Hepatocellular carcinoma is the major cause of cancer death in Taiwan and the most endemic cancer in a large region of the world. Tumour metastasis, both intrahepatic and extrahepatic, is a major factor of mortality in hepatocellular carcinoma patients. Tumour cell metastasis is characteristic of tumour progression involving complex processes including the ability to dissolve the basement membrane and the extracellular matrix (ECM) and to migrate through the ECM. The degradative process is mediated largely by matrix metalloproteinases (MMP), cathepsins and plasminogen activator systems(Reference Ulisse, Baldini and Sorrenti1). MMP-2, MMP-9 and urokinase-type plasminogen activator (uPA) are the most vital proteases for degradation of base membrane and, therefore, are deeply involved in cancer metastasis(Reference Ulisse, Baldini and Sorrenti1–Reference Yu, Lin and Chang3). MMP-2 (72 kDa) and MMP-9 (92 kDa) activities are regulated extracellularly, and their regulations are primarily affected by the balance of pro-enzyme activation and inhibition by tissue inhibitors of matrix metalloproteinase (TIMP), TIMP-2 and TIMP-1, respectively(Reference Yu, Lin and Chang3–Reference Park, Lim and Kim5). In addition, serine protease uPA is a protease that cleaves the ECM and activates the conversion of plasminogen to plasma(Reference Blasi6). The conversion of plasminogen to active plasmin is regulated by two specific and fast-acting plasminogen activator inhibitors (PAI), PAI-1 and PAI-2, with PAI-1 being more important(Reference Itoh, Hayashi and Kanamaru7). The inhibition of MMP and uPA activity has been adopted as an anti-metastasis therapeutic strategy.
Focal adhesion kinase (FAK) is the most extensively studied focal adhesion protein in hepatocellular carcinoma(Reference Yam, Tse and Ng8). FAK is a non-receptor tyrosine kinase that is involved in ECM/integrin-mediated signalling pathways, and has been suggested to play an essential role in metastasis through the modulation of tumour cell adhesion, migration and invasion(Reference Zhao and Guan9) probably by the regulation of MMP(Reference Mon, Ito and Senga10). Inhibition of FAK leads to reduced secretion of MMP-9 in carcinoma cells, and this effect is associated with the selective loss of an invasive cell phenotype(Reference Mon, Hasegawa and Thant11). FAK has also been shown to regulate cell migration by modulating the assembly and disassembly of the actin cytoskeleton through its effects on the Rho subfamily of small GTPase, a member of the Ras superfamily of small (approximately 21 kDa) GTPase(Reference Zhao and Guan9). Rho GTPase, which comprises Rho, Cdc42 and Rac1, is involved in various cellular functions such as cell growth, division, morphology, polarity and migration(Reference Etienne-Manneville and Hall12). Furthermore, altered expression of the putative metastasis suppressor gene nm23-H1 is considered to be an important step during the acquisition of metastatic ability(Reference Huang, Shih and Chuang13).
Epidemiological studies have suggested that elevated intakes of fruit and vegetables are associated with a reduced risk of several types of cancer, and these effects have drawn attention to the possibility that biologically active plant secondary metabolites exert anti-carcinogenic activity(Reference Johnson14). Isoprenoids, which are widely distributed in fruits, vegetables and grains, are a class of phytochemicals that encompasses approximately 22 000 individual components(Reference Bach15). β-Ionone (BI), a cyclic isoprenoid, is a precursor for carotenoids, some of which exert anti-carcinogenic and anti-tumour activities in vitro and in vivo such as induction of cell-cycle arrest in various types of cancer cells(Reference Kim, Moon and Kang16–Reference Sun, Liu and Sun22). We have recently shown that β-carotene (BC), which has a similar ionone ring structure to BI, exhibits anti-metastatic effects both in vitro and in vivo (Reference Huang, Liao and Hu2, Reference Huang, Shih and Chuang13). Lin et al. (Reference Lin, Chen and Yang23) have reported that BI exerts inhibitory effects on the proliferation of SGC-7901 human gastric adenocarcinoma cells and up-regulates TIMP-1 and TIMP-2 mRNA expression. However, it is unclear whether BI may exert anti-metastatic effects in hepatic cancer cells. Therefore, in the present study, we employed a highly invasive human hepatocarcinoma, the SK-Hep-1 cells, to examine the effects of BI on cell invasion, migration and adhesion as well as the possible mechanisms underlying these actions.
Materials and methods
Materials
The cell line SK-Hep-1 (BCRC 67005) was purchased from the Food Industry Research and Development Institute, Hsin Chu, Taiwan. All chemicals used were of reagent or higher grade. BI (97 %; Acros organics, Morris Plains, NJ, USA) was delivered to the cell using ethanol (99 %; Sigma, St Louis, MO, USA) solvent. BC was delivered to the cell using tetrahydrofuran (Merck, Germany) as solvent. Dulbecco's minimal essential medium (DMEM), fetal bovine serum, trypsin, penicillin, streptomycin, sodium pyruvate, non-essential amino acid and Giemsa stain were from GIBCO/BRL (Gaitherburg, MD, USA). Transwells were from Costar (Cambridge, MA, USA). Matrigel®, anti-human-nm23-H1, anti-Cdc42, anti-Rac1 and anti-Rho mouse monoclonal antibodies were from BD Biosciences (San Diego, CA, USA). Anti-TIMP-2, anti-PAI-1, anti-FAK and anti-β-actin monoclonal antibodies and anti-mouse IgG horseradish peroxidase were purchased from Santa Cruz Biotechnology Company (Santa Cruz, CA, USA). The phosphorylated form of FAK (FAK-p, Y397), TIMP-1 rabbit monoclonal antibodies and anti-rabbit IgG horseradish peroxidase were purchased from Epitomics (Burlingame, CA, USA).
Cell culture and β-ionone incorporation
SK-Hep-1 cells were grown in DMEM containing 10 % (v/v) fetal bovine serum, 0·37 % (w/v) NaHCO3, penicillin (100 units/ml) and streptomycin (100 units/ml) in a humidified incubator under 5 % CO2 and 95 % air at 37°C. The cells were harvested at approximately 90 % confluence (106 cells/dish). The survival rate of cells was always higher than 95 % by trypan blue assay(Reference Phillips24). A stock BI solution (50 mm) and a BC solution (10 mm) were prepared freshly before each experiment.
Cell migration assay
Tumour cell migration was assayed in transwell chambers (Costar) according to the methods reported by Repesh(Reference Repesh25) with some modifications. Briefly, transwell chambers (Costar) with 6·5 mm-polycarbonate filters of 8 μm pore size were used. After pre-incubation with BI or BC for 24 and 48 h, SK-Hep-1 cells (5 × 105 cells/ml) were finally suspended in DMEM (100 μl, serum free) and placed in the upper transwell chamber, and then incubated for 5 h at 37°C. After incubation for 5 h at 37°C, the cells on the upper surface of the filter were completely wiped away with a cotton swab. The cells on the lower surface of the filter were fixed in methanol, stained with Giemsa, and then counted under a microscope. For each replicate, the tumour cells in ten randomly selected fields were determined, and the counts were averaged. The percentage inhibition of invasion was calculated by the following formula: (1 − (treatment/control)) × 100 .
Cell invasion assay
The procedure reported by Repesh(Reference Repesh25) for the cell invasion assay was similar to cell migration. The invasion of tumour cells was assessed in transwell chambers with a 6·5 mm diameter polyvinyl/pyrrolidone-free polycarbonate filter of 8 μm pore size. Each filter was coated with 100 μl of a 1:20 diluted Matrigel® in cold DMEM to form a thin continuous film on the top of the filter. After pre-incubation with BI or BC for 24 and 48 h, SK-Hep-1 cells (5 × 105 cells/ml) were suspended in DMEM (100 μl, serum free) and placed in the upper transwell chamber, and then incubated for 24 h at 37°C. After incubation for 24 h, cells were stained and counted as described earlier, and the number of cells invading the lower side of the filter was measured as the invasive activity. For each replicate, the tumour cells in ten randomly selected fields were determined, and the counts were averaged. The percentage inhibition of invasion was calculated by the aforementioned formula.
Cell adhesion assay
The procedure reported by Yang et al. (Reference Yang, Liu and Liao26) for the cell adhesion was used. The twenty-four-well plates were coated with 100 μl of 1:20 diluted Matrigel® in cold DMEM to form a thin continuous film and dried in a laminar hood overnight. Cells were adjusted to 5 × 104 cells/well in DMEM containing 1–50 μm-BI and incubated at 37°C for 24 and 48 h. After the incubation, cells were washed twice in PBS and then incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide for 1 h, after which the supernatant was removed and dimethyl sulfoxide was added to dissolve the solid residue cells. Optical density at 570 nm of each well was then determined by using a microplate reader (FLUOstar OPTIMA; BMG Labtechnologies GmbH, Offenburg, Germany).
Gelatin zymography
MMP-2 and MMP-9 activities were assayed using gelatin zymography according to the methods described previously(Reference Yang, Kuo and Tsai27). The cells (5 × 104 cells/ml) were treated with BI for 24 h in DMEM containing 10 % (v/v) fetal bovine serum and incubated for 24 h at 37°C in serum-free DMEM, and then the culture medium was harvested and stored at − 20°C until use. For the assay of gelatin zymography, the culture medium of an appropriate volume (adjusted by viable cell number) was used to avoid the possible effect of BI on cell viability, as adopted from the approach reported by Yang et al. (Reference Yang, Kuo and Tsai27). Then, the gel was electrophoresed in a 10 % SDS-PAGE gel containing 0·1 % (w/v) gelatin. The gel (MMP-gel) was washed for 30 min at room temperature in a solution containing 2·5 % (v/v) Triton X-100 with two changes and subsequently transferred to a reaction buffer for enzymatic reaction containing 1 % NaN3, 10 mm-CaCl2 and 40 mm-Tris–HCl (pH 8·0) at 37°C with shaking overnight (for 15 h). Finally, the MMP-gel was stained for 30 min with 0·25 % (w/v) Coomassie blue in 10 % acetic acid (v/v) and 50 % methanol (v/v) and de-stained in 10 % acetic acid (v/v) and 50 % methanol (v/v). The relative MMP-2 and MMP-9 activities were quantified by Matrox Inspector 2.1 software (Matrox Imaging, Dorval, QC, Canada).
Casein–plasminogen zymography
The procedure for the casein–plasminogen zymography, which was adopted from that reported by Yoon et al. (Reference Yoon, Song and Lee28), was similar to that of gelatin zymography as described earlier. The culture medium (20 μl) were separated by electrophoresis in 10 % SDS-PAGE gel containing 1 mg/ml of casein (Sigma) and 13 μg/ml of human plasminogen (Sigma) under non-reducing conditions. After electrophoresis, the gels were washed twice in 2·5 % Triton X-100 for 30 min, incubated with reaction buffer (1 % NaN3, 10 mm-CaCl2 and 40 mm-Tris–HCl, pH 8·0) for 15 h at 37°C, and stained with Coomassie blue G-250. The relative uPA activities were quantified by Matrox Inspector 2.1 software.
Western blotting
TIMP-1, TIMP-2, PAI-1, FAK, FAK-p, Rho GTPase and nm23-H1 protein levels were assayed using Western blotting as described previously(Reference Yang, Kuo and Tsai27). Total cellular proteins were prepared in lysis buffer containing 20 % SDS and 1 mm-phenylmethyl sulfonyl fluoride. The lysate was sonicated for 30 s on ice, followed by centrifugation for 30 min at 4°C. The protein concentrations of extracts were determined by the Bio-Rad assay as outlined by the manufacturer (Bio-Rad, Hercules, CA, USA). The relative protein levels were quantified by Matrox Inspector 2.1 software.
Statistical analysis
Values are expressed as means and standard deviations and analysed by using one-way ANOVA followed by Duncan's multiple range test for comparisons of group means. The statistical analysis was performed using SPSS for Windows, version 10 (SPSS, Inc., Chicago, IL, USA). A P value < 0·05 was considered statistically significant.
Results
β-Ionone inhibits cell invasion, migration and adhesion in vitro
Table 1 shows that incubation of SK-Hep-1 cells with BI (1–50 μm) for 24 and 48 h resulted in the concentration-dependent inhibition of cell invasion, and the longer incubation time (48 h) caused a somewhat stronger inhibition than the shorter incubation time (24 h). No further increase in inhibition was observed, when BI concentrations reached 20 μm, which inhibited cell invasion by 40 % (P < 0·001). Similarly, BI caused a concentration-dependent inhibition of cell migration and adhesion, with a 42 % (P < 0·001) inhibition of cell migration and a 22 % (P < 0·001) inhibition of cell adhesion at 20 μm-BI, but the extent of inhibition was somewhat lower at the incubation time of 48 h than at 24 h. BC (10 μm) also significantly inhibited cell invasion (35 and 49 % at 24 and 48 h, respectively), migration (34 % at 48 h) and adhesion (30 and 32 % at 24 and 48 h, respectively). Based on the time-course experiment, we chose an incubation time of 24 h for BI in the following studies.
Table 1 Effects of β-ionone (BI) and β-carotene (BC) on cell invasion, migration and adhesion of SK-Hep-1 cells† (Mean values and standard deviations, n≥3)
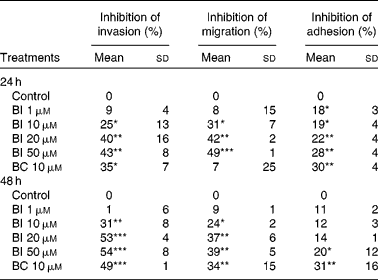
Mean values were significantly different from those of control: *P < 0·05; **P < 0·001; ***P < 0·0001.
† Cells were pre-incubated with BI (1, 10, 20 and 50 μm) or BC (10 μm) for 24 and 48 h. Ethanol (1 %) is the solvent for BI.
β-Ionone inhibits the activities of matrix metallo-proteinase-2, -9 and urokinase-type plasminogen activator
Western blots (Fig. 1(a)) show that BI (1–50 μm) inhibited the activities of MMP-2, MMP-9 and uPA at 24 h incubation. When added at 50 μm, BI inhibited the activities of MMP-2, MMP-9 and uPA by 25 % (P < 0·005), 29 % (P < 0·005) and 20 % (P < 0·005), respectively (Fig. 1(b)). BC (10 μm) also significantly inhibited the activities of MMP-2, MMP-9 and uPA by 19, 23 and 24 %, respectively.

Fig. 1 Effects of β-ionone (BI) and β-carotene (BC) on the matrix metalloproteinase (MMP)-2, MMP-9 and urokinase-type plasminogen activator (uPA) activity in SK-Hep-1 cells. Cells were pre-incubated with BI (1, 20 and 50 μm) or BC (10 μm) for 24 h. Ethanol (1 %) is the solvent for BI. (a) Zymography of MMP-2, MMP-9 and uPA. (b) Densitometric analysis of (a). Values are means, with standard deviations represented by vertical bars (n ≥ 3). Mean values were significantly different from those of control: *P < 0·05; **P < 0·005. ![]() , BI 0 μm;
, BI 0 μm; ![]() , BI 1 μm;
, BI 1 μm; ![]() , BI 10 μm;
, BI 10 μm; ![]() , BI 20 μm;
, BI 20 μm; ![]() , BI 50 μm;
, BI 50 μm; ![]() , BC 10 μm.
, BC 10 μm.
β-Ionone increases the protein expression of tissue inhibitor of matrix metalloproteinase-1, -2 and plasminogen activator inhibitor-1
Western blots show (Fig. 2(a)) that incubation of SK-Hep-1 cells with BI (1–50 μm) for 24 h resulted in concentration-dependent increases in protein levels of TIMP-1, TIMP-2 and PAI-1, the endogenous inhibitors of MMP-9, MMP-2 and uPA. Although the highest increase in TIMP-1, TIMP-2 and PAI-1 levels all occurred at 50 μm-BI (66 %, P < 0·005; 254 %, P < 0·0001; 175 %, P < 0·0001), there were no significant differences in these protein levels between 20 and 50 μm-BI (Fig. 2(b)). BC (10 μm) also significantly increased the protein expression of TIMP-1, TIMP-2 and PAI-1 by 48, 159 and 100 %, respectively.

Fig. 2 Effects of β-ionone (BI) and β-carotene (BC) on tissue inhibitor of matrix metalloproteinase (TIMP)-1, TIMP-2 and plasminogen activator inhibitor (PAI)-1 protein expression in SK-Hep-1 cells. Cells were incubated with BI (1, 10, 20 and 50 μm) or BC (10 μm) for 24 h. Ethanol (1 %) is the solvent for BI. (a) Western blots of TIMP-1, TIMP-2, PAI-1 and β-actin. (b) Densitometric analysis of (a). For loading control, expression levels of β-actin were analysed using the same lysate. Values are means, with standard deviations represented by vertical bars (n ≥ 3). Mean values were significantly different from those of control: *P < 0·05; **P < 0·005; ***P < 0·0001. ![]() , BI 0 μm;
, BI 0 μm; ![]() , BI 1 μm;
, BI 1 μm; ![]() , BI 10 μm;
, BI 10 μm; ![]() , BI 20 μm;
, BI 20 μm; ![]() , BI 50 μm;
, BI 50 μm; ![]() , BC 10 μm.
, BC 10 μm.
β-Ionone inhibits the protein expression of focal adhesion kinase and phosphorylated focal adhesion kinase
Western blots show that BI (20 and 50 μm) significantly inhibited the protein expression of FAK and FAK-p at 24 h incubation (Fig. 3(a)), with 32 % (P < 0·05) and 49 % (P < 0·0001) inhibition, respectively, at 50 μm-BI (Fig. 3(b)). There was no significant difference between 20 and 50 μm-BI. BC (10 μm) also significantly inhibited the protein expression of FAK-p by 36 %.

Fig. 3 Effects of β-ionone (BI) and β-carotene (BC) on focal adhesion kinase (FAK, ■)/phosphorylated form of FAK (FAK-p, ![]() ) protein expression in SK-Hep-1 cells. Cells were incubated with BI (1, 10, 20 and 50 μm) or BC (10 μm) for 24 h. Ethanol (1 %) is the solvent for BI. (a) Western blots of FAK/FAK-p and β-actin. (b) Densitometric analysis of (a). For loading control, expression levels of β-actin were analysed using the same lysate. Values are means, with standard deviations represented by vertical bars (n ≥ 3). Mean values were significantly different from those of control: *P < 0·05; **P < 0·005; ***P < 0·0001.
) protein expression in SK-Hep-1 cells. Cells were incubated with BI (1, 10, 20 and 50 μm) or BC (10 μm) for 24 h. Ethanol (1 %) is the solvent for BI. (a) Western blots of FAK/FAK-p and β-actin. (b) Densitometric analysis of (a). For loading control, expression levels of β-actin were analysed using the same lysate. Values are means, with standard deviations represented by vertical bars (n ≥ 3). Mean values were significantly different from those of control: *P < 0·05; **P < 0·005; ***P < 0·0001.
β-Ionone inhibits the protein expression of Rho GTPase
To test whether BI inhibits cell migration through the down-regulation of Rho GTPases, we examined the levels of Rho, Rac1 and Cdc42 by Western blotting. We showed that BI (10–50 μm) significantly inhibited the protein expression of Rho, Rac1 and Cdc42 at 24 h incubation (Fig. 4(a)), with 46 % (P < 0·001), 42 % (P < 0·0001) and 36 % (P < 0·0001) inhibition, respectively, at 50 μm-BI (Fig. 4(b)). BC (10 μm) also significantly inhibited the protein expression of Rho, Rac1 and Cdc42 by 39, 40 and 13 %, respectively. In addition, there were negative correlations between the protein expression and migration for Rho (r 2 0·97, P = 0·002), Rac1 (r 2 0·97, P = 0·002) and Cdc42 (r 2 0·91, P = 0·012) in SK-Hep-1 cells (data not shown).

Fig. 4 Effects of β-ionone (BI) and β-carotene (BC) on Rho GTPase protein expression in SK-Hep-1 cells. Cells were incubated with BI (1, 10, 20 and 50 μm) or BC (10 μm) for 24 h. Ethanol (1 %) is the solvent for BI. (a) Western blots of Rho, Rac1, Cdc42 and β-actin. (b) Densitometric analysis of (a). For loading control, expression levels of β-actin were analysed using the same lysate. Values are means, with standard deviations represented by vertical bars (n ≥ 3). Mean values were significantly different from those of control: *P < 0·05; **P < 0·001; ***P < 0·0001. ![]() , BI 0 μm;
, BI 0 μm; ![]() , BI 1 μm;
, BI 1 μm; ![]() , BI 10 μm;
, BI 10 μm; ![]() , BI 20 μm;
, BI 20 μm; ![]() , BI 50 μm;
, BI 50 μm; ![]() , BC 10 μm.
, BC 10 μm.
β-Ionone increases the protein expression of nm23-H1
The expression of nm23-H1 protein was significantly increased by BI (10–50 μm; Fig. 5). At 50 μm, BI induced the highest expression of nm23-H1 protein (62 %, P < 0·005). BC (10 μm) also significantly increased the protein expression of nm23-H1 (55 %, P < 0·005). In addition, nm23-H1 protein expression was negatively correlated with migration (r 2 0·90, P < 0·001) and invasion (r 2 0·89, P < 0·005) in SK-Hep-1 cells (data not shown).

Fig. 5 Effects of β-ionone (BI) and β-carotene (BC) on nm23-H1 protein expression in SK-Hep-1 cells. Cells were incubated with BI (1, 10, 20 and 50 μm) or BC (10 μm) for 24 h. Ethanol (1 %) is the solvent for BI. (a) Western blots of nm23-H1 and β-actin. (b) Densitometric analysis of (a). For loading control, expression levels of β-actin were analysed using the same lysate. Values are means, with standard deviations represented by vertical bars (n ≥ 3). Mean values were significantly different from those of control: *P < 0·05; **P < 0·005.
Discussion
In the present study, we show that BI dose-dependently inhibited the metastasis of SK-Hep-1 cells, as indicated by decreased cell invasion, migration and adhesion. We further show that BI significantly and dose-dependently down-regulated the expression of MMP-2, MMP-9 and uPA, whereas it increased the expression of endogenous protease inhibitors, TIMP-2, TIMP-1 and PAI-1, respectively. These results demonstrate that BI is able to inhibit the in vitro metastatic activity of SK-Hep-1 cells.
Several probable mechanisms may be involved in the anti-metastatic actions of BI (Fig. 6); one is the inhibition of MMP activity, especially MMP-2 (gelatinase A) and MMP-9 (gelatinase B). Numerous studies have indicated that inhibition of MMP expression or enzyme activity can be used as early targets for preventing cancer metastasis(Reference Huang, Liao and Hu2, Reference Yang, Kuo and Tsai27, Reference Libra, Scalisi and Vella29). The present study demonstrates that BI significantly inhibited the invasion, migration and adhesion of SK-Hep-1 cells and suppressed the activities of MMP-2, MMP-9 and uPA; the latter (uPA) is an upstream enzyme of MMP and an extremely specific serine protease that catalyses plasminogen degradation to plasmin(Reference Blasi6). Indeed, it has been suggested that the inhibition of MMP is of great promise with inhibitors as anti-tumour (anti-angiogenic, anti-proliferative and anti-metastatic) agents in preclinical models(Reference Libra, Scalisi and Vella29).

Fig. 6 Proposed anti-metastatic mechanisms of β-ionone in SK-Hep-1 cells. ![]() , Promoted by β-ionone;
, Promoted by β-ionone; ![]() , inhibited by β-ionone;
, inhibited by β-ionone; ![]() , dependent inhibition as a result of activation or inactivation of upstream signalling molecules by β-ionone. PAI, plasminogen activator inhibitor; TIMP, tissue inhibitor of matrix metalloproteinase; FAK, focal adhesion kinase; FAK-p, phosphorylated form of focal adhesion kinase; uPA, urokinase-type plasminogen activator; MMP, matrix metalloproteinase.
, dependent inhibition as a result of activation or inactivation of upstream signalling molecules by β-ionone. PAI, plasminogen activator inhibitor; TIMP, tissue inhibitor of matrix metalloproteinase; FAK, focal adhesion kinase; FAK-p, phosphorylated form of focal adhesion kinase; uPA, urokinase-type plasminogen activator; MMP, matrix metalloproteinase.
Another possible anti-metastatic mechanism of BI is through increased protein expression of TIMP-1, TIMP-2 and PAI-1, as TIMP and PAI-1 have been shown to play an important role in the invasion and metastasis of cancerous cells(Reference Cairns, Khokha and Hill4, Reference Park, Lim and Kim5, Reference Itoh, Hayashi and Kanamaru7). The activities of MMP are inhibited by TIMP, which are specific inhibitors of MMP, and the imbalance between MMP and TIMP may promote degradation of the ECM(Reference Yu, Lin and Chang3–Reference Park, Lim and Kim5). Indeed, it has been shown that the imbalance between MMP and TIMP produced by tumour tissue may be a major determinant of the progression in hepatocarcinoma(Reference Altadill, Rodríguez and González30). The transfection of TIMP-1 cDNA into HepG2 cells was shown to result in the suppression of metastasis potential of proliferation and invasion(Reference Xia, Yan and Xie31). For instance, overexpression of TIMP-1 and TIMP-2 has been shown to inhibit the pulmonary metastasis in a rat model of bladder carcinoma(Reference Kawamata, Kawai and Kameyama32). The present findings demonstrate that the significantly increased TIMP-1 and TIMP-2 protein expression in SK-Hep-1 cells is probably an important anti-metastatic feature of BI.
Still another possible anti-metastatic mechanism of BI is the inhibition of cancer cell migration, as we demonstrated that BI significantly decreased the FAK signalling pathway, including down-regulation of FAK, FAK-p and Rho GTPase expression. FAK is an attractive therapeutic target because it is a key convergence point for many growth factor pathways required for survival and metastatic functions of cancer cells(Reference Yam, Tse and Ng8, Reference Zhao and Guan9). A well-understood regulation of FAK is phosphorylation, particularly tyrosine phosphorylation(Reference Zhao and Guan9). Phosphorylation of FAK on the Y397 site promotes the Src homology domain 2-dependent binding of Src family tyrosine kinases and the formation of an activated FAK(Y397)–Src complex(Reference Schlaepfer, Hanks and Hunter33). Activated FAK(Y397)/Src signal transduction through multiple downstream targets, such as PI3K/AKT and Ras/ERK1/2 cascades, and the promotion of MMP-9 and Rho A signalling mechanisms in cancer cells result in cellular invasion and cytoskeletal rearrangements, cellular adhesion and migration, respectively(Reference Meng, Jin and Yu34). In addition to anti-metastasis, BI has been shown to down-regulate extracellular signal-regulated kinase and mitogen-activated protein kinase/ERK kinase expression, leading to reduced cancer cell proliferation(Reference Liu, Yang and Dong35).
Rho GTPases are overexpressed in human tumours and are involved in a variety of cellular processes such as organisation of the actin cytoskeleton, cell–cell contact and malignant transformation(Reference Grise, Bidaud and Moreau36, Reference Kim, Vigil and Der37). Both Cdc42 and Rac1 promote actin polymerisation at the leading edge, and thereby the formation of filopodia and lamellipodia is required for carcinoma migration and invasion(Reference Keely, Westwick and Whitehead38). Rho induces the assembly and contraction of the actomyosin fibres, which contributes to pulling the trailing edge forwards during migration(Reference Guo and Giancotti39). Here, we show that BI significantly decreased the expression of Rho, Cdc42 and Rac1, and these actions of BI were highly correlated with migration (r 2 0·99, P < 0·0001; r 2 0·91, P = 0·012; r 2 0·97, P = 0·002, respectively) in SK-Hep-1 cells.
The decrease in Rho protein expression and MMP activity by BI may be mediated in part by its up-regulation of nm23-H1 protein expression, a tumour metastasis suppressor gene. The expression of MMP, including MMP-9 and MMP-2, has been shown to be down-regulated by nm23-H1 protein(Reference Cheng, Alfonso-Jaume and Mertens40, Reference Ohba, Miyata and Koga41). In addition, nm23-H1 has been reported to negatively regulate cell migration and tumour metastasis by modulating the activity of Cdc42 and other Rho family members(Reference Murakami, Meneses and Lan42). Miyamoto et al. (Reference Miyamoto, Iwashita and Yamaguchi43) have suggested that the binding of nm23-H1 to Tiam1 and Db1 activates Rac and Cdc42 small GTPase, respectively. The same authors(Reference Miyamoto, Iwashita and Yamaguchi43) have also suggested that nm23 functions as a negative regulator for cell motility and migration by binding to Rho-type specific guanine-nucleotide exchange factors and suppressing Rho GTPase.
An interesting observation of the present study is that the anti-metastatic actions and mechanisms of BI were similar to those of BC. It appears that the anti-metastatic effects of BI and BC may be related to their common chemical structure, i.e. an ionone ring. The chemical structure of BI is similar to that of 9-cis-retinoic acid, vitamin A and BC. Retinoids, which have significant anti-cancer effects, regulate gene transcription through two families of nuclear receptor, i.e. retinoic acid receptors and retinoid X receptors (RXR)(Reference Altucci and Gronemeyer44). It is well established that regulation of RXR-α may lead to several molecular/cellular changes, which in turn lead to reduced proliferation, migration and invasion and to enhanced apoptosis in cancer cells. Janakiram et al. (Reference Janakiram, Cooma and Mohammed20) found that BI up-regulates the expression of RXR-α dose-dependently in human colon cancer cells, indicating that BI may act as an RXR agonist. It has been suggested that the use of RXR agonists in conjunction with pharmacological or genetic approaches to elevating RXR-α protein levels in target tumours may be effective therapies for cancers(Reference Xiao, Ghosn and Hinchman45). Further studies are needed to prove that BI may exert anti-metastatic effects through the RXR pathway.
In summary, we have demonstrated that BI effectively inhibits the metastasis of SK-Hep-1 cells in vitro, and that this effect involves the regulation of gene expression and signal pathways related to invasion and migration. The anti-metastatic potential of BI warrants further studies in vivo.
Acknowledgements
The present study was funded by grants from the National Science Council, Taiwan, ROC (NSC98-2320-B-005-005-MY3 and NSC99-2320-B-468-001-MY3). There is no conflict of interest for any of the authors. C.-S. H. and M.-L. H. contributed equally in the study design, supervision of experimental execution, writing and revising of the manuscript; S.-C. L. performed the study; J.-Y. C. contributed to the statistical analysis and data interpretation. All authors read and approved the final manuscript.









