Soya and its isoflavones (IFL) are reported to prevent cancer, heart diseases and other chronic disorders(Reference Yan and Spitznagel1–Reference Setchell and Lydeking-Olsen6), especially when the dietary exposure occurs early in life(Reference Thanos, Cotterchio and Boucher7–Reference Wu, Wan and Hankin10). Similar to the situation with flavonoids, several human studies have established that isoflavonoid glycosides, the main IFL form present in a soya containing diet, taken orally, appear in the circulation as glucuronides and sulphates(Reference Setchell, Brown and Zimmer-Nechemias11–Reference Walle, Otake and Walle18). The available evidence suggests that during digestion, isoflavonoid glycosides are hydrolysed mainly by intestinal bacteria, followed by passive diffusion through the mucosa and reconjugation to glucuronides and sulphates on the basolateral side of the enterocyte and/or in the liver(Reference Franke, Custer and Hundahl19, Reference Setchell20). Gut bacteria play an additional vital role because they also form the major IFL metabolites (dihydrodaidzein, dihydrogenistein, equol (EQ), O-desmethylangolensin) and further degrade IFL(Reference Setchell, Brown and Lydeking-Olsen21–Reference Bannwart, Adlercreutz and Fotsis23).
We and others have shown recently that blood and urine isoflavonoid values, including those in children, are highly correlated, particularly when timing of collection is considered accurately(Reference Franke, Custer and Hundahl19, Reference Franke, Halm and Custer24–Reference Grace, Taylor and Low30). Since bioavailability is defined based on circulating levels, we suggest the term ‘apparent bioavailability’ (AB) when using urinary excretion data as surrogate to describe systemic exposure(Reference Franke, Halm and Ashburn29, Reference Franke, Halm and Ashburn31).
In a variety of studies on IFL bioavailability after soya intake, either men or women (often in one menopausal state) alone(Reference Faughnan, Hawdon and Ah-Singh32–Reference Anupongsanugool, Teekachunhatean and Rojanasthien38), solid soya alone(Reference Xu, Wang and Murphy36) or highly processed foods alone(Reference Anupongsanugool, Teekachunhatean and Rojanasthien38) was investigated. Despite variabilities, in particular related to the patterns of the individual IFL daidzein (DE), genistein (GE) and glycitein (GLYE), the unifying result of previous investigations was that background diet plays an important role(Reference Xu, Wang and Murphy36). For example, fibre decreases IFL uptake(Reference Tew, Xu and Wang37) and fat decreases EQ production(Reference Rowland, Wiseman and Sanders39). Additionally, the IFL from liquid soya foods was absorbed more quickly(Reference Franke, Custer and Hundahl19, Reference de Pascual-Teresa, Hallund and Talbot35) and more extensively(Reference Cassidy, Brown and Hawdon40). Aglycons as present in fermented soya products were found to result in higher IFL uptake by some(Reference Hutchins, Slavin and Lampe33, Reference Cassidy, Brown and Hawdon40–Reference Izumi, Piskula and Osawa42), but not others(Reference Franke, Custer and Hundahl19, Reference Tsangalis, Wilcox and Shah34, Reference Setchell, Brown and Desai43) including non-distinguishable differences(Reference Richelle, Pridmore-Merten and Bodenstab44, Reference Maskarinec, Watts and Kagihara45).
While considerable knowledge exists in experimental settings(Reference Kano, Takayanagi and Harada41, Reference Setchell, Brown and Desai43, Reference Zubik and Meydani46, Reference Setchell, Faughnan and Avades47), the uptake efficiency and pharmacokinetic parameters of IFL from various soya foods are little understood in human subjects. By measuring urinary isoflavonoid excretion (UIE), the intent of this research was to find out whether the isoflavonoid exposure differs between different types of soya foods consumed. We aimed to compare liquid (soya milk and soya protein drink referred to as ‘milk’ and ‘powder’) v. solid (soya nuts and soya protein bars referred to as ‘nuts’ and ‘bars’) and native v. processed soya foods. An important part of the present study was to keep the foods consumed together with the respective study soya item constant in order to avoid confounding effects by the food environment. In addition, we aimed to determine the time frame needed within 26 h after soya intake to determine IFL excretion accurately considering the biphasic UIE profile(Reference Franke, Custer and Hundahl19).
Methods
Population
The University of Hawaii Committee on Human Studies approved the study protocol and consent form. The latter was signed by all participants. In total fourteen participants took part in the study. The subjects recruited were healthy, with no known soya allergies, who did not report antibiotic use within any 4-week period before consuming the study soya food. Each participant consented to donating their full volume of urine immediately before and 2, 8, 24, and 26 h after intake of at least four different soya foods (nuts, milk, health bar and protein drink), which was designed to happen on four separate days, each of them approximately 1-week apart.
Data from urines that were collected with more than 10 % difference to the scheduled time point were omitted. For soya nuts, eleven people correctly completed the 2 and 8-h collections, ten completed the 24-h collection and thirteen completed the up to 26-h collections. For soya milk, eleven participants completed the 2, 8 and 24-h collections, and thirteen completed the up to 26-h collection correctly. For the soya bar, eleven people correctly collected the 2, 8 and 24-h collections, and all fourteen completed the up to 26-h collections. Finally, for the soya powder drink, nine were able to correctly collect the 2, 8 and 24-h collections, with thirteen completing the up to 26-h collections (Table 1).
Table 1 Participant characteristics and urine collection details
(Mean values and standard deviations)
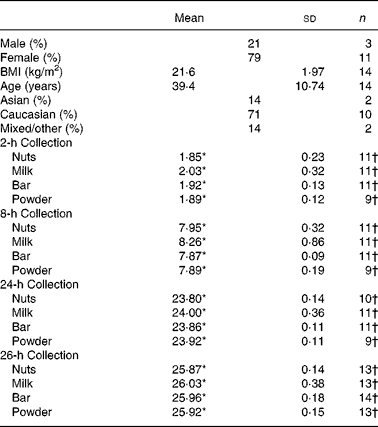
Sum of percentage may differ from 100 owing to rounding.
* Mean hours since respective soya intake.
† Total number of participants collecting urine correctly during the given time periods.
Study procedures
After emptying their bladders for the spot urine collection, the participants had breakfast with the study soya food and subsequently donated all urine during 0–2, 2–8, 8–24 and 24–26-h time periods. According to HPLC analysis(Reference Franke, Hankin and Yu48), the serving sizes of the respective soya foods, namely 13·6 g lightly salted and roasted soya nuts (referred to in text as nuts), 12·7 g Peanut Pal soya bar (referred to in text as bar) containing as IFL source soya protein, 12·6 g Strawberry Banana Bliss soya protein powder drink (referred to in text as powder) mixed with water (all from Revival®, Physician's Pharmaceuticals, Kernersville, NC, USA) and 95·2 g WestSoy organic unsweetened soya milk (referred to in text as milk; Hain Celestial Group, Boulder, CO, USA) contained on average 23·7 (sd 0·03 mg IFL (Table 2). The soya food was consumed together with a breakfast of the participants' choice (some participants brought their own breakfast). The study staff kept the breakfast exactly the same across each of the 4–5 study days. After an approximately 1-week washout period, the participants repeated the same procedure as described earlier with a different soya food during the following weeks.
Table 2 Isoflavone doses of study soya foods consumed*
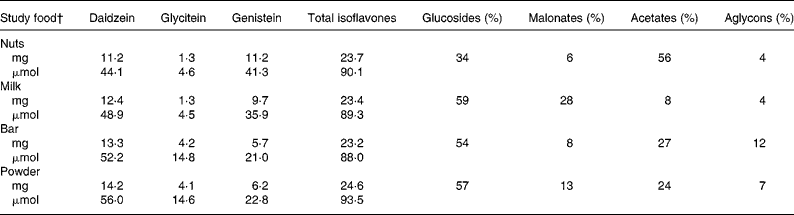
Total isoflavone values may deviate from the sum of individual isoflavones owing to rounding.
Total isoflavones = all isoflavones as the sum of native aglycons plus all glycosides.
* According to HPLC analysis(Reference Franke, Hankin and Yu48).
† Lightly salted roasted soya nuts 13·6 g (Revival®), unsweetened organic soya milk 95·2 g (Westsoy Comp.), Peanut Pal bar 12·7 g (Revival®) and Strawberry Banana Bliss protein powder 12·6 g (Revival®).
Urine containers included approximately 200 mg boric and 100 mg ascorbic acid as preservatives(Reference Franke and Custer49). Urine samples were immediately weighed, followed by storage of 2 ml aliquots at − 20°C; if collected urine needed to be stored longer than 2 h, it was kept chilled in coolers that contained ice packs. Participants completed a validated two-page soya intake and a twenty-six-page diet and health questionnaire(Reference Williams, Maskarinec and Hebshi50), and provided information regarding their height and weight. On their worksheets, they recorded time and date of urine collections, food consumption during the study day and the time at which the study food was consumed. Participants avoided eating soya foods for a full 48 h before and during the 26 h of the study day. Preliminary data suggested that allowing participants to consume soya food(s) within 24 h before the baseline urine collection interferes with the study results. We confirmed by questionnaire that the participants had refrained from taking oral antibiotics within 4 weeks before and during their participation, that they had avoided eating any soya products 48 h before the start of the study, that they had fasted overnight, and that they did not have any gastrointestinal problems before or on the study day.
Urinary isoflavonoid analysis
DE, GE, GLYE, EQ, O-desmethylangolensin, dihydrogenistein and dihydrodaidzein (the sum of these were calculated as ‘Total IFL’) were analysed by HPLC with electrospray ionisation (negative mode) tandem mass spectrometry (model TSQ Ultra, Thermo, San Jose, CA, USA) similar to our earlier reports(Reference Blair, Appt and Franke51, Reference Franke, Custer and Wilkens52) and as have been detailed most recently(Reference Franke, Halm and Kakazu53). Limits of quantitation for all analytes were 1 nm for DE and GE, 2 nm for EQ and 5 nm for the other analytes. Between-day coefficients of variation ranged between 4 and 18 % for all analytes, while intra-day variation was half or less than that.
Creatinine levels were determined by a clinical autoanalyzer (Roche Cobas MiraPlus; Roche Diagnostics, Indianapolis, IN, USA) using a test kit based on the Jaffé reaction (Randox Laboratories, Crumlin, UK). Isoflavonoid excretion in urine is expressed in nanomoles per hour by adjusting for urine volume and collection times. Baseline urine was converted from nanomoles per milligram creatinine (nmol/mg) units to nmol/h units using hourly creatinine excretion as available from each participant from the timed urine collections(Reference Franke, Halm and Ashburn29, Reference Halm, Ashburn and Franke54). Instrument calibration was performed daily using stock solutions that were measured for concentrations using absorbance readings as described previously(Reference Franke, Custer and Wilkens52).
Calculations
Since the IFL composition was slightly different between the study foods (Table 2), UIE was adjusted to the IFL doses of nuts; factors applied (to divide measured UIE values by) for milk, bar and powder to that in nuts were for DE 1·107, 1·188 and 1·268; for GE 0·866, 0·509 and 0·554; for glycitein 1·000, 3·231 and 3·154; and for Tot IFL 0·987, 0·979 and 1·038, respectively. The UIE 2, 8, 24 and 26 h after soya intake was adjusted for the UIE contribution by the baseline spot urine if the latter had IFL levels >1000 nm as described in detailed recently(Reference Franke, Halm and Ashburn29, Reference Halm, Ashburn and Franke54). This cut-off can be considered conservative because at this level the theoretical amount after 0–2, 6–8, 8–24 and 24–26 h is only 96, 217, 212 and 22 nmol, respectively, given a creatinine excretion of 1060 mg/l (equivalent to 55 mg/h leading to an excretion rate of 52 nmol/h in our cohort) and a 8-h half-life of IFL(Reference Nielsen and Williamson55). This excretion is negligible compared with the amounts found after soya challenge. Five subjects had levels < 1000 nm in baseline spot urine, three participants had IFL in the baseline spot urine at levels of 1000–3000 nm and only one participant had consistently at least one IFL at levels 3000–5000 nm in baseline spot urine, but overall results did not change when this participant was removed from all calculations. The correctness of urine collections was checked by comparing the measured to the theoretically expected urinary creatinine excretion according to the accepted (sex, body weight and age-dependent) values from healthy adults(Reference Franke, Halm and Ashburn29, Reference Halm, Ashburn and Franke54).
Ethical approval
The present study was conducted according to the guidelines laid down in the Declaration of Helinski, and all procedures involving human subjects were approved by the University of Hawaii Committee on Human Subjects. Written informed consent was obtained from all subjects.
Statistical analysis
Paired t tests were performed using Excel 2004 for the Macintosh (Microsoft Inc., Redmond, WA, USA). These tests were performed with log-transformed UIE values to account for the non-normality of distributions. A P-value of < 0·05 was considered suggestive and a P-value < 0·05/6 = 0·008 was considered significant, after Bonferroni correction for the six pairwise multiple comparisons of products at each time point.
Results
Fourteen subjects were recruited and thirteen completed the collection correctly for the soya foods nuts, milk and bar, whereas fourteen completed the collection correctly for powder during the cumulative up to 26-h time point, respectively. High compliance was observed by individual monitoring of the collections during the day, since participants were all part of our institution. Correct urine collections were also confirmed by the measured creatinine values (data not shown).
There were a total of nine repeat collections for the soya nuts, four for the soya milk, three for the soya bar and two for the powder drink, but only the average within each repeat was used in the present calculations. The mean coefficient of variation of the four types of repeated soya products by analyte was DE 19 %, GE 29 %, GLYE 28 % and total IFL 14 %, which can be considered consistent.
The total IFL dose was consistent in all four soya items, namely 23·7, 23·4, 23·2 and 24·6 mg for nuts, milk, bar and powder, respectively (mean = 23·7 mg, CV = 2·6 %); however, the IFL composition was somewhat different (Table 2). We therefore adjusted all UIE data to doses as present in nuts (see ‘Methods’ for details), in order to perform an accurate comparison of the AB between each food item by evaluating each IFL individually. After 2, 8 and 24 h relative to 26 h, DE was 10–17, 58–69 and 96–100 % excreted, respectively; for GE, these numbers were 10–17, 49–62 and 85–100 and for GLYE, 7–13, 59–72 and 91–100, respectively (Fig. 1).
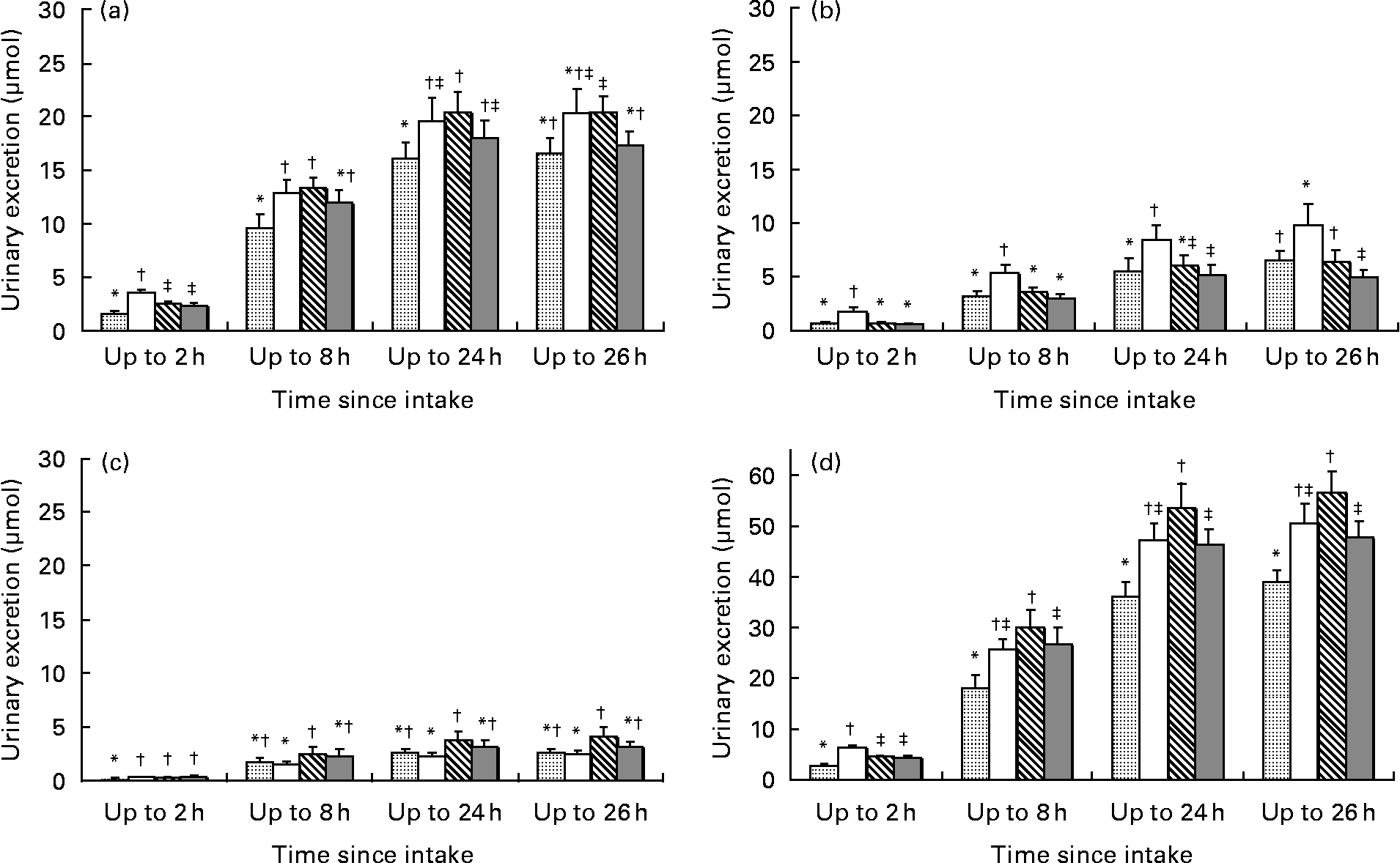
Fig. 1 Urinary excretion of isoflavonoids ((a) daidzein, (b) genistein, (c) glycitein and (d) total isoflavones) over four collection periods after adjustment to dose present in nuts (![]() , nut;
, nut; ![]() , milk;
, milk; ![]() , bar;
, bar; ![]() , powder). * Values for individual and total isoflavones within a collection period with different symbols are significantly different (P < 0·05); error bars indicate standard error; total isoflavones = daidzein+genistein+glycitein+dihydrodaidzein+dihydrogenistein+equol+O-desmethylangolensin.
, powder). * Values for individual and total isoflavones within a collection period with different symbols are significantly different (P < 0·05); error bars indicate standard error; total isoflavones = daidzein+genistein+glycitein+dihydrodaidzein+dihydrogenistein+equol+O-desmethylangolensin.
In general, the liquid foods, particularly the milk, showed initially higher UIE values than the solid foods, but this difference was much reduced or disappeared entirely after 24 and 26 h. Significant differences (P < 0·01 by paired t test) in DE excretion between the foods were observed at early collection periods, especially at the 2-h time point, but these were partially lost for later collections. At the 26-h time point, the milk and bar groups showed urinary DE excretion of 20 μmol, while nuts and powder groups showed 17 μmol, respectively. Significant differences (P < 0·05 by paired t test) in DE excretions were observed for the groups bar v. nuts (+23 %), milk v. powder (+18 %) and bar v. powder (+18 %). For GE, the pattern changed between the four soya foods much less over time, in fact stayed very similar in the last two collection periods (24 and 26-h collections). After 26 h, the urinary GE excretion was the highest in the milk group (10 μmol), followed by the nut, bar (both 6 μmol) and powder groups (5 μmol). The differences were significant (P < 0·05 by paired t test) between all groups except between nuts and bars, i.e. milk v. nuts +52 %, nuts v. powder +32 %, milk v. bar +53 %, milk v. powder +101 % and bar v. power +31 %. The UIE for GLYE was the highest for the bars (4 μmol) 26 h after soya intake, followed by powder and nuts (3 μmol), and milk (2 μmol). The up to 2-h collection showed significance (P < 0·05 by paired t test) for milk v. nuts (+52 %), bar v. nuts (+38 %) and powder v. nuts (+52 %). For the up to 8, 24 and 26-h collections, only the pair bar v. milk showed a significant difference (+65–67 %; P < 0·05 by paired t test).
Total IFL 26 h after soya intake showed the highest UIE for the bars (57 μmol), followed by those of milk (51 μmol), powder (48 μmol) and nuts (39 μmol), respectively. This was significant (P < 0·05 by paired t test) for the UIE pairs bar v. nuts (+45 %), milk v. nuts (+30 %), powder v. nuts (+23 %) and bar v. powder (+18 %; Appendix 1). The same pattern was observed for the up to 8 and 24-h periods, but not for the up to 2-h collection.
The urinary appearance patterns of the metabolites dihydrodaidzein, dihydrogenistein, EQ and O-desmethylangolensin are shown in detail in Appendix 1, but the relative short time of specimen collection after soya intake possibly led to an incomplete recovery. The urinary recovery relative to dose 26 h after soya intake (Table 3) showed a similar pattern as described earlier for the individual IFL. Recovery of DE and its metabolites were higher in milk (74 %) and bars (73 %) than in nuts (61 %) and powder (61 %). The urinary GE recovery was the highest in the milk group (28 %), followed by the nut (20 %), bar (19 %) and powder groups (14 %). Recovery of GLYE was the highest for the powder (59 %), followed by bars (56 %), milk (54 %) and nuts (49 %).
Table 3 Recovery relative to dose in urine collected up to 26 h after soya food consumption
(Mean values with their standard errors of urinary recovery)
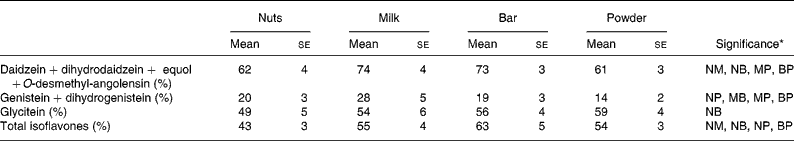
NM, nuts v. milk; NB, nuts v. bar; NP, nuts v. powder, MB, milk v. bar; MP, milk v. powder; BP, bar v. powder.
* Significant difference for food pairs at P < 0·05 by Student's paired t test of logged values if letters are shown.
Discussion
It was previously demonstrated that UIE profiles reflect circulating IFL levels accurately(Reference Franke, Custer and Hundahl19, Reference Arai, Uehara and Sato28, Reference Faughnan, Hawdon and Ah-Singh32) (and also soya intake(Reference Ritchie, Morton and Deighton25)) prompting us to use UIE as a reliable surrogate to determine IFL bioavailability. We emphasise that bioavailability is strictly based on measuring levels in the circulation and we therefore refer to AB when using urinary values. In the present context, it is important to note that the plasma:urine ratio is different between the various IFL (for example different by a factor of 3 comparing DE with GE), but it will remain unchanged within each IFL(Reference Franke, Custer and Hundahl19). Therefore, accurate AB conclusions can be drawn for each individual IFL when comparing various soya foods or various individuals exposed to or adjusted to the same dose.
In the present study, we examined the UIE of fourteen participants after they had a breakfast, which was kept consistent within each subject during the consumption of all four different soya foods. The soya items were dosed to assure a consistent total IFL exposure. We allowed for approximately 1-week of washout between the consumption of each soya food to avoid interferences. Although a previous study reported no significant difference of UIE when diverse soya foods were consumed(Reference Xu, Wang and Murphy36), we found that the UIE profiles differed significantly between the four soya foods we tested regarding total IFL as well as individual IFL when adjusted to dose. DE was approximately 18 % more bioavailable when consumed from milk or bars relative to nuts or powder. By contrast, GE was the most bioavailable from milk, while nuts and bars were 35 % lower, and powder was 50 % lower. Again different was the sequence for glycitein among the soya foods tested with bars leading, followed by powder (22 % lower), nuts (36 % lower) and milk (40 % lower). This differential IFL bioavailability pattern depending on the type of soya food consumed is in agreement with other studies(Reference Faughnan, Hawdon and Ah-Singh32, Reference Anupongsanugool, Teekachunhatean and Rojanasthien38). When comparing our urinary recoveries, slight deviations from earlier differences occurred for GE and glycitein owing to the different doses applied in each soya food. By and large though, they followed the described trend even when considering that the urinary recovery data included the metabolites dihydrodaidzein, EQ and O-desmethylangolensin with the DE data and dihydrogenistein with the GE data.
In agreement with other studies(Reference Faughnan, Hawdon and Ah-Singh32), we found that DE appeared in much larger amounts in urine than GE, GLYE, or the metabolites (Appendix 1). Therefore, DE is the largest contributor to the total IFL value in urine (in the present study, approximately 40 %) and predominates the latter value. Consequently, we discourage using the total IFL value as a reliable UIE data point, because it is biased towards DE and suggest considering each IFL individually. This approach will also take into consideration that each IFL has very distinct pharmacologic properties, for example binding to and transactivation of the oestrogen receptors(Reference Kuiper, Carlsson and Grandien56).
The present results indicate that specimen collections over at least 24 h are needed to determine conclusive bioavailability data. Shorter periods are insufficient probably owing to the biphasic IFL appearance pattern in plasma and urine(Reference Franke, Custer and Hundahl19, Reference Zubik and Meydani46).
Since the IFL profiles in each of the foods were different, we adjusted the UIE values to the dose present in nuts; this made AB comparisons between the foods accurate. This was also performed for the total UIE values, although little adjustment was needed since serving sizes were designed to keep the total IFL dose consistent. Some studies have shown that IFL from liquid soya foods such as soya milk are absorbed and excreted more quickly than from solid forms of soya foods(Reference Faughnan, Hawdon and Ah-Singh32, Reference Cassidy, Brown and Hawdon40). The present results agree with this finding only for soya milk, but not for the soya protein powder drink. It appears that not all liquid forms of soya are equivalent. This seems to also apply for solid foods because the bars and nuts we tested showed vast differences in AB. The underlying mechanisms for the observed differences in urinary IFL appearance patterns are uncertain, but the matrix of the type of soya food might have had a distinct influence. For example, the considerable fat content of the nuts may have caused a slower uptake(Reference Tew, Xu and Wang37), while fibre and the solid nature of this food might have decreased general bioaccesibility. Additional factors that could have affected the IFL absorption after soya intake are the intestinal bacteria. IFL absorption will be decreased when these bacteria that hydrolyse the native IFL glycosides to the bioavailable aglycons are impaired. On the other hand, IFL absorption will be increased when bacteria that degrade IFL aglycons during digestion are impaired. This fine line of dynamic changes of IFL bioavailability caused by the gut flora has recently been suggested as an explanation for the variable AB of IFL after oral antibiotic therapy(Reference Franke, Halm and Ashburn29) and may have also played a role in the findings of the present study. Additional causes for variabilities are intra-individual differences in IFL uptake depending for example on hormonal, immunologic or other factors that play a role during the complex steps of digestion. We tried to address this issue by including repeated challenges with the same soya food in a few of our subjects. More extensive elaborations in this respect would have been desirable, but could not be done owing to budget restrictions and would need to be considered in future studies in order to optimise the robustness of data.
In the present study, the UIE profiles changed significantly between the time periods of urine collection investigated. We therefore realise that urine collections of up to 24–26 h are necessary in order to obtain accurate values for AB of IFL from soya foods. We observed generally no changes UIE patterns between 24 and 26-h collection periods and therefore assume that longer times of collection will not add significantly more information for the unmetabolised IFL. The reason for less significance between UIE patterns found at 24 v. 26 h are likely due to the lower number of participants in the 24-h time period. An up to 8-h urine collection may give an approximate ‘snapshot’ of AB, which, however, could change over later times and should therefore not be used as basis for final conclusions. Some of the strengths of the present study include that subjects were highly compliant, health professionals, the washout period of 1-week between consumption of the different soya foods, utilisation of urinary creatinine to confirm accurate urine collections and several collections of urine during 26 h in order to determine the appropriate time frame needed to obtain conclusive results about UIE profiles. Similar to others, we kept the background diet consistent(Reference de Pascual-Teresa, Hallund and Talbot35, Reference Kano, Takayanagi and Harada41), an important factor since the food matrix has been shown to have an effect of IFL absorption(Reference Cassidy57). This eliminated possible confounding factors that would have been present if participants were allowed to eat different meals with each soya food. Additional strengths of the present study were the examination of the participants' diet history that assured consistent and healthy dietary behaviours without carnivorous or vegetarian extremes and lack of medication use for at least 1 month before and during the study, particularly antibiotics.
Although our sample size was relatively small and made up primarily of women, we were able to demonstrate significant differences in AB of IFL. The highly compliant nature of the present study participants, mostly health professionals, possibly contributed to our success. Further studies that include a larger sample size, more equal sex distribution and repeated challenges with the same soya food type are needed to confirm the presented effect of soya food type (liquid v. solid; processed v. native) on IFL bioavailability.
Acknowledgements
We thank the participants for their time and cooperation and Physician's Pharmaceuticals, Inc. for partial support of the present study including the donation of soya foods. L. A. A. was responsible for study coordination, urine collection and assistance with data compilation and manuscript preparation, K. K. for the urine analysis by LCMS, S. S. for the completion of the manuscript, L. R. W. for statistical evaluations, B. M. H. for the study design (she also acted as a consultant in study performance and medical issues) and A. A. F. for the overall supervision of all aspects of the study, securing of research funds and manuscript preparation. We also acknowledge the NIH for instrumentation support S10-RR020890 (there are no conflicts of interest).
Appendix 1 Urinary isoflavone excretion adjusted for exposure to doses in soya nuts
(Mean values with their standard errors)
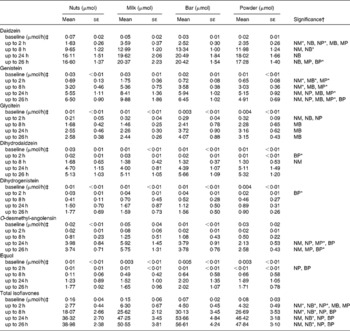
NM, nuts v. milk; NB, nuts v. bar; NP, nuts v. powder; MB, milk v. bar; MP, milk v. powder; BP, bar v. powder.
* P < 0·008 (Bonferroni correction for multiple comparisons = 0·05/6).
† Significant difference for food pairs at P < 0·05 by Student's paired t test of logged values if letters are shown.
‡ Calculated from creatinine data (mg/h) owing to the lack of values for duration and volume of collected urine at baseline (spot urine).







