in the same way that the tumour control animals (which were not fed fermented milk) had been. Four days after the tumour injection, fermented milks were added again to the diet for 2 or 7 consecutive d (depending on the group), followed by a 5 d break, and then added again for a further 2 or 7 d. Feeding was done in this manner cyclically until the end of the experiment (28 d after tumour induction).
Mice receiving two d cyclical fermented milk feeding did not show significant differences in tumour volume, compared to the tumour control group. Seven d cyclical administration of both bacterial strains delayed or stopped tumour development. There were no significant differences between both bacterial strains used in milk fermentation either 2 or 7 d cyclical feeding17.
Cytokines have been shown to regulate oestrogen synthesis in breast tumours, stimulating research questions around these important molecules. Therefore, we evaluated cytokines in different samples to have a spectrum at a systemic level, and to measure the local response in mammary glands or tumours to study the effect of the fermented milks cited above on the immune response.
Serum TNF-α levels increased as a function of time, as did the tumour volume in the control groupReference de Moreno de LeBlanc, Matar, LeBlanc and Perdigón17. Mice receiving cyclical feeding with milk fermented by L. helveticus R389 or L89 for 7 days showed a significant increase of TNF-α in the basal sample, compared to the tumour control group. This increase prior to tumour induction could be related to the decrease of tumour growth. The P(+)7d group maintained the TNF-α concentration at close to the basal level throughout the trial, demonstrating a regulation of this cytokine, whereas the P(-)7d group showed increased TNF-α in the last sample (similar to the control group), which is considered a typical immune response to the tumourReference de Moreno de LeBlanc, Matar, LeBlanc and Perdigón17.
IL-6 is a cytokine implicated in oestrogen synthesisReference Purohit, Newman and Reed18, a hormone that the tumour needs to grow. It is also a pro-angiogenic factorReference Urban, Shepard, Rothstein and Sugarman19, supporting the growth of new blood vessels that are essential for tumour growth. The three groups in which the tumour grew at a faster rate showed elevated levels of IL-6. However, the P(+)7d and P( − )7d groups did not show increased levels of this cytokine during the study, suggesting that this IL-6 decrease could be involved in one of the mechanisms for tumour growth delay.
Serum IL-10 levels were significantly increased in the P(+)7d group in relation to the tumour control group, beginning at day 18 after tumour injection, which could explain the regulation of the immune response observed for TNF-α and IL-6 in these animals. This regulatory response was not observed in the P(-)7d group.
Study of cytokine-positive cells in mammary gland tissues
Systemic cytokine levels were different depending on the tumour growth. In fact, a regulatory immune response was observed in the P(+)7d group, but not in the P( − )7d group.
The study of cytokine-positive cells in mammary glands furthered an understanding of the local cell response, after mice were fed with fermented milk as well as after tumour injection, in tumour control and different test groupsReference de Moreno de LeBlanc, Matar, LeBlanc and Perdigón17.
TNF-α secreting cells in mammary glands showed very similar patterns to those obtained for this cytokine level in serum (Table 2).
Table 2 Cytokine positive cells in mammary glands or breast tumour

* For mammary gland tissues, cytokine-positive cells were analysed using indirect immunofluorescence. Results are expressed as means and SD of cytokine-positive cells counted in 10 fields of vision at 1000 × of magnification.
** For cells isolated from tumour, cytokine-positive cells were analysed by immunoperoxidase technique and results are expressed as means and SD of cytokine-positive cells each 100 counted cells (cells/100). Means for each cytokine and for tissue or isolated cells without a common letter differ significantly (P < 0·05).
ND = Not determined.
IL-6 secreting cell numbers were constant and similar in all groups until 18 days after tumour injection. This result can be explained due to the relationship between this cytokine and oestrogen synthesis in the mammary gland; oestrogens being essential to promote proper growth of this tumour cell line. These cytokine-positive cells increased 18 days after tumour cell injection, in the control, P(+)2d and P( − )2d groups, whereas IL-6 secreting cell numbers remained unmodified in both P(+)7d and P( − )7d groups and showed significantly lower levels in relation to the other groups (Table 2).
Mice fed L. helveticus R389 showed increased IL-10 secreting cell numbers throughout the time of the entire study, but only the P(+)7d group showed significantly higher numbers compared to the tumour control on days 18 and 22 (Table 2). This cytokine can be related to the regulation of the other cytokines observed in this group, where increases in TNF-α secreting cell numbers in mammary glands were observed. This outcome did not seem to occur in the P( − )7d group, since IL-10 secreting cell numbers were not higher than those from the tumour control group (Table 2).
Determination of cytokines in tumour-infiltrative cells
Breast tumour tissue contains malignant epithelial cells, stromal cells, adipocytes, lymphocytes and macrophages. The role of tumour-infiltrating immune cells in anti-tumour immunity, as well as their potential for cancer immunotherapy, has been investigated extensivelyReference Ferrarini, Ferrero, Dagna and Zocchi20, Reference Bingle, Brown and Lewis21.
TNF-α showed differences in the isolated cells compared to the other samples (serum and mammary glands). Cytokines produced by tumour-infiltrating immune cells have previously been reported to play an important role in the anti-tumour responseReference Brandtzaeg and Pabst22. The TNF-α secreting cells increased in the groups fed with fermented milk where the tumour growth was delayed (P(+)7d and P( − )7d), leading to an induction of the cytokine production by fermented milk, which may play a biological role in the induction of cellular apoptosisReference de Moreno de LeBlanc, Matar, LeBlanc and Perdigón17 (Table 2). IL-10 is a regulatory cytokine that can be released by tumour-infiltrating immune cells such as macrophages and lymphocytes. IL-10+ cell numbers decreased in both the control and the P(+)2d groups throughout the time of the entire study. P(+)7d showed the highest number of IL-10+ cells (29 ± 7) at 22 days in comparison with the rest of the groups. In addition, increases of IL-10+ cells in mice from P(+)7d group were observed in the other samples tested (serum and mammary gland tissues) (Table 2). These could occur in order to regulate the proinflammatory cytokines (TNF-α and IL-6) produced. All mice fed with fermented milk showed decreases in the number of IL-6+ cells compared to the tumour control group (Table 2). This outcome shows a protective effect of LAB in this oestrogen-dependent tumour, due mainly to the decrease in IL-6. IL-6+ secreting cell numbers remained significantly lower only in the P(+)7d group in comparison to the rest of the groups tested in all the samples, which shows once again the best anti-tumour response.
IgA+ cells and CD4+ and CD8+T lymphocytes in mammary glands of mice fed fermented milk and injected with tumour cells or not
The existence of the common mucosal immune system permits the study of the influence of antigens orally administered on mucosal sites different to the intestine. Both B and T cells can migrate from Peyer's patches, found in the small intestine, to the respiratory, gastrointestinal and genitourinary tracts, as well as to exocrine glands such as lacrimal, salivary, mammary and prostate glandsReference Brandtzaeg and Pabst22.
Previous observations led us to study the immune response in mammary glands induced by fermented milk. It is important to highlight the cells which are involved in the local immune response observed in the mice fed cyclically (7 d) with one of both fermented milks and not injected with the tumour cells.
It was demonstrated that milk fermented by L. helveticus R389 again showed different responses compared with the other groups (control and milk fermented using the deficient proteolytic variant strain)Reference de Moreno de LeBlanc, Matar, LeBlanc and Perdigón17. This fermented milk produced increases in the IgA+ cells in mammary glands after tumour injection. However, this increase was not observed when a tumour was not induced, which could mean that enhancement of IgA+ cells in mammary glands needs a stronger stimulation such as that induced by tumour cellsReference de Moreno de LeBlanc, Matar, Thériault and Perdigón23.
T cell population studies are of great interest, since tumour antigens are recognised by T cells as the principal target, especially in the solid tumours by the protective anti-tumour immune response. CD8+ cytotoxic T lymphocytes can carry out a surveillance function by recognising and killing potentially malignant cells. Although CD4+T-helper cells are not generally cytotoxic, they can play an important role in preventing tumour development by releasing cytokines, such as IFN-γ in order to regulate the immune response. When T cells were studied in our model, it was possible to observe changes in the balance between CD4+ and CD8+ cells in mammary glands in mice from the group fed with milk fermented by L. helveticus R389 and injected with tumour cellsReference de Moreno de LeBlanc, Matar, Thériault and Perdigón23. CD4+ cell numbers increased whereas CD8+ cell numbers remained unmodified. This outcome was different in the tumour control group, which maintained the balance of these cells in mammary glands towards CD8+ cells more than towards CD4+ cellsReference de Moreno de LeBlanc, Matar, Thériault and Perdigón23.
These results agree with the colon cancer model in mice injected with DMH and fed with yoghurt, where the tumour development was inhibited with a balance between CD4+/CD8+ cells favouring the first population, different from the DMH control group which showed increased cytotoxic cellsReference Perdigón, de Moreno de LeBlanc, Valdez and Rachid10, as already explained.
When the effect of the administration of both fermented milks on the immune cell populations of mammary glands without tumour cells stimulus was analysed, neither IgA+ nor T cells were found to increaseReference de Moreno de LeBlanc, Matar, Thériault and Perdigón23. These findings are in agreement with previous works where L. casei CRL 431 administered orally was able to stimulate the IgA cycle, increasing IgA+ cells in intestine, bronchus and mammary glands tissues in short periods of time (5 d)Reference de Moreno de LeBlanc and Perdigón24. These results are important to take into account since fermented milks are only confirmed as changing immune cell balances in mammary glands when immune cells have to act against a target like tumour cells, avoiding any exacerbated immune response.
We can conclude that it is possible in this breast cancer model to obtain an immune stimulation in distant mucosal sites with the oral administration of fermented products. The probiotic strain would play an important role in the mucosal activation observed. Seven days of cyclical feeding with milk fermented by L. helveticus R389 or L89 delayed tumour development and consequently decreased IL-6 secreting cells. However, milk fermented by L. helveticus R389 induced not only a decrease of IL-6, but also an increase of regulatory cytokine IL-10 and cell apoptosis in the tumour. These effects were observed when a local stimulus such as tumour cells was present.
Conflict of interest statement
The authors have no conflict of interest to declare.





