In recent years, the need to find new bioactive molecules has raised the scientific community’s interest on marine and freshwater microalgae. These photosynthetic prokaryotic organisms have the capacity to modulate their metabolism to synthesise different metabolites depending on environmental conditions. Moreover, microalgae are known to be a diverse source of bioactive molecules that make them attractive to the pharmaceutical industry( Reference Apel and Weuster-Botz 1 ). In this regard, microalgae are rich sources of n-3 PUFA such as α-linolenic acid (ALA), EPA and DHA( Reference Martins, Custodio and Barreira 2 ). Moreover, the oxidative transformation of these fatty acids to different types of oxylipins is currently being investigated to be used as nutraceuticals and therapeutic agents. Oxylipins are present in different kingdoms in nature and are involved in cellular development, defensive system and stress responses( Reference Sasso, Pohnert and Lohr 3 ); moreover, they can act as inter-kingdom signalling molecules( Reference Pohl and Kock 4 ). In this regard, many oxylipins in animals are known as a family of pro-resolving lipid mediators, which have an important role in the prevention and resolution of the inflammatory process( Reference Serhan 5 – Reference Barden, Mas and Mori 7 ). In a previous study, microalgal biomass from Chlamydomonas debaryana was fractioned and chemically characterised, leading to the isolation of different oxylipins including (9Z,11E,13S,15Z)-13-hydroxyoctadeca-9,11,15-trienoic acid ((13S)-HOTE), produced by the oxidation of ALA. This compound inhibited the production of the pro-inflammatory cytokine TNF-α in lipopolysaccharide-stimulated THP-1 monocyte-derived macrophages( Reference de Los Reyes, Ávila-Román and Ortega 8 ). The fractions also were investigated in vitro by using a bio-guide study to evaluate their cytotoxic, antioxidant and anti-inflammatory activities. The more active fraction proved was that containing oxylipins, mainly (13S)-HOTE (data not published). Another study from our group reported that the lyophilised biomass of C. debaryana and (13S)-HOTE as its major oxylipin constituent had preventive effects on 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced acute ulcerative colitis (UC) in rats. The beneficial effects of these products in the active phase of inflammatory bowel disease (IBD) were related to an inhibition of TNF-α production as well as the suppression of cyclo-oxygenase 2 (COX-2) and inducible nitric oxide (iNOS) expression( Reference Ávila-Román, Talero and Alcaide 9 ).
IBD is a chronic, idiopathic and relapsing inflammatory disorder of the gastrointestinal tract that encompasses UC or Crohn’s disease (CD). The current IBD treatment is based on standard therapies such as salicylates, corticosteroids, immunomodulators and immunosuppressive drugs( Reference Engel and Neurath 10 ) to achieve and maintain the remission and prevent disease progression. These pharmaceutical treatments are not effective in many patients, who can acquire opportunist infections or develop cancers( Reference Engel, Khalil and Neurath 11 ). Nutrition plays a pivotal role in the clinical care of IBD patients as demonstrated since years( Reference Davies and Rhodes 12 , Reference Goh and O’Morain 13 ). It has been shown that the use of probiotics, prebiotics or the Western diet are beneficial to improve symptoms of IBD( Reference Durchschein, Petritsch and Hammer 14 ). In this sense, modulating the diet in these patients with an n-3 PUFA-rich diet( Reference Calder 15 ) has been suggested as an alternative strategy to ameliorate the effects of the relapses and allow a wide clinical remission in these patients.
The aim of the present study was to explore the effects of the oxylipin-containing lyophilised microalgae (OLM) biomass from C. debaryana on a recurrent TNBS colitis model in mice, on the basis of different TNBS applications. Given the clinical course of IBD, considered as a chronic disease with periods of relapse and remission, we were interested in analysing the prophylactic effects of OLM as a pre-treatment, as well as further exploring its effects as curative treatment during the relapsing phases of the disease. Specifically, we report that OLM biomass can effectively ameliorate relapsing colitis by down-regulating pro-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-17) and COX-2, iNOS and NF-κB protein expressions as well as up-regulating the antioxidant heme oxygenase 1 (HO-1) protein levels through nuclear factor E2-related factor 2 (Nrf-2) signalling pathway.
Methods
Mice
For the present study, 8-week-old female BALB/c mice (18–22 g) were supplied by Janvier Labs and were maintained in our Animal Laboratory Center under standard conditions (temperature of 24–25°C, humidity of 70–75 % and 12 h light–12 h dark cycle). Mice were allowed free access to a standard diet (Panlab) and water ad libitum, and were deprived of food for 12 h before the induction of colitis. All animal experiments were approved by the Animal Ethics Committee of the University of Seville, and all experiments were in accordance with the recommendations of the European Union regarding animal experimentation (Directive of the European Council 2010/63/EU).
Induction of colitis and treatments
Colitis induction was performed as described previously( Reference Mariman, Kremer and van Erk 16 ), with slight modifications. For ethical use of animals tested, ten mice per group were used following the 3R principle. In brief, mice were sensitised by application of 3·75 mg of TNBS (Sigma-Aldrich) in 50 % (v/v) ethanol to the shaved dorsal skin on day 0. Experimental colitis was induced by administration of increasing doses (0·75, 1·0 and 2·5 mg/mouse) of TNBS in 50 % ethanol on days 7, 14 and 21, respectively. Mice were anaesthetised with ketamine (100 mg/kg of animal) and diazepam (5 mg/kg of animal), and subsequently administered TNBS in ethanol via a sterile catheter (external diameter 2 mm) inserted 35-mm intra-rectally. Following this, mice were maintained in a head-down position for 30 s to ensure appropriate distribution of TNBS in the colon. A reference control group was included for comparison with the TNBS-induced group; the sham group received physiological saline instead of the TNBS solution in a comparable volume. On day 23, that is, 2 d after the third TNBS instillation, mice were killed and colons were removed. After cleaning colons in physiological saline to remove faecal residues, they were weighed, measured and subjected to macroscopic evaluation, before further processing for histology, cytokine production and protein expression studies.
The OLM biomass of the microalga C. debaryana was obtained and characterised as previously described( Reference de Los Reyes, Ávila-Román and Ortega 8 ). The microalgal biomass was emulsified in 0·9 % saline solution and <2 % Tween-80 (Sigma Chemical Company), and administered by the oral route at doses of 50, 100 and 200 mg/kg of animal. These doses were chosen on the basis of our previous experience( Reference Ávila-Román, Talero and Alcaide 9 ) and also according to preliminary experiments with a reduced number of animals (unpublished results). The three doses assayed (50, 100 and 200 mg/kg) contained 0·008, 0·017 and 0·035 mg of (13S)-HOTE, respectively, the main oxylipin constituent of the OLM biomass. Mice were treated three times a week with the OLM biomass, starting 14 d before the first intra-rectal challenge with TNBS and until being killed( Reference Mariman, Kremer and van Erk 16 ). The sham and TNBS groups also received the vehicle (0·9 % saline solution and <2 % Tween-80) by the oral route.
Histological study
For histological analysis, portions of the middle colon (cut at 6–7 cm from the anus) from different groups were fixed in 4 % buffered paraformaldehyde, dehydrated by increasing concentrations of ethanol and embedded in paraffin. Thereafter, sections of tissue were cut at 5 μm on a rotary microtome (Leica Microsystems), mounted on clean glass slides and dried overnight at 37°C. The sections were cleared, hydrated and stained with haematoxylin–eosin for histological evaluation of colonic inflammation, according to standard protocols. All tissue sections were examined under an Olympus BH-2 microscope (GMI Inc.) for characterisation of histopathological changes. The tissues were analysed by a blinded observer to establish a composite histological score as previously described( Reference Santiago, Pagan and Isidro 17 ) with slight modifications. Criteria include mucosal architecture (0, absent; 1, mild; 2, medium; 3, severe), cellular infiltration (0, none; 1, infiltrate around the crypt basis; 2, infiltrate reaching the muscularis mucosae; 3, infiltrate reaching the submucosa), and goblet cell depletion (0, absent; 1, present). The semi-quantitative histopathological score of each variable was added to give a total microscopic damage score (maximum of 7).
Analysis of cytokine levels
TNF-α, IL-1β, IL-6, IL-17 and IL-10 concentrations in colon tissues were measured by quantitative enzyme immunoassay kits according to the manufacturer’s protocol (Diaclone). A random subset of colon samples (ten per group) from the three representative parts of the colonic tissue (proximal, middle and distal) was weighed and homogenised at 4°C in PBS (pH 7·2) and 1 % bovine serum albumin containing 0·01 mg/ml leupeptin, 0·01 mg/ml pepstatin, 0·01 mg/ml aprotinin and 1 mm-phenylmethylsulphonyl fluoride. Next, the homogenates were centrifuged at 12 000 g for 10 min. Cytokine levels were determined in duplicate and expressed as picograms per milligram of tissue.
Isolation of cytoplasmic proteins and Western blot assay
A random subset of frozen colonic tissue samples (six per group) from the three representative parts of the colon (proximal, middle and distal) was weighed and homogenised in ice-cold buffer (50 mm-Tris-HCl, pH 7·5, 8 mm-MgCl2, 5 mm-ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′-tetra acetic acid, 0·5 mm-EDTA, 0·01 mg/ml leupeptin, 0·01 mg/ml pepstatin, 0·01 mg/ml aprotinin, 1 mm-phenylmethylsulphonyl fluoride and 250 mm-NaCl). The homogenates were centrifuged (12 000 g , for 15 min, at 4°C), and the supernatants were collected and stored at −80°C. Protein concentration of the homogenates was determined following the Bradford colorimetric method( Reference Bradford 18 ). Aliquots of the supernatants containing equal amounts of protein (100 μg) were separated on 10 % acrylamide gel by SDS PAGE. In the next step, the proteins were electrophoretically transferred onto a nitrocellulose membrane and incubated with specific primary antibodies: rabbit anti-iNOS (1:1000; Stressgen-Enzo Life Sciences), rabbit anti-COX-2 (1:3000; Cayman Chemical), rabbit anti-Nrf-2 (1:500; Santa Cruz Biotechnology), rabbit anti-NF-κB (1:1000; Cell Signaling), rabbit anti-PPAR-γ (1:1000; Cell Signaling) and rabbit anti-HO-1 (1:500; Enzo Life Sciences). Each membrane was washed three times for 15 min and incubated with secondary horseradish peroxidase-linked anti-rabbit (Pierce Chemical Company). To prove equal loading, the blots were analysed for β-actin expression using an anti-β-actin antibody (Sigma-Aldrich). Immunodetection was performed using an enhanced chemiluminescence light-detecting kit (SuperSignal West Pico Chemiluminescent Substrate). Next, the immunosignals were captured by using LAS-3000 Imaging System (Fujifilm Image Reader), and densitometric data were obtained after normalisation to the control (housekeeping gene). The signals were analysed and quantified with Scientific Imaging Systems (Biophotonics ImageJ Analysis Software; National Institute of Mental Health) and expressed as a percentage with respect to the sham control group.
Statistical analysis
All values in the figures and text are expressed as arithmetic means with their standard errors. Data were evaluated using GraphPad Prism version 5.00 software (GraphPad Software, Inc.). In all cases, the Shapiro–Wilk test was used to verify the normality of the data. The Mann–Whitney U test was chosen for non-parametric values. ANOVA was used for parametric value groups. P values <0·05 were considered to be statistically significant. In the histological experiment, the results shown are representative of at least three independent experiments performed on different days. The statistical test used for individual analyses is provided in the figure legends.
Results
Treatment with lyophilised microalgae biomass reduces primary outcome parameters of recurrent 2,4,6-trinitrobenzenesulfonic acid colitis
Mice were subjected to oral treatment with an OLM biomass in 0·9 % saline solution, or with saline solution as vehicle, for 2 weeks before the first TNBS challenge (sensitisation on day 0). During the experimental period, the sham group showed body weight gain as compared with baseline (day −1) (104·4 (sem 1·11) %). In contrast, mice subjected to colitis showed a transient weight loss for 2 d immediately following each TNBS instillation (Fig. 1(a)). At the end point (day 23), mice subjected to colitis induction (TNBS group) showed a significant loss in body weight (84·54 (sem 1·87) %) as compared with the sham group (P<0·05). Mice treated with the different doses of OLM biomass also showed transient weight loss after each TNBS instillation; however, they presented less weight loss over the entire follow-up period compared with the TNBS group (repeated measurement ANOVA, P<0·01 and P<0·05 for 100 and 200 mg/kg, respectively), especially in the recovery period at doses of 100 and 200 mg/kg of OLM biomass (Fig. 1(a)).
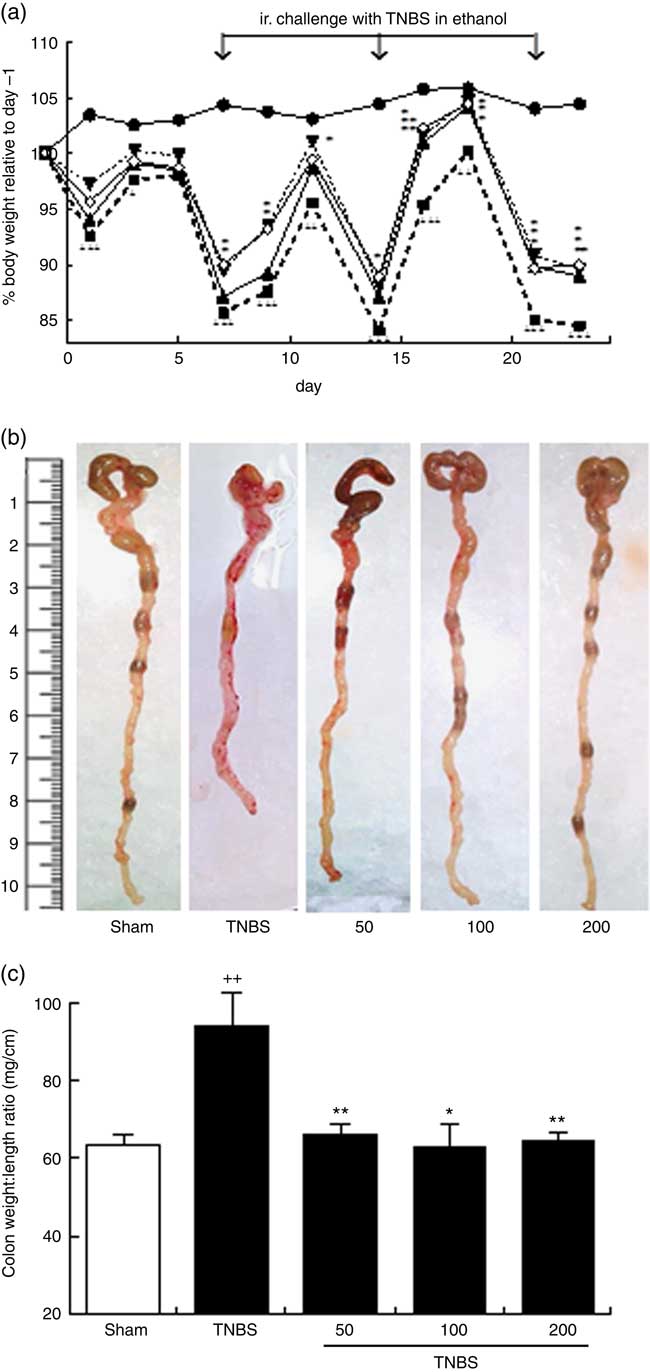
Fig. 1 (a) Effects of the oxylipin-containing lyophilised microalgae (OLM) biomass (50, 100 and 200 mg/kg, by mouth) on body weight development in the recurrent trinitrobenzene sulfonic acid (TNBS) colitis model in mice (n 10 mice/group). Body weights were normalised against body weight on day –1. (b) Representative photographs of the colonic tissue of the different groups; colon shortening is a marker of a higher tissue inflammation. (c) Colon weight:length ratio is a parameter reflecting changes in colon morphology (n 10 mice/group). Mean was significantly different compared with the TNBS group: *P < 0.05, **P < 0.01 (Mann–Whitney U test). †† Mean value was significantly different compared with the sham group (P < 0.01; Mann–Whitney U test). ![]() , Sham;
, Sham; ![]() , TNBS;
, TNBS; ![]() , 50;
, 50; ![]() , 100;
, 100; ![]() , 200.
, 200.
After 2 d of the third TNBS instillation (on day 23), mice were killed and colons were removed and evaluated macroscopically. Colons of TNBS mice revealed a shortening (Fig. 1(b)), accompanied by an increased colon weight:length ratio, a marker of tissue inflammation (P<0·01), in comparison with the sham group (Fig. 1(c)). Colons of the OLM biomass mice, treated with 50, 100 or 200 mg/kg, exhibited a marked suppression in colon inflammation, as demonstrated by the reduction in the weight:length ratio of the colon (P<0·01, P<0·05, P<0·01, respectively) (Fig. 1(b) and (c)). Although haemorrhages and the absence of solid stool are a hallmark during the acute phase following each rectal TNBS instillation, these features were unremarkable on day 23, and for this reason they were not included in the evaluation of treatment effects. Adhesions of the colon to adjacent tissues were only sporadically observed.
Lyophilised microalgae biomass alleviates microscopic colon damage, preserves the epithelial glandular architecture and decreases neutrophil infiltration
Colitis-associated histological features were examined on H&E-stained sections of the middle part of the colon to establish a composite score as described previously( Reference Santiago, Pagan and Isidro 17 ), on the basis of mucosal architecture, cellular infiltration and goblet cell depletion. Colon samples of sham mice revealed typical features of a normal structure (Fig. 2(a)). In contrast, on day 23, a marked inflammation involving all layers of the bowel wall and a higher histological score with loss of mucosal architecture, massive cell infiltration and goblet cell depletion were evident in mice with colitis in relation to the sham group (P<0·05) (Fig. 2(b) and (f)). However, mice treated with OLM biomass showed an improvement in the microscopic features of colitis with all the doses used, evidenced by preservation of the colonic mucosal structure and a reduction of inflammatory cells in the lamina propria when compared with the TNBS group (Fig. 2(c–e)). In addition, administration of 200 mg/kg of OLM biomass significantly inhibited the microscopic damage score (P<0·05) (Fig. 2(f)).

Fig. 2 Histological appearance of mouse colonic mucosa after haematoxylin/eosin staining: (a) sham group; (b) trinitrobenzene sulfonic acid (TNBS) group; (c, d, e) oxylipin-containing lyophilised microalgae (OLM) biomass groups (50, 100 and 200mg/kg, by mouth). OLM biomass administration attenuated microscopic colon damage induced by TNBS administration evidenced by a preservation of the colonic mucosal structure, a reduction of inflammatory cells (black star) in the lamina propria and an improvement in the appearance of the goblet cells (black arrows). (f) Histopathological score of the colon (0–7) (n 5) was evaluated as indicated in the Methods section. *Mean was significantly different compared with the TNBS group (P < 0.05; Mann–Whitney U test). † Mean value was significantly different compared with the sham group (P < 0.05; Mann–Whitney U test). Original magnification 100x.
Effect of lyophilised microalgae biomass on cytokine levels
To support the beneficial effects of OLM biomass on colon inflammation and explore its possible mechanism of action, we examined the production of several inflammatory cytokines that are highly involved in IBD and the anti-inflammatory cytokine IL-10. Immune cell infiltration detected in the histological examination of the colon from TNBS mice correlated with increased levels of pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-17, in relation to control animals (P<0·05, P<0·05, P<0·05 and P<0·01, respectively) (Fig. 3). In accordance with the suppression of the histological score, the colonic production of TNF-α and IL-1β was significantly reduced in animals treated with the highest dose of OLM biomass (P<0·05) (Fig. 3(a) and (b)). Moreover, administration of OLM biomass resulted in a significant suppression, under basal levels, of IL-6 production at the three doses assayed (P<0·01) (Fig. 3(c)). With regard to IL-17 levels, exposure to OLM biomass significantly attenuated the colonic production of this cytokine at doses of 100 and 200 mg/kg (P<0·05) (Fig. 3(d)). IL-10 production analysis revealed significantly increased levels in mice with colitis in comparison with sham animals (P<0·05). In contrast, levels of this cytokine were lower in mice treated with the OLM biomass, reaching a significant value at the dose of 200 mg/kg (P<0·05).
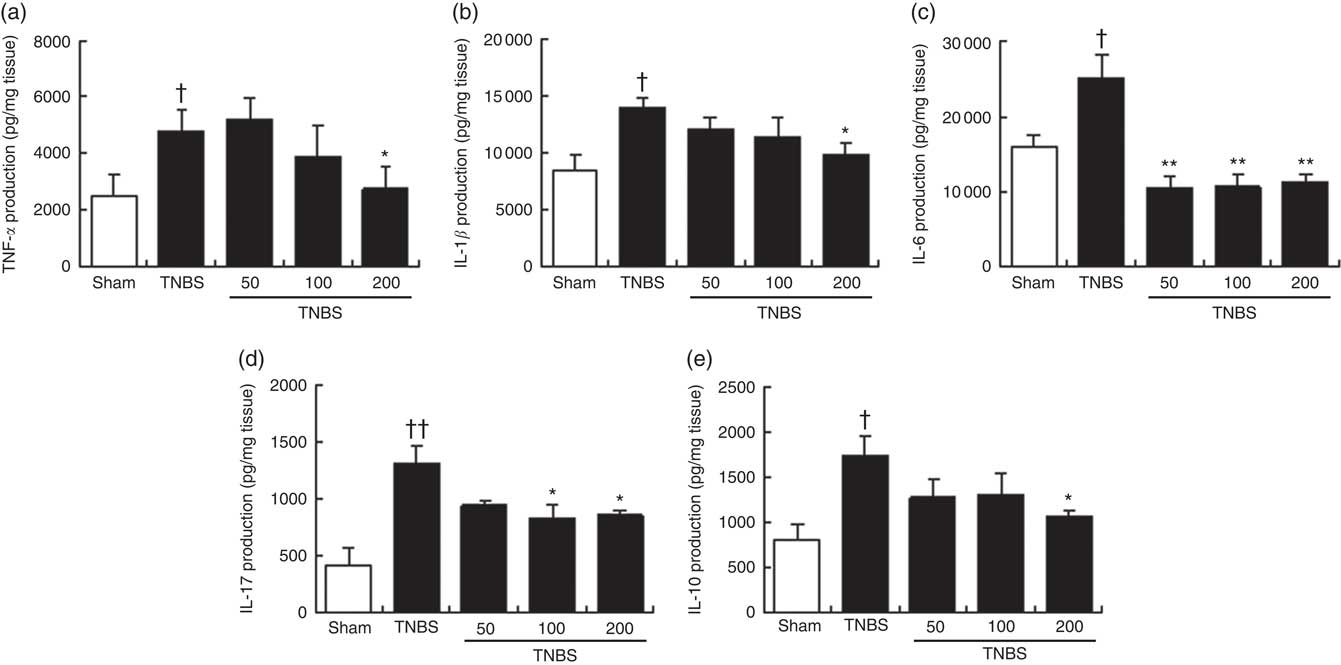
Fig. 3 Effects of the oxylipin-containing lyophilised microalgae (OLM) biomass (50, 100 and 200mg/kg, by mouth) on production of cytokines in colon tissue in relapsing trinitrobenzenesulfonic acid (TNBS)-induced colitis in mice. (a) TNF-α (pg/mg tissue), (b) IL-1β (pg/mg tissue), (c) IL-6 (pg/mg tissue), (d) IL-17 (pg/mg tissue) and (e) IL-10 (pg/mg tissue). OLM biomass reduced the production of the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-17 and the anti-inflammatory cytokine IL-10. Results are representative of six experiments performed on different samples. Values are means, with standard errors represented by vertical bars. Mean value was significantly different compared with the TNBS group: *P < 0.05, **P < 0.01 (Mann–Whitney U test). Mean value was significantly different compared with the sham group: † P < 0.05, †† P < 0.01 (Mann–Whitney U test).
Effect of lyophilised microalgae biomass on inducible nitric oxide and cyclo-oxygenase 2 expressions
We examined iNOS and COX-2 expressions by Western blotting of cytosolic extracts from colonic mucosa (Fig. 4(a)). Exposure of the colon to TNBS-induced a pronounced increase in iNOS (P<0·05) and COX-2 (P<0·05) protein levels when compared with sham animals. Oral treatment with OLM biomass resulted in a significant decrease in iNOS expression (100 and 200 mg/kg, P<0·05) and COX-2 levels (200 mg/kg, P<0·05). Interestingly, both protein expressions drastically decreased to basal levels with the highest dose (Fig. 4(b) and (c)).
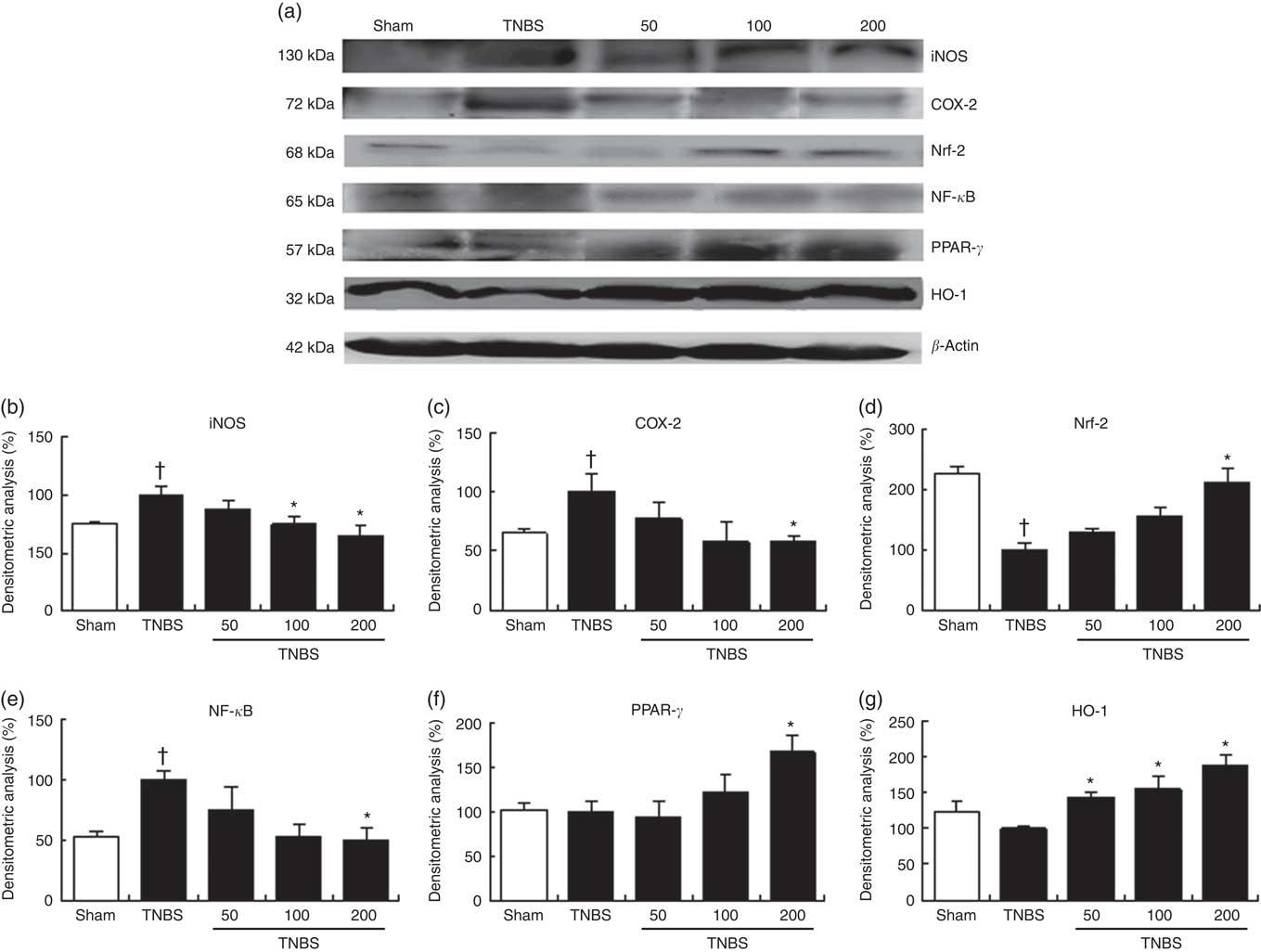
Fig. 4 Effect of the oxylipin-containing lyophilised microalgae (OLM) biomass administration on colonic protein levels. Protein expression was quantified in mice treated with trinitrobenzenesulfonic acid (TNBS) alone or receiving TNBS plus OLM biomass (50, 100 and 200 mg/kg, by mouth). (a) Densitometric data were studied following normalisation to the control (housekeeping gene, β-actin). (b) Representative Western blot analysis of inducible nitric oxide synthase (iNOS), (c) cyclo-oxygenase-2 (COX-2), (d) nuclear factor (erythroid-derived 2)-like 2 (Nrf-2), (e) nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), (f) PPAR-γ and (g) heme oxygenase (HO-1) proteins. Results are representative of six experiments performed on different samples. Values are means, with standard errors represented by vertical bars. *Mean value was significantly different compared with the TNBS group (P < 0.05; Mann–Whitney U test). † Mean value was significantly different compared with the sham group (P < 0.05; Mann–Whitney U test).
Effect of lyophilised microalgae biomass on regulation of NF-κB and PPAR-γ expressions
To further explore the molecular action mechanisms underlying the anti-inflammatory effects of lyophilised microalgal biomass in TNBS-induced recurrent colitis, we also determined the role of NF-κB and PPAR-γ signalling pathways (Fig. 4(a)). Accumulating evidence has demonstrated that PPAR-γ activation may palliate the NF-κB-mediated inflammatory process( Reference Hou, Lee and Lewis 19 ). Mice with TNBS-induced colitis showed a pronounced increase in NF-κB-p65 protein expression levels (P<0·05), which was significantly decreased in mice treated with 200 mg/kg of lyophilised microalgae (P<0·05). These findings were accompanied by a significant increase in PPAR-γ protein expression in mice treated with the highest dose of OLM biomass (P<0·05) (Fig. 4(e) and (f)).
Effect of lyophilised microalgae biomass on regulation of heme oxygenase 1 expression via nuclear factor E2-related factor 2 activation
It is well known that oxidative stress plays an important role in chronic inflammation promoting its maintenance along time. In stressful conditions, Nrf-2 transcriptional factor is activated to achieve the expression of antioxidant proteins such as HO-1 to ameliorate the oxidative effects. To identify whether lyophilised biomass from C. debaryana could attenuate the TNBS-induced colon injury through regulation of the antioxidant transcriptional factor Nrf-2-dependent signalling, we measured Nrf-2 and HO-1 protein expressions by Western blotting (Fig. 4(a)). Nrf-2 protein expression was significantly down-regulated in TNBS-induced colitis mice in comparison with the sham group (P<0·05). On the contrary, a significant up-regulation of Nrf-2 was observed in the colon of mice treated with the highest dose of lyophilised microalgae (P<0·05). In accordance with these observations, oral treatment with OLM biomass (50, 100 and 200 mg/kg) significantly up-regulated HO-1 protein expression (P<0·05) (Fig. 4(d) and (g)).
Discussion
IBD, whose major forms are UC and CD, are multifactorial disorders of the gastrointestinal tract characterised by uncontrolled and relapsing chronic inflammation of the intestinal mucosa. IBD affects millions of people worldwide, and current therapies have modest results with many side-effects. It has been reported that diet plays a key role in the development of the disease, and therefore developing nutritional interventions against uncontrolled inflammation remains important( Reference Durchschein, Petritsch and Hammer 14 , Reference Hashemi, Villa and Comelli 20 , Reference Vargas Robles, Citalán Madrid and García Ponce 21 ). In this respect, n-3 PUFA have been suggested as a pivotal treatment for IBD because of their anti-inflammatory effects( Reference Marion-Letellier, Savoye and Beck 22 , Reference Hou, McMurray and Chapkin 23 ). Microalgal species are a promising source of n-3 PUFA and derived oxylipins, which are lipid mediators with a key role in the resolution of many inflammatory disorders. Our group has recently demonstrated the anti-inflammatory effects of an OLM biomass of the microalgae C. debaryana and its main oxylipin constituent, (13S)-HOTE, in an acute colitis model in rats. Given the clinical course of IBD, considered as a chronic disease with periods of relapse and remission, which frequently remains as quiescent colitis, the aim of this study was to assess not only the preventive effects of this OLM biomass but also its therapeutic effects in the acute phase of an experimental recurrent IBD.
Clinically, IBD is a disease with cycles of remission and relapse, characterised by the infiltration of innate and adaptive immune cells in the colonic mucosa. These responses can be mimicked in an animal model of relapsing colitis induced by weekly intracolonic instillations of low doses of TNBS( Reference Mariman, Kremer and van Erk 16 ). In this model, an infiltration of CD4+ and CD8+ T cells, macrophages and mast cells in the intestinal mucosa as well as the initial production of Th1 cytokines, followed by a Th17 profile after 3 weeks of initial TNBS application, have been reported. In the present study, we selected a time point of 2 d after the third intra-rectal TNBS instillation to examine the effects of the OLM biomass on the acute phase of relapsing colitis. Our data showed that treatment of mice with OLM biomass inhibited body weight loss, colon shortening and microscopic colon damage. Colonic samples from the TNBS group showed increased levels of pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-17, which were decreased in OLM biomass-treated mice. These findings are of interest, as a recent study has confirmed the predominant Th1/Th17 profile in IBD, where cells with this mixed phenotype commonly produce these cytokines( Reference Riaz, Sollid and Olsen 24 ). On the other hand, IL-10 has been reported to be an anti-inflammatory cytokine because it inhibits pro-inflammatory cytokine production as well as T cell stimulatory capacities of macrophages and dendritic cells( Reference Geginat, Larghi and Paroni 25 ). Our results evidenced reduced levels of IL-10 in OLM biomass-treated mice when compared with the TNBS group. These findings suggest that, owing to the lower levels of pro-inflammatory cytokines detected in these animals, colonic production of IL-10 was not required, and confirm a lower hyperactive immune response after treatment with the OLM biomass of C. debaryana.
The NF-κB signalling pathway is activated persistently in IBD and stimulates the transcription of pro-inflammatory genes including TNF-α, IL-1β, IL-6, COX-2 and iNOS( Reference Lau, Liu and Pahlevan 26 ). On the other hand, PPAR-γ is a regulator of fatty acid storage and glucose metabolism transcriptional factor that has been shown to be deficient in IBD. Therefore, PPAR-γ agonists, mainly lipid mediators, have been used in experimental IBD diseases( Reference Celinski, Dworzanski and Fornal 27 , Reference Choo, Lee and Yan 28 ). Moreover, activation of PPARγ down-regulates IBD-induced stimulation of the NF-κB signalling pathway, and thus reduces the production of pro-inflammatory factors( Reference Soubh, Abdallah and El-Abhar 29 ). In the present study, the OLM biomass administered orally significantly down-regulated colonic expressions of iNOS, COX-2 and NF-κB induced by TNBS administration as well as increased levels of PPAR-γ. Our findings suggest that the anti-inflammatory effect of this OLM biomass may be partly due to the decrease in NF-κB protein levels and the increase in PPAR-γ, with a consequent reduction in the production of pro-inflammatory mediators. These data are in agreement with previous studies, which showed that the family of oxylipins including resolvins, lipoxins, protectins and maresins have potent anti-inflammatory effects in the acute phase of inflammation. These actions are related to inhibition of the production of cytokines and chemokines and expressions of pro-inflammatory genes, as well as activation of apoptosis( Reference Arita, Clish and Serhan 30 – Reference Willenberg, Ostermann and Giovannini 32 ). In addition, many of these lipid mediators have been valued as PPAR-γ agonists, with consequent interaction and inhibition of the NF-κB signalling pathway( Reference Arita 33 – Reference Schwanke, Marcon and Bento 35 ). These molecules are also believed to be pro-resolving, thereby actively involved in wound healing and return of tissue homoeostasis after an acute inflammatory process( Reference Serhan, Chiang and Dalli 36 ). In addition, these findings have shown an important role of oxylipins in chronic inflammation( Reference Hsiao, Thatcher and Colas 37 ) as well as host defence, pain, organ protection and tissue re-modelling( Reference Serhan 38 , Reference Barden, Moghaddami and Mas 39 ). It has been recently suggested that these pro-resolution-inducing agents could suppress reactive oxygen species (ROS) production, enhance wound healing and have cytoprotective properties in IBD without causing immunosuppression( Reference Das 40 ). Although their mechanisms of action are poorly understood, supplementation with specialised pro-resolving lipid mediators may be an important therapeutic strategy in chronic inflammatory diseases( Reference Hsiao, Thatcher and Colas 37 , Reference Jia, Jin and Xiao 41 , Reference Börgeson, Johnson and Lee 42 ).
Inflammatory cells infiltrate the wounded colonic mucosa and create oxidative stress, producing ROS and nitrogen reactive species, respectively, which contribute to chronic inflammation-associated neoplastic transformation( Reference Khor, Huang and Prawan 43 ). Nrf-2 plays a key role as a transcription factor regulating the antioxidant response against extracellular stresses. This factor is translocated into the nucleus where it binds with antioxidant response elements (ARE) and transactivates antioxidant genes such as HO-1 ( Reference Casili, Cordaro and Impellizzeri 44 ). Numerous in vitro and in vivo studies indicate that Nrf-2 also plays a main role in the inhibition of pro-inflammatory pathways( Reference Wang, Zhang and Zhang 45 , Reference Shang, Shi and Wang 46 ), suggesting that this factor may be used as a novel target for the treatment of IBD. It has been suggested that the antioxidant and anti-inflammatory effects of Nrf-2 activators may be mediated through suppression of NF-κB activation( Reference Yao, Zhao and Zhao 47 ). In addition, connections between PPAR-γ and Nrf-2 pathways have been observed as PPAR-γ stimulation activated Nrf-2 in an in vitro model of oxidative stress-mediated lung inflammation( Reference Hsu, Lee and Pan 48 ). In the present study, we showed reduced Nrf-2 expression levels in colon samples from the TNBS group when compared with the sham group, which were recovered after oral administration of the OLM biomass. These results were accompanied by a significant increase in the expression of the Nrf-2 target gene HO-1 in OLM biomass-treated mice. In agreement with our observations, a recent in vitro study has reported that a plant-derived oxylipin is able to provide protection against H2O2-induced oxidative stress( Reference Taki-Nakano, Ohzeki and Kotera 49 ). Altogether, these data indicate that this microalgal biomass may protect colonic mucosa from TNBS damage by reducing oxidative stress through regulation of the Nrf-2-ARE signalling pathway and potential intracellular connections with PPAR-γ and NF-κB pathway.
In conclusion, our results clearly demonstrate for the first time the prophylactic and curative effects of an OLM biomass from C. debaryana in a recurrent TNBS-induced colitis mice model. These actions may be related to PPAR-γ stimulation and NF-κB inhibition in parallel with potentiated antioxidant defence by activated Nrf-2 pathway, with subsequent reduction of pro-inflammatory mediators. Further investigations are needed to obtain complete knowledge of the mechanism of action of these lipid products and their function as local pro-resolving factors. These studies support the potential use of this microalga, or derived oxylipins, as a therapeutic agent in the treatment of relapsing IBD.
Acknowledgements
This study was supported by grants from Ministerio de Economía y Competitividad MICIIN PSE-0632/0151 and MICIIN INNPACTO-IPT-2012-1370-060000. The authors thank ‘Centro de Investigación, Tecnología e Innovación’ of the University of Seville for providing technical assistance. The authors also thank Adelina de la Jara (Instituto Tecnológico de Canarias, Spain) for providing lyophilised microalgal samples and Dr Eva Zubía and Dr Carolina de los Reyes (Department of Organic Chemistry, Faculty of Marine and Environmental Sciences, University of Cádiz, Spain) for performing chemical characterisation of OLM from C. debaryana.
The authors’ contributions are as follows: J. A.-R., S. G.-M. and V. M. designed the study protocol; J. A.-R., E. T. and A. R.-L. conducted the in vivo experiments; J. A.-R. and E. T. performed the histological experiments and analysed the data; J. A.-R. and E. T. wrote the draft of the manuscript. All the authors critically reviewed and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.







