Diabetes mellitus has a history dating back about 3000 years as described by the ancient Egyptians(Reference Lakhtakia1). Over the passage of the time, progressively more knowledge has been gathered concerning the aetiology and pathogenesis of the disease which is now defined as prominent metabolic disorder that leads to hyper-glycaemia due to deficiency of insulin action and/or secretion(2). The major form, type 2 diabetes (T2DM) is a rapidly increasing global phenomenon(2). It is a leading cause of complications such as CVD, retinopathy, neuropathy, nephropathy and infertility which exert profound psychological and physical distress on patients and their families(Reference King, Park and Li3). Since T2DM has developed deep and increasing inroads into society across globe, fuelled by Western diet and obesity, early detection, treatment and prevention are key strategies to reduce morbidity, mortality and both personal and economic costs of the disease(Reference Patti, Cavallari and Andreotti4).
Many treatment options have been utilised to tackle T2DM since ancient times. Currently, mainstay treatment strategies are based on dietary intervention plus the single or combined use of metformin, sulphonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-IV) enzyme inhibitors, sodium-glucose co-transporter-2 (SGLT-2) inhibitors and injections of glucagon-like peptide-1 (GLP-1) mimetics or insulin(Reference Wang, Zhao and Yang5). However, these medications are not always accessible in some parts of the world; they can be very expensive and if not used with diligence can induce undesirable side effects. This directs attention towards addition of natural products found in plants to the fight against diabetes as a means of safe, economical and useful anti-diabetic therapy that can be used as dietary adjuncts or as an avenue towards new pharmaceutical products. Thus, many herbs/plants and their phytochemicals such as flavonoids have been reported to exhibit anti-diabetic properties mediated by various actions such as inhibition of α-glucosidase or preservation of enhancement endocrine pancreatic function(Reference Ghorbani6).
Interestingly, the importance of food in medicine was recognised in the 5th century BC by Hypocrates, the father of Western medicine, by his most popular phrase ‘let food be thy medicine and let medicine be thy food’ which vividly indicated the significance and medicinal benefit of herbs. Similar citations can be found in ancient traditional Chinese medicinal texts where tea was used in treatment of all type of disease(Reference Yang, Chen and Wu7). Black tea has attracted great interest for its various therapeutic and nutritional effects(Reference Yang, Chen and Wu7). Several bioactive agents in black tea are particularly interesting compared with many other herbal plants(Reference Li, Lo and Pan8). The potential of these natural products and the breadth of their effects are notable(Reference Hayat, Iqbal and Malik9). As a result, the consumption of tea had been studied extensively for its therapeutic benefits in different diseases including diabetes where it has been suggested to be protective(Reference Babu, Liu and Gilbert10,Reference Pastoriza, Mesias and Cabrera11) . Indeed, Sri Lankan physicians have recommended that pre-diabetic and mildly diabetic subjects should take heavy consumption (6–10 cups/d) of Sri Lankan Broken Orange Pekoe Fanning’s black tea (tea grade containing leaf particles with medium size) based on its reputed beneficial actions(Reference Hayat, Iqbal and Malik9).
Camellia sinensis (black tea) gained attention for its polyphenol compounds including epigallocatechin-3-gallate, epigallocatechin, epicatechin-3-gallate and epicatechin, which have been reported to have wide range of health promoting effects(Reference Li, Wang and Huang12). Recent studies suggest that consumption of this tea is effective in lowering blood glucose in diabetes(Reference Fu, Li and Lin13,Reference Pathak, Millar and Pathak14) , possibly due to ability of polyphenols to improve insulin action(Reference Abeywickrama, Ratnasooriya and Amarakoon15).
Consumption of black tea has also been reported to reduce the total cholesterol and LDL(Reference Fujita and Yamagami16) and decrease cardiovascular risk factors such as TAG and LDL-cholesterol and increase in HDL-cholesterol(Reference Bahorun, Luximon-Ramma and Neergheen-Bhujun17). This is associated with lowered risk of CVD(Reference Li, Wang and Huang12). Furthermore, several studies have positively correlated healthy heart with the consumption of black tea(Reference Hakim, Alsaif and Alduwaihy18). The tea is known to inhibit the clot formation and increase coronary vasodilatation(Reference Vasko, Vaskova and Fejercakova19), flow-mediated dilatation of the brachial artery(Reference Grassi, Mulder and Draijer20), lower blood pressure(Reference Hodgson, Croft and Woodman21) and inhibit the LDL oxidation(Reference Davies, Judd and Baer22).
Despite this wealth of positive observations which potentially counter pathogenic features of T2DM, little detailed information exists on the spectrum of actions responsible for the observed anti-diabetic activity of black tea. In the present study, we have employed in vitro and in vivo techniques to study a wide range of insulinotropic, insulinometic and other anti-diabetic actions of C. sinensis leaves to understand the precise mechanisms of action. Based on previous claims suggesting potential anti-diabetic activity, we have tested the hypothesis that the anti-diabetic actions of C. sinensis plant extract stem from the ability of constituent phytochemicals to interfere with carbohydrate digestion/absorption, enhance insulin action and/or, in particular, promote β-cell function and insulin secretion.
Materials and methods
Collection and preparation of plant extracts
C. sinensis leaves were collected from Jahangirnagar University, Dhaka, Bangladesh, and were identified and documented by the botanical taxonomist, Bangladesh National Herbarium, Mirpur, Dhaka, with assignment of 43 207 as botanical accession number. Plant leaves were washed and air-dried, before proceeding with hot water extraction. A quantity of 25 g of the dried powder of leaves was added into 1 litre of water and heated until boiling. Afterwards, the decoction was allowed to stand for 15 min prior to separation of solid materials utilising filter paper (Whatman no. 1). The oil-separated solution was then dried under a vacuum (Savant Speed vac) to leave a final sticky residue which was stored at 4°C until use(Reference Hannan, Ansari and Haque23). Dimethyl sulphoxide (0·6 %, v/v) was used for dissolving the extract and included in experimental controls. A wide range of in vitro and in vivo tests were performed to test activity as described briefly below. Full details are given in the references cited.
In vitro insulin-releasing studies
Clonal pancreatic β-cells (BRIN-BD11 cells) represent an insulin-releasing cell line generated via electrofusion of RINm5f cells with New England Deaconess Hospital rat pancreatic islet cells(Reference McClenaghan, Barnett and O’Harte24). The insulin-releasing effects were tested using BRIN-BD11 cells(Reference McClenaghan, Barnett and O’Harte24) and isolated mouse islets(Reference Ansari, Azam and Hannan25). Plant extract (1·6–200 µg/ml), peak samples derived from HPLC purification of crude extract described below in the Purification of crude extracts section (P-1–P-13) (60 µl/5 ml; 1·6–1 × 103 µm for peak 8 and peak 9) or isoquercitrin (1·62–50 µm), was incubated with BRIN BD11 cells in the presence or absence of known modulators of insulin secretion at various glucose concentrations for 40 or 20 min at 37°C. The concentrations of 1·1, 5·6 or 16·7 mm glucose were selected as representing non-stimulatory, basal and stimulatory glucose concentrations, respectively. Islets were isolated from the pancreas of Swiss albino mice as previously described(Reference Ojo, Srinivasan and Owolabi26) using collagenase P obtained from Clostridium histolyticum (Sigma-Aldrich). Islets were cultured for 24–48 h in an atmosphere of 5 % CO2 at 37°C in Roswell Park Memorial Institute media prior to pre-incubation in Krebs–Ringer bicarbonate (KRB) buffer at 1·4 mm glucose for 1 h. Test incubations were conducted in the presence of 16·7 mm glucose for 1 h. Supernatant samples were aliquoted and stored at −20°C for insulin measurement using a dextran-coated charcoal RIA developed by Flatt & Bailey(Reference Flatt and Bailey27). Islets were also retrieved to measure insulin content following acid–ethanol extraction (1·5 % HCl, 75 % ethanol and 23·5 % H2O)(Reference Ojo, Srinivasan and Owolabi26). In brief, islets were disrupted using pipette tips and extracted at 4°C overnight. Tubes containing the islets were centrifuged at 1200 rpm for 2 min, and the supernatant was kept at −20°C for further analysis. GLP-1 (7–36) (10−6 and 10−8 m), used as a positive control, was supplied by EZBiolab Ltd at 95 % purity. In order to elucidate the secretory pathway activated by C. sinensis, these studies included assessment of the effects of established insulin secretion modulators including diazoxide (KATP-channel opener), tolbutamide (sulphonylurea and KATP-channel blocker), verapamil (voltage-dependent Ca2+ channel blocker), isobutylmethylxanthine (phosphodiesterase inhibitor), 30 mm KCl and 10 mm alanine, both of which depolarise the plasma membrane and cause Ca2+ influx. This is primarily accomplished by alanine through co-transport of the amino acid with Na+ plus metabolism and the production of ATP(Reference Ansari, Flatt and Harriott28).
Membrane potential and intra-cellular calcium concentration
Changes in membrane potential and intra-cellular Ca of BRIN-BD11 cells in response to C. sinensis extract were determined using a FLIPR Membrane Potential Assay Kit and a FLIPR Calcium Assay Kit (Molecular Devices), respectively, as described previously(Reference Abdel-Wahab, Marenah and Flatt29). In brief, BRIN-BD11 cells were seeded in ninety-six-well microplates and allowed to attach overnight at 37°C for 18 h. The medium was discarded, and the cells were incubated for 10 min at 37°C with 100 μl of KRB buffer containing 5·6 mm glucose. After this, the cells were incubated with 100 μl of FLIPR membrane potential or calcium dye (reconstituted using assay buffer) at 37°C for 60 min. Changes in signal intensity were detected in a FlexStation 3 scanning fluorimeter (Molecular Devices).
Cellular glucose uptake assay
3T3 L1 fibroblast cell monolayers were differentiated into adipocytes as previously described(Reference Hannan, Ali and Khaleque30). These cells were then incubated for 30 min at 37°C with plant extract plus or minus 100 nm insulin prior to the addition of 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)-2-deoxyglucose (50 nm) for 5 min. The wells were washed with ice-cold PBS, and three to four coverslips were mounted in each slide. Four images were taken of the coverslips using a 10× magnification on the microscope. The fluorescence intensity was measured as an indicator of glucose uptake.
Glycation of insulin
Effects of C. sinensis on in vitro protein glycation were assessed as described previously(Reference Kasabri, Flatt and Abdel-Wahab31). d-Glucose (246·5 mm) in sodium phosphate buffer (10 mm) was incubated with human insulin (1 mg/ml) and NaBH3CN (0·0853 g/ml) in the absence (control) and presence of leaf extract (50, 100 and 200 μg/ml). After 24 h incubation at 37°C, the reaction was stopped with the addition of 0·5 m acetic acid. Separation and quantification of glycated and non-glycated insulin were performed using reversed-phase HPLC (RP-HPLC)(Reference O’Harte, Højrup and Barnett32).
Dipeptidyl peptidase-4 enzyme activity in vitro
Effects of C. sinensis extract on DPP-IV enzyme activity in vitro were determined using a fluorometric method. Enzyme activity was determined using ninety-six-well black-walled, clear-bottomed microplates (Greiner) containing 8 mU/ml of DPP-IV enzyme and 200 µm of substrate (Gly-Pro-AMC) as described previously(Reference Duffy, Green and Irwin33). Flex Station 3 (Molecular Devices) was used to measure the changes in fluorescence at excitation/emission of 370/440 nm and 2·5 nm slit width.
Starch digestion
Starch digestion studies were performed as described previously(Reference Thomson, Ojo and Flatt34). Starch (100 mg) (Sigma-Aldrich) was dissolved in water with or without plant extract or acarbose as a positive control. Heat-stable α-amylase (0·01 %; from Bacillus licheniformis; Sigma-Aldrich) was added, and after incubation at 80 ºC for 20 min, the mixture was diluted and incubated with 0·1 % amyloglucosidase from Rhizopus mold (Sigma-Aldrich). After a further 30 min at 60°C, samples were taken and stored for subsequent analysis of glucose liberation using the glucose oxidase/four-aminophenazone-phenol method (Randox GL 2623).
Glucose diffusion in vitro
A previously described in vitro glucose diffusion model(Reference Gallagher, Flatt and Duffy35) was employed using cellulose ester dialysis tubes (20 cm × 7·5 mm, Spectra/Por®CE layer, MWCO: 2000, Spectrum). Briefly, a 2 ml volume supplemented with 220 mm glucose, with or without leaf extract, gaur gum or pectin, was loaded into dialysis tubing. Both ends of each tube were sealed tightly and placed in 45 ml 0·9 % NaCl (45 ml). After 24 h shaking at 37°C, glucose was measured outside the tube.
Animals
Sprague–Dawley male rats (Envigo UK, approximately 350–400 g) were fed a high-fat diet (20 % protein, 45 % fat and 35 % carbohydrate; 26·15 kJ/g total energy percentage, Special Diet Service) for 5–6 weeks before experiments. Age-matched rats maintained on a standard rodent diet (10 % fat, 30 % protein and 60 % carbohydrate making 12·99 kJ/g total energy; Trouw Nutrition) were used as controls. We used twenty-four rats in total, namely a normal control group (n 8), high-fat-fed (HFF) group (n 8) and HFF group receiving plant treatment (n 8). The dose of extract employed (250 mg/5 ml per kg) was selected on the basis of previous studies and was considered to represent a high but reasonable level of ingestion not associated with any ill effects. Longer-term effects of daily treatment with C. sinensis in HFF rats were assessed at intervals over the 9-d period without any signs of discomfort, altered behaviour or other side effects. Additional experiments to test insulin secretion from isolated mouse pancreatic islets and the in vivo effects of isoquercitrin on oral glucose tolerance were performed using 6–8 weeks old, male Swiss albino mice (n 7; Envigo UK). All animal experiments were performed in accordance with the Principles of Laboratory Animals Care and the UK animals Scientific Procedures Act 1986.
Oral glucose tolerance
To evaluate effects of the extract of C. sinensis leaves (250 mg/5 ml per kg) and the isolated phytochemical (isoquercitrin) (100 mg/kg) on glycaemic control, oral glucose tolerance tests (18 mmol/kg) were conducted using 6 or 12 h fasted HFF rats or mice in both acute or chronic studies (6 d) as described previously(Reference Ansari, Flatt and Harriott28). Blood was collected at times indicated in Figs. 3(c) and (d), 5(a) and (b) and 10(e) and (f) for measurement of glucose and plasma insulin by RIA(Reference Flatt and Bailey27).
Dipeptidyl peptidase-4 enzyme activity in vivo
HFF rats were used to study DPP-IV inhibitory activity of orally administered C. sinensis (250 mg/5 ml per kg) extract following acute administration or chronic treatment over nine consecutive days. The activity of DPP-IV was determined in plasma by a fluorometric assay(Reference Duffy, Green and Irwin33) as mentioned above.
Glucose homoeostasis after 9-d treatment with hot water extract of Camellia sinensis leaves in high-fat-fed rats
Sprague–Dawley rats fed a high-fat diet received twice-daily oral gavage of either saline vehicle (0·9 % (w/v)) or plant extract (250 mg/5 ml per kg body weight) for nine consecutive days. Body weight, food and fluid intakes, blood glucose and plasma insulin were measured at regular intervals. Glucose tolerance (18 mmol/kg) was evaluated after 6 d of treatment. At the end of the study, pancreatic tissues were collected to measure islet morphology and pancreatic insulin content(Reference Ansari, Flatt and Harriott28).
Islet morphology after 9-d treatment with extract of Camellia sinensis leaves (250 mg/5 ml per kg body weight) in high-fat-fed rats
Pancreatic tissues were fixed, processed, stained and analysed as described previously(Reference Srinivasan, Ojo and Owolabi36). Sections (5–8 μm) were incubated overnight at 4 °C with primary anti-body (mouse anti-insulin (1:500) and guinea pig anti-glucagon (1:400)). The slides were incubated at room temperature with secondary anti-body mixture (Alexa Fluor 594 goat anti-mouse anti-body and Alexa Fluor 488 goat anti-guinea pig anti-body). After staining the nucleus with 4′,6-diamidino-2-phenylindole, the slides were mounted and subjected to analysis using tetramethylrhodamine isothiocyanate (594 nm) or fluorescein isothiocyanate filters (488 nm) using a fluorescent Olympus System Microscope BX51 (Olympus instruments). Images were captured using a DP70 camera adapter system. Cell^F imaging software (Olympus System Microscope BX51, Olympus instruments) was used to determine islet distribution, islet area, β-cell and α-cell area.
Purification of crude extracts
Crude leaf extracts were re-dissolved in 0·12 % (v/v) TFA/water and purified by RP-HPLC. The prepared crude extract solution was injected into a (22 × 250 mm) Vydac 218TP1022 10 μm (C-18) RP-HPLC column (Grace, Deerfield) equilibrated with 0·12 % (v/v) TFA/water at a flow rate of 5 min/ml. The concentration of acetonitrile within the eluting solvent was expanded using linear gradients to 20 % over 10 min, 70 % over a period of 40 min. Further, insulinotropic fractions were purified using a Vydac 208TP510 (10 × 250 mm) semi-preparative stainless steel 5 μm C-18 column (Phenomenex) at a flow rate of 1 ml/min. The wavelengths of 254 nm and 360 nm were used to measure the absorbance, and fractions were collected according to the appearance of peaks in individual time points(Reference Kissoudi, Sarakatsianos and Samanidou37).
Structural characterisation of purified extracts
Molecular weights of fractions (peak samples) were determined using LC-MS via Electrospray Ionization MS. Fractions (peak samples) were separated on a Spectra System LC (Thermo Separation Products) using a Kinetex 5 µm F5 LC column ((150 × 4·6 mm) (Phenomenex)) with UV detection at 220–360 nm as described previously(Reference Zhang, Zhao and Ohland38).
Confirmation of extract purity and identity
All NMR spectra were physically staged and routinely baseline-corrected as described previously by Grace et al.(Reference Grace, Warlick and Neff39) for compound identity confirmation followed by HPLC and LC-MS analyses. NMR spectra had been recorded on a 600 MHz Bruker AVIII HD spectrometer outfitted with a 5 mm BBO H & F cryogenic test. Standard one-dimensional composite pulse sequencing (zgcppr) was used to obtain 1H NMR spectra. The 13C NMR spectra were obtained with the aid of the use of the reverse-gated decoupling pulse sequence (zgig), and the purchase parameters were set as described previously(Reference Grace, Warlick and Neff39).
Statistical analysis
A statistical analysis software for windows, Graph Pad prism 5, was used for analysis of data using unpaired Student’s t test (nonparametric, with two-tailed P values) and one-way ANOVA with Bonferroni post hoc tests as appropriate. AUC values were calculated using concentrations at individual data points following trapezoidal rules with baseline subtraction. Values were presented as mean values with their standard errors; the significant limit was determined as P < 0·05.
Results
Effects of Camellia sinensis on insulin release from BRIN-BD11 cells
Concentration-dependent effects of C. sinensis (1·6–200 μg/ml) extract on insulin release are shown in Fig. 1(a) and (b). Alanine (10 mm) and KCl (30 mm) were used as positive controls. The extract produced dose-dependent increase in insulin secretion by 1·5–2·6-fold in comparison with 5·6 mm or 16·7 mm glucose controls (P < 0·05–P < 0·001; Fig. 1(a) and (b)). Cells were viable at up to 200 μg/ml concentrations, but LDH release was increased by 20–75 % at higher concentrations indicating loss of cellular viability (Supplementary Fig. S1(a) and (b)).
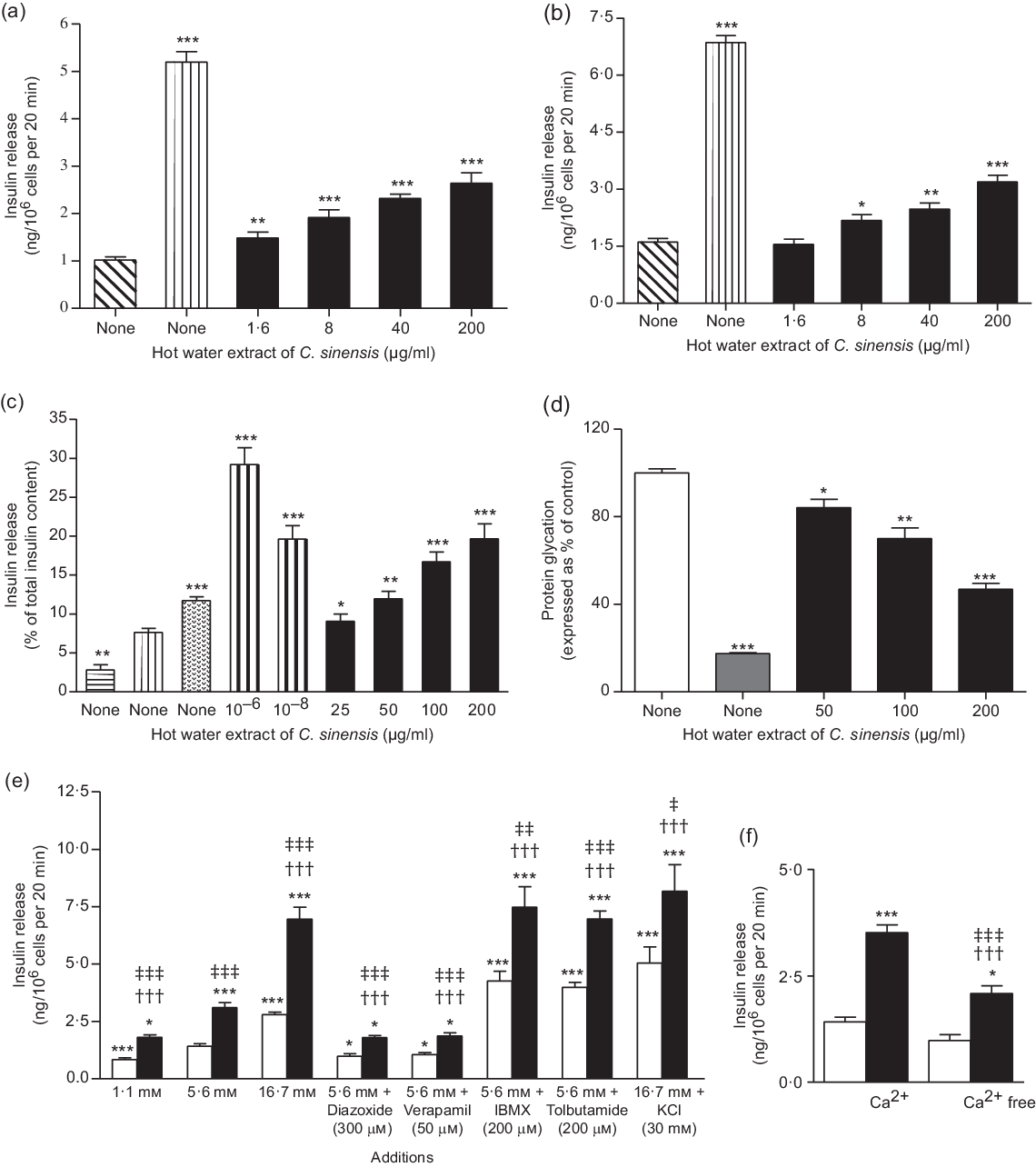
Fig. 1. Dose-dependent effects hot water extract of Camellia sinensis leaves on insulin release from (a and b) BRIN-BD11 cells and (c) islets of Langerhans, (d) protein glycation, (e) insulin secretion in the presence of established stimulators or inhibitors and (f) absence of extracellular calcium from BRIN BD11 cells. Values are means with their standard errors for n 4–8 for insulin release and n 3 for protein glycation. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with control 5·6 mm glucose for (a) and (e), 16·7 mm glucose for Fig. 1(b) and (c) and 220 mm glucose + insulin (1 mg/ml) for (d). ††† P < 0·001 compared with 5·6 mm glucose in the presence of the extract for (e) and (f). ‡ P < 0·05, ‡‡ P < 0·01 and ‡‡‡ P < 0·001 compared with respective incubation in the absence of the extract for (e) and (f). (a) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 10 mm alanine;
, 5·6 mm glucose + 10 mm alanine; ![]() , 5·6 mm glucose + C. sinensis (µg/ml). (b)
, 5·6 mm glucose + C. sinensis (µg/ml). (b) ![]() , 16·7 mm Glucose;
, 16·7 mm Glucose; ![]() , 16·7 mm glucose + 30 mm KCl;
, 16·7 mm glucose + 30 mm KCl; ![]() , 16·7 mm glucose + C. sinenesis (µg/ml). (c)
, 16·7 mm glucose + C. sinenesis (µg/ml). (c) ![]() , 1·4 mm Glucose;
, 1·4 mm Glucose; ![]() , 16·7 mm glucose;
, 16·7 mm glucose; ![]() , 16·7 mm glucose + 10 mm alanine;
, 16·7 mm glucose + 10 mm alanine; ![]() , 16·7 mm glucose + glucagon-like peptide-1 (10–6 and 10–8 m);
, 16·7 mm glucose + glucagon-like peptide-1 (10–6 and 10–8 m); ![]() , 16·7 mm glucose + C. sinensis (μg/ml). (d)
, 16·7 mm glucose + C. sinensis (μg/ml). (d) ![]() , 220 mm Glucose + 1 mg/ml insulin;
, 220 mm Glucose + 1 mg/ml insulin; ![]() , 200 mm glucose + 1 mg/ml insulin + aminoguaninine;
, 200 mm glucose + 1 mg/ml insulin + aminoguaninine; ![]() , 200 mm glucose + 1 mg/ml insulin + C. sinensis (µg/ml). (e)
, 200 mm glucose + 1 mg/ml insulin + C. sinensis (µg/ml). (e) ![]() , Glucose alone;
, Glucose alone; ![]() , glucose + hot water extract of C. sinensis (200 µg/ml). (f)
, glucose + hot water extract of C. sinensis (200 µg/ml). (f) ![]() , Glucose (5·6 mm);
, Glucose (5·6 mm); ![]() , glucose (5·6 mm) + C. sinensis (200 µg/ml).
, glucose (5·6 mm) + C. sinensis (200 µg/ml).
Effects of Camellia sinensis on insulin release from isolated mouse islets
Fig. 1(c) shows C. sinensis effects on insulin release from islets in the presence of 16·7 mm glucose. Increasing the concentrations of extract from 25 to 200 μg/ml potentiated glucose-induced insulin secretion by 1·6–2·8-fold (P < 0·05–0·001, Fig. 1(c)). GLP-1 (10−6 and 10−8 m) and alanine (10 mm) were used as positive controls (P < 0·001).
Effects of Camellia sinensis on glycation of insulin
Insulin glycation was inhibited by 16–53 % (P < 0·05–0·001; Fig. 1(d)) by the leaf extract in a concentration-dependent manner (50–200 µg/ml). The positive control, aminoguanidine (44 mm), inhibited insulin glycation by 82 % (P < 0·001; Fig. 1(d)).
Insulinotropic effects of Camellia sinensis with known modulators of insulin release
A non-toxic concentration (200 µg/ml) of C. sinensis extract was used to probe the mechanisms underlying its insulin secretory actions as shown in Fig. 1(e) and (f). A significant stimulation of insulin release (P < 0·05–0·001) was observed in the absence and presence of modulators such as 16·7 mm glucose (P < 0·001), isobutylmethylxanthine (P < 0·01) and tolbutamide (P < 0·001). Incubation of extract with 30 mm KCl depolarised cells also significantly increased insulin secretion (P < 0·05; Fig. 1(e)). This insulin-releasing effects were partially inhibited by diazoxide, verapamil and in the absence of extracellular Ca (P < 0·05 and P < 0·001; Fig. 1(e) and (f)).
Effects of Camellia sinensis on BRIN-BD11 cell membrane depolarisation and intra-cellular calcium concentration
Extract significantly depolarised (P < 0·001) the plasma membrane and increased intra-cellular Ca concentration of BRIN-BD11 cells (Fig. 2(a) and (b)). Pharmacological depolarisation with KCl (30 mm) and alanine (10 mm) which triggers insulin release by elevation of intra-cellular Ca was used as positive controls.
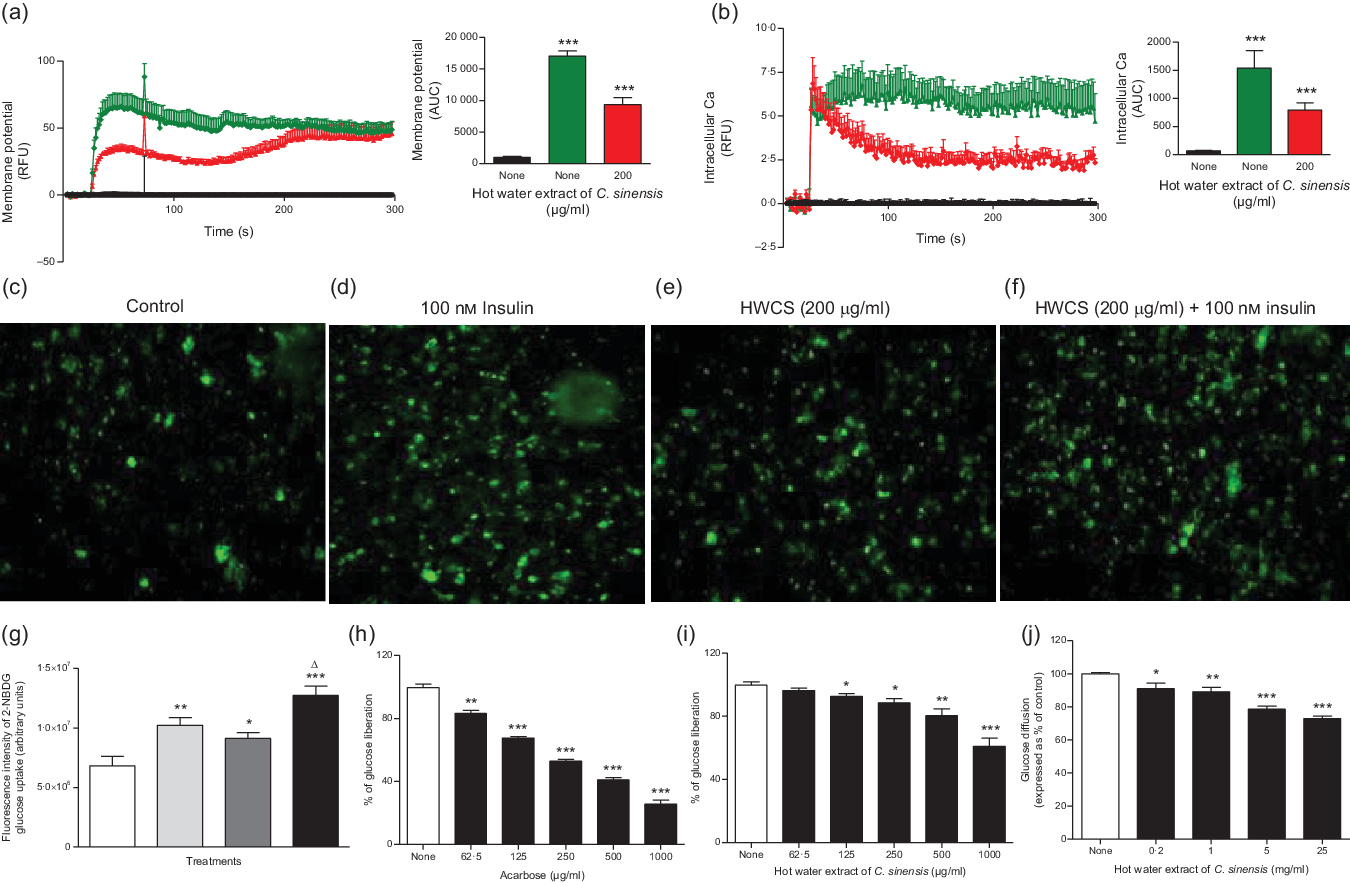
Fig. 2. Effects of hot water extract of Camellia sinensis (HWCS) leaves on (a) membrane potential and (b) intra-cellular calcium in BRIN BD11 cells (c, d, e, f and g) glucose uptake in differentiated 3T3L1 adipocyte cells (h and i) starch digestion and (j) glucose diffusion in vitro. Changes of fluorescence intensity in differentiated 3T3L1 adipocytes incubated with extract in the (e) absence or (f) presence of 100 nm insulin. Images were taken at 10× magnification. Values are means with their standard errors for n 6 for membrane potential and intra-cellular calcium, n 4 for glucose uptake, starch digestion and glucose diffusion. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with control. (a) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 30 mm KCl;
, 5·6 mm glucose + 30 mm KCl; ![]() , 5·6 mm glucose + C. sinensis (200 μg/ml). (b)
, 5·6 mm glucose + C. sinensis (200 μg/ml). (b) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 10 mm alanine;
, 5·6 mm glucose + 10 mm alanine; ![]() , 5·6 mm glucose + C. sinensis (200 μg/ml). (c)
, 5·6 mm glucose + C. sinensis (200 μg/ml). (c) ![]() , Control. (d)
, Control. (d) ![]() , 100 nm Insulin. (e)
, 100 nm Insulin. (e) ![]() , HWCS (200 μg/ml). (f)
, HWCS (200 μg/ml). (f) ![]() , HWCS (200 μg/ml) + 100 nm insulin. (g)
, HWCS (200 μg/ml) + 100 nm insulin. (g) ![]() , Control;
, Control; ![]() , 100 nm insulin;
, 100 nm insulin; ![]() , HWCS (200 μg/ml);
, HWCS (200 μg/ml); ![]() , 100 nm insulin + HWCS (200 μg/ml). (h)
, 100 nm insulin + HWCS (200 μg/ml). (h) ![]() , Control;
, Control; ![]() , acarbose (μg/ml). (i)
, acarbose (μg/ml). (i) ![]() , Control;
, Control; ![]() , C. sinensis (μg/ml). (j)
, C. sinensis (μg/ml). (j) ![]() , 220 mm Glucose alone;
, 220 mm Glucose alone; ![]() , 220 mm glucose + C. sinensis (mg/ml).
, 220 mm glucose + C. sinensis (mg/ml).
Effects of Camellia sinensis on glucose uptake and insulin action
The uptake of the fluorescent glucose analogue 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)-2-deoxyglucose by 3T3L1 differentiated adipocyte cells. Fig. 2(c)–(g) represents changes in fluorescence intensity after glucose absorption by differentiated 3T3L1 adipocyte cells. The number of cells taking up glucose is proportional to the intensity of the fluorescence. However, the fluorescence intensity in controls was low, but it was increased by 100 nm insulin, hot water extract of C. sinensis (HWCS) (200 μg/ml) and HWCS (200 μg/ml) + 100 nm insulin (Fig. 2(c)–(g)). C. sinensis (200 μg/ml) in the absence or presence of insulin (100 nm) significantly (P < 0·05–0·001) enhanced the glucose uptake (Fig. 2(g)) by 25 and 43 %, respectively.
Effects of Camellia sinensis on starch digestion
C. sinensis extract dose dependently (125–1000 µg/ml) inhibited starch digestion by 8–39% (P < 0·05–0·001; Fig. 2(i)). Acarbose (62·5–1000µg/ml), used as positive control, produced a 17–72 % decrease in starch digestion (P < 0·05–0·001, Fig. 2(h)).
Effects of Camellia sinensis on glucose diffusion in vitro
Leaf extract of C. sinensis (mg/ml) significantly reduced glucose diffusion in concentration-dependent manner by 9–27 % (P < 0·05–0·001, Fig. 2(j)).
Effects of Camellia sinensis on dipeptidyl peptidase-4 enzyme activity in vitro
As shown in Fig. 3, sitagliptin, an established inhibitor, decreased in vitro DPP-IV enzyme activity by 15–98 % (P < 0·01–0·001; Fig. 3(a)) at 1 × 10−3–10 µm. C. sinensis extract inhibited DPP-IV activity by 5–59 % at 40–5000 µg/ml (P < 0·05 and P < 0·001; Fig. 3(b)).
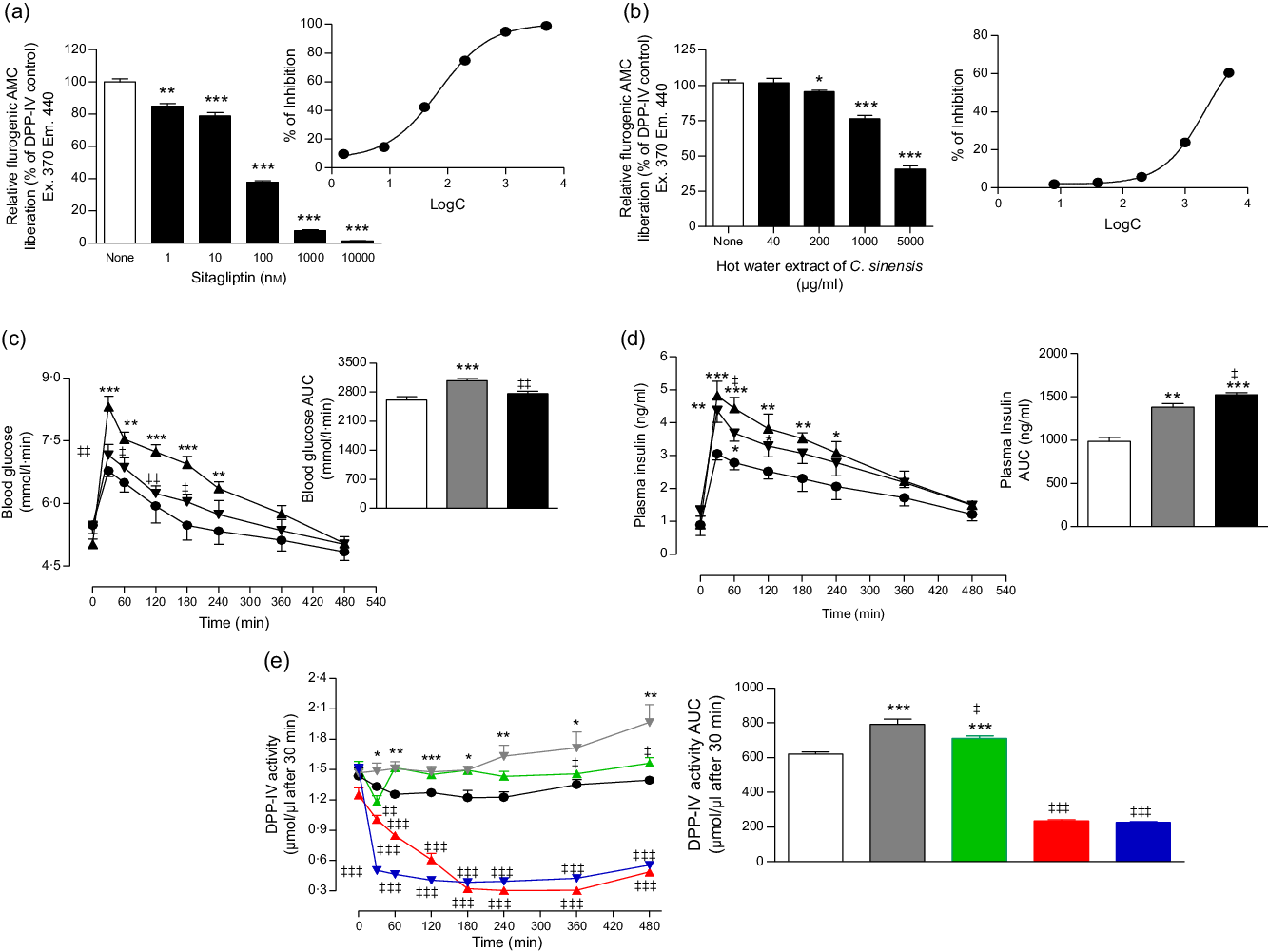
Fig. 3. Effects of hot water extract of Camellia sinensis leaves on (a and b) dipeptidyl peptidase-4 (DPP-IV) enzyme activity in vitro, (c) glucose tolerance, (d) plasma insulin and (e) plasma DPP-IV in high-fat-fed rats. Parameters were measured prior to and after oral administration of glucose alone (18 mmol/kg body weight, control) or with C. sinensis extract (250 mg/5 ml per kg body weight). Established DPP-IV inhibitors: sitagliptin and vildagliptin, were used as positive controls. Values are means with their standard errors, n 3 for DPP-IV enzyme activity in vitro and n 6 for glucose tolerance, plasma insulin and DPP-IV in vivo. *P < 0·05, **P < 0·01 and ***P < 0·001, compared with normal control and ‡ P < 0·05, ‡‡ P < 0·01 and ‡‡‡ P < 0·001 compared with high-fat-fed diet control. (a) ![]() , Control;
, Control; ![]() , sitagliptin (nm). (b)
, sitagliptin (nm). (b) ![]() , Control;
, Control; ![]() , C. sinensis (μg/ml). (c)
, C. sinensis (μg/ml). (c) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (d)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (d) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (e)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (e) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg);
, high fat diet + C. sinensis (250 mg/5 ml per kg); ![]() , high fat diet + sitagliptin (10 μmol/5 ml per kg);
, high fat diet + sitagliptin (10 μmol/5 ml per kg); ![]() , high fat diet + vildagliptin (10 μmol/5 ml per kg).
, high fat diet + vildagliptin (10 μmol/5 ml per kg).
Acute effects of Camellia sinensis on oral glucose tolerance
HFF rat groups exhibited significantly increased levels of blood glucose and plasma insulin compared with normal rats (P < 0·05–0·001, Fig. 3(c) and (d)). Following a single dose of HWCS (250 mg/5 ml per kg), glucose tolerance was significantly (P < 0·05–0·01) improved compared with the HFF diet control (glucose alone) (Fig. 3(c)). Similarly, rats treated with the extract displayed a sharper increase in plasma insulin release after 60 min of administration (P < 0·05; Fig. 3(d)).
Acute effects of Camellia sinensis on dipeptidyl peptidase-4 enzyme activity in vivo
C. sinensis (250 mg/5 ml per kg) significantly inhibited DPP-IV enzyme activity (P < 0·05–0·01; Fig. 3(e)) in vivo. The extract evoked a 20 % decrease in a time-dependent manner compared with HFF diet controls. Sitagliptin and vildagliptin (10 µmol/kg), established inhibitors, produced a significant (P < 0·001) approximate 70 % reduction in DPP-IV enzyme activity after 30 min (Fig. 3(e)).
Effects of longer-term administration of Camellia sinensis on body weight, food intake, fluid intake, non-fasting blood glucose, plasma insulin and dipeptidyl peptidase-4 levels
Oral administration of extract (250 mg/5 ml per kg) twice daily for nine consecutive days reduced body weight significantly (P < 0·01; Fig. 4(d)) compared with HFF control rats. Treatment also reduced food intake, fluid intake and blood glucose by 23, 18·5 and 13 %, respectively. The changes were significant from 6 d (P < 0·05–0·001; Figs. 4(a)–(c), (e) and 5(d)). Plasma insulin levels were also increased from 6 d (P < 0·01; Figs. 4(f) and 5(e)). The hot water extract also decreased plasma DPP-IV activity by 22 % (P < 0·05–0·01; Figs. 4(g) and 5(f)).

Fig. 4. Effects of 9 d treatment of twice daily oral administration of hot water extract of Camellia sinensis leaves on (a) food intake, (b) energy intake, (c) fluid intake, (d) body weight, (e) blood glucose, (f) plasma insulin and (g) dipeptidyl peptidase-4 (DPP-IV) enzyme activity in high-fat-fed rats. Parameters were measured, prior to and after oral administration of C. sinensis leaves (250 mg/5 ml per kg body weight) twice daily. Values are means with their standard errors for n 8 rats. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with lean control. ‡ P < 0·05, ‡‡ P < 0·01 and ‡‡‡ P < 0·001 compared with high-fat-fed diet control at corresponding time point. (a) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (b)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (b) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (c)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (c) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (d)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (d) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (e)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (e) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (f)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (f) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (g)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (g) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg).
, high fat diet + C. sinensis (250 mg/5 ml per kg).
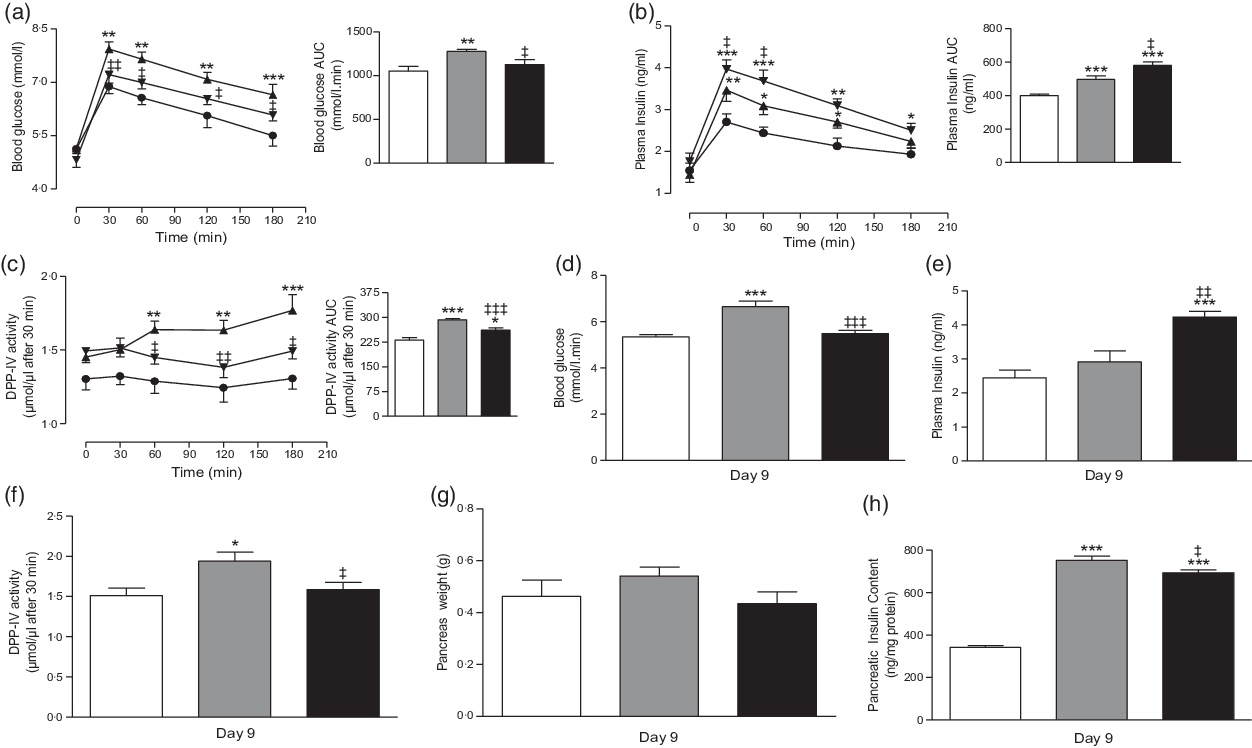
Fig. 5. Chronic effects of twice daily oral administration of hot water extract of Camellia sinensis leaves at day 6 on (a) glucose tolerance, (b) plasma insulin and (c) plasma dipeptidyl peptidase-4 (DPP-IV) and at day 9 on (d) blood glucose, (e) plasma insulin, (f) plasma DPP-IV, (g) pancreas weight and (h) pancreatic insulin content in high-fat-fed rats. Parameters were measured after treatment for 6 or 9 d with twice daily oral administration of hot water extract of C. sinensis leaves (250 mg/5 ml per kg body weight). Values are means with their standard errors with n 8. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with lean control. ‡ P < 0·05, ‡‡ P < 0·01 and ‡‡‡ P < 0·001 compared with high-fat-fed diet control at corresponding time point. (a) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (b)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (b) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (c)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (c) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat diet control (saline);
, high fat diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (d)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (d) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (e)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (e) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (f)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (f) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (g)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (g) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (h)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (h) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg).
, high fat diet + C. sinensis (250 mg/5 ml per kg).
Effects of 6-d administration of Camellia sinensis on glucose tolerance and plasma dipeptidyl peptidase-4
Treatment with C. sinensis (250 mg/5 ml per kg) for 6 d significantly (P < 0·05–0·01) improved oral glucose tolerance compared with the HFF control rats (Fig. 5(a)). This was paralleled by sharper and more prominent increase in insulin secretion within 30 and 60 min following the oral glucose load (P < 0·05; Fig. 5(b)). This effect was accompanied by reduced DPP-IV enzyme activity (P < 0·05–0·01; Fig. 5(c)).
Effects of longer-term administration of Camellia sinensis on pancreatic insulin content
Pancreatic weights of all groups of rats were similar (Fig. 5(g)). Hot water extract reduced the pancreatic insulin content by 8 % (P < 0·05, Fig. 5(h)) compared with HFF control rats. Levels remained increased by 51 % compared with lean control rats (P < 0·001, Fig. 5(h)).
Effects of longer-term administration of Camellia sinensis on islet size distribution and islet cell areas
Pancreatic islet morphology was assessed at the end of the treatment period as shown in Fig. 6(a), (b) and (c)). The number of large islets decreased, while the medium- and small-sized islets number increased (Fig. 6(g)) in the C. sinensis-treated group. There were no significant differences in the number of islets per mm2 in pancreas (Fig. 6(j)). High-fat-feeding increased islet area compared with the lean controls by 2–2·3-fold (P < 0·001, Fig. 6(d)). The β- and α-cell areas were also increased significantly by high-fat feeding (P < 0·001, Fig. 6(e) and (f)). C. sinensis treatment significantly (P < 0·05) reduced islet area (Fig. 6(d)) and β-cell area (Fig. 6(f)) by 15 and 16 %, respectively. The β-cell mass was higher than α-cells in HFF rats compared with normal rats (P < 0·05–0·01 Fig. 6(h) and (i)).

Fig. 6. Effects of 9-d treatment of hot water extract of Camellia sinensis leaves on islet morphology in high-fat-fed rats. Representative images of islets of (a) lean control, (b) high-fat-fed control and (c) high-fat-fed plus hot water extract of C. sinensis (250 mg/5 ml per kg) rats showing insulin in red, glucagon in green and 4′,6-diamidino-2-phenylindole in blue with scale bar of 100 μm, (d) islet area, (e) α-cell area, (f) β-cell area, (g) islet size distribution, (h) α-cell percentage, (i) β-cell percentage and (j) number of islets (per mm2). Values are means with their standard errors for n 8 (about 100 islets per group). *P < 0·05, **P < 0·01 and ***P < 0·001 compared with lean control. ‡ P < 0·05 compared with high-fat-fed diet alone (control). Saline, lean control; HFFR, high-fat-fed rats; HFFR + CS, high-fat-fed rats + C. sinensis. (d) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (e)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (e) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (f)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (f) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (g)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (g) ![]() , Small (<10 000 μm2);
, Small (<10 000 μm2); ![]() , medium (10 000–25 000 μm2);
, medium (10 000–25 000 μm2); ![]() , large (>25 000 μm2). (h)
, large (>25 000 μm2). (h) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (i)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (i) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg). (j)
, high fat diet + C. sinensis (250 mg/5 ml per kg). (j) ![]() , Lean control (saline);
, Lean control (saline); ![]() , high fat fed diet control (saline);
, high fat fed diet control (saline); ![]() , high fat diet + C. sinensis (250 mg/5 ml per kg).
, high fat diet + C. sinensis (250 mg/5 ml per kg).
Purification and structural characterisation of insulinotropic agents from Camellia sinensis
The chromatograms resulting from RP-HPLC of hot water leaf extract are shown in Fig. 7(a)–(d). Fig. 7 shows the identical retention time for (B) rutin, (C) peak 8 and (D) peak 8 plus rutin. Molecular masses (m/z) of peaks 5, 6, 7, 8, 9 and 10 were determined as 596·2, 594·3, 622·5, 610·3, 464·2 and 448·2 Da, respectively (Fig. 8(a)–(f)). The characteristics of the unknown compound using 1H (Fig. 9(a)) and 13C NMR (Fig. 9(b)) are: EI-MS m/z 664·2 Da [M]; (600 MHz, CD3OD, 1H-NMR (δ in ppm): 7·65 (d, 1 H, J = 2·4 Hz), 7·49 (dd, 1 H, J = 2·0 Hz, 12 Hz), 6·81(1 H, d, J = 8·5 Hz), 6·33 (d, 1 H, J = 2·2 Hz), 6·10 (d, 1 H, J = 2·0 Hz), 5·19 (d, 1 H, J = 7·2 Hz), 3·62–3·24 (m, 6 H). 13C NMR (600 MHz, CD3OD, δ in ppm); 157·0 (C-2), 134·2 (C-3), 178·1 (CO-4), 161·6 (C-5), 98·5 (CH-6), 164·6 (C-7), 93·3 (CH-8), 157·6 (C-9), 104·2 (C-10), 121·6 (C-1’), 116·1 (C-2’), 148·4 (C-3’), 144·5 (C-4’), 114·8 (C-5’), 121·8 (C-6’), 104·2 (C1-G), 74·3 (C2-G), 76·7 (C3-G), 69·8 (C4-G’), 76·9 (C5-G), 61·1 (C6-G) [G: glucose].
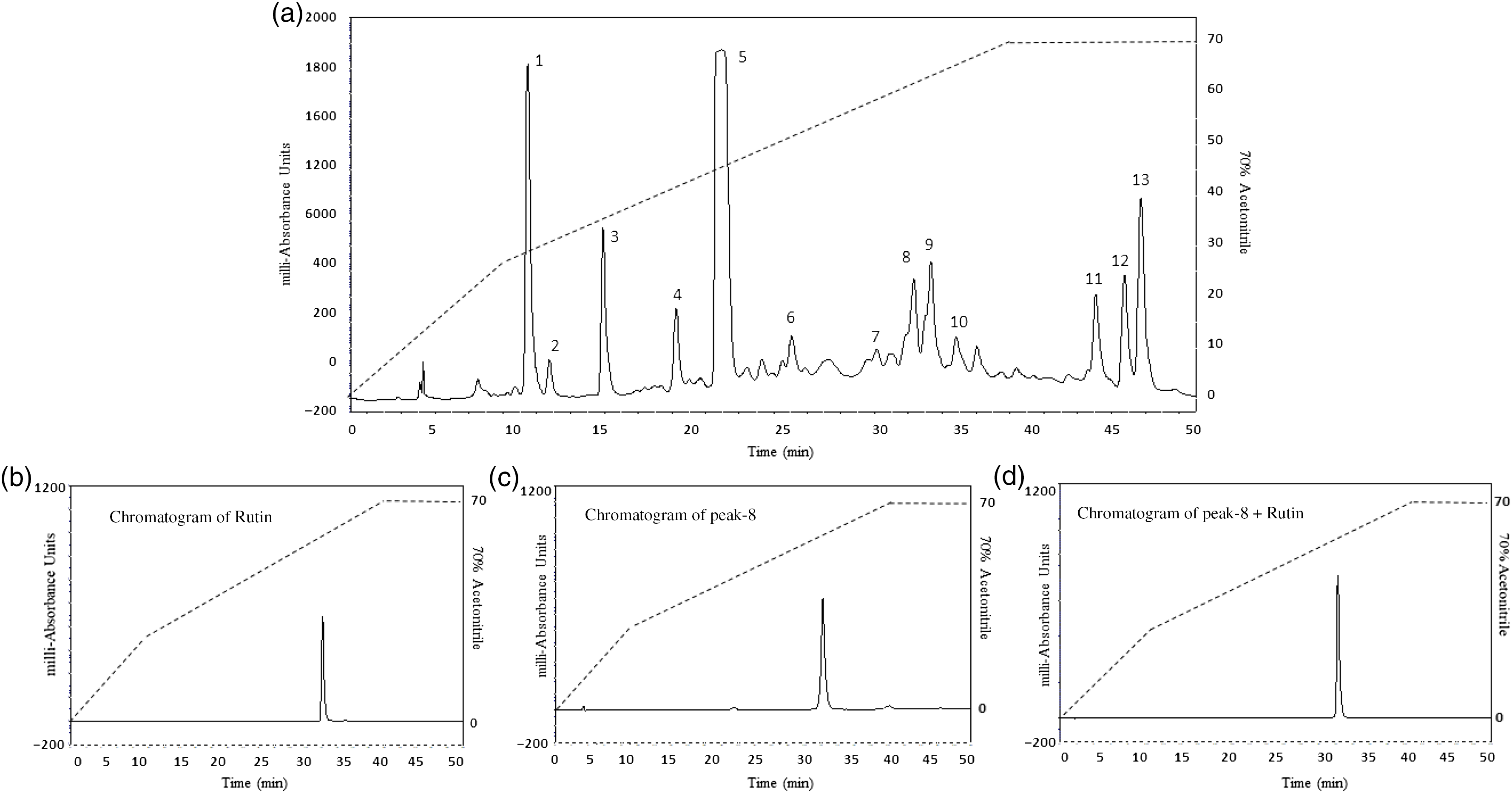
Fig. 7. Representative HPLC profile of (a) hot water extract of Camellia sinensis leaves, (b) rutin, (c) peak 8 and (d) peak 8 + rutin. The extract was chromatographed at a flow rate of 5·0 ml/min on a (22 × 250 mm) Vydac 218TP1022 10 μm (C-18) reversed-phase HPLC column (Grace, Deerfield). The concentration of the eluting solvent was raised using linear gradients of acetonitrile (0–20 % from 0 to 10 min, 20–70 % from 10 to 40 min). Further, insulinotropic fractions were purified using Vydac 208TP510 (10 × 250 mm) semi-preparative stainless steel 5 μm C-18 column (Phenomenex) at a flow rate of 1 ml/min. Compounds were detected by measurement of absorbance at 254 and 360 nm. Details of peaks corresponding to C. sinensis leaves and rutin are presented in the chromatogram, whereas peaks (P) are present in different retention times (RT).

Fig. 8. Molecular mass of peak samples of hot water extract of Camellia sinensis leaves by LC-MS analysis. Peaks were separated on a Spectra System LC using a Kinetex 5 µm F5 LC column (150 × 4·6 mm) (Phenomenex). The mass:charge ratio (m/z) v. peak intensity was determined. Samples of ‘peaks (P) 5 to 10’ with retention times of 22·0, 26, 30·4, 32·2, 33·5 and 35 min were used to determine the molecular weights of unknown compounds with m/z 596·2, 594·3, 622·5, 610·3, 464·2 and 448·2 Da, respectively.

Fig. 9. (a) 1H-NMR, (b) C13-NMR spectrum, isolated compounds (c) isoquercitrin, (d) rutin and (e) quercitrin of peaks 8, 9 and 10 samples obtained from reversed-phase HPLC of hot water extract of Camellia sinensis leaves. Proton-decoupled natural abundance C13- NMR and 1H-NMR spectrum of peak-9 sample of hot water extract of C. sinensis leaves (obtained from chromatograph over the period of 70 % acetonitrile from 10 to 40 min with retention time of 34·2 min) at 40°C. The spectrum was obtained at 600 MHz after 119 044 transients (14 h) by the pulsed Fourier transform method on a Varian XL-100 A spectrometer. Representative structure of flavonoids, corresponding to the molecular formula of rutin (quercetin 3-rutinoside), isoquercitrin and quercitrin are C27H30O16, C21H20O12 and C21H20O11.
The yield of pure isoquercitrin isolated from 1 mg/ml of C. sinensis leaf was 10–15 %. Fig. 9(c)–(e) depicts the molecular structure of predicted compounds: isoquercitrin, rutin and quercitrin isolated from the C. sinensis leaf extract. These results are in strong agreement with previous studies of the same compound(Reference Fernandez, Reyes and Ponce40). With the demonstration that isoquercitrin was present in the crude extract of C. sinensis, further experiments were performed to test if it might contribute to the insulin-releasing and glucose-lowering effects of the extract.
Effects of HPLC peak samples of Camellia sinensis and synthetic isoquercitrin on insulin release
Fig. 10(a) shows the effects of the peak samples (P-1–P-13) of C. sinensis leaf (60 µl/5 ml) on insulin secretion from BRIN-BD11 cells. The peaks labelled P-1, P-2, P-5, P-6, P-7, P-8, P-9 and P-10 increased insulin release significantly (P < 0·05–0·001). However, P-1, P-2, P-6, P-7 and P-10 were associated with toxicity of cells (online Supplementary Fig. S1(c)). Alanine (10 mm) was used as a positive control. Peaks 8 (610·3 Da) and 9 (664·2 Da) stimulated concentration-dependent insulin release (8–1 × 103 μm; P < 0·05–0·001, Fig. 10(b) and (c)) compared with 5·6 mm glucose control. However, at higher concentrations, cell viability was decreased (online Supplementary Fig. S1(d) and (e)). Commercially available isoquercitrin also increased insulin secretion in a concentration-dependent manner between 1·62 and 50 µm (P < 0·05–0·001, Fig. 10(d)). However, at a higher concentration of 50 µm, the compound increased the LDH release significantly (online Supplementary Fig. 1(f)).

Fig. 10. Insulin-releasing effects of (a) peak samples (1–13) of hot water extract of Camellia sinensis leaves, (b) peak 8, (c) peak 9 and (d) isoquercitrin from BRIN-BD11 cells, (e) glucose tolerance and (f) plasma insulin. Mice were fasted for 12 h and administered glucose (18 mmol/kg body weight) by oral gavage in the presence or absence of isoquercitrin (100 mg/kg body weight). Values are means with their standard errors for n 8 for insulin release and n 7 for glucose tolerance and plasma insulin. *P < 0·05, **P < 0·01 and ***P < 0·001 compared with control. (a) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 10 mm alanine;
, 5·6 mm glucose + 10 mm alanine; ![]() , 5·6 mm glucose + peak samples. (b)
, 5·6 mm glucose + peak samples. (b) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 10 mm alanine;
, 5·6 mm glucose + 10 mm alanine; ![]() , 5·6 mm glucose + peak-8 (m/z = 610·3). (c)
, 5·6 mm glucose + peak-8 (m/z = 610·3). (c) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 10 mm alanine;
, 5·6 mm glucose + 10 mm alanine; ![]() , 5·6 mm glucose + peak-9 (m/z = 464·2 Da). (d)
, 5·6 mm glucose + peak-9 (m/z = 464·2 Da). (d) ![]() , 5·6 mm Glucose;
, 5·6 mm Glucose; ![]() , 5·6 mm glucose + 10 mm alanine;
, 5·6 mm glucose + 10 mm alanine; ![]() , 5·6 mm glucose + isoquercitrin (μm). (e)
, 5·6 mm glucose + isoquercitrin (μm). (e) ![]() , Glucose alone;
, Glucose alone; ![]() , glucose + isoquercitrin (100 mg/kg). (f)
, glucose + isoquercitrin (100 mg/kg). (f) ![]() , Glucose alone;
, Glucose alone; ![]() , glucose + isoquercitrin (100 mg/kg).
, glucose + isoquercitrin (100 mg/kg).
Acute effects of isoquercitrin on oral glucose tolerance and insulin responses
Isoquercitrin (100 mg/kg) administered together with oral glucose (18 mmol/kg body weight) significantly (n 7; P < 0·01–0·001) improved blood glucose at 30 and 60 min with overall improvement of glucose tolerance of approximately 20 % (Fig. 10(e)). Isoquercitrin treatment also increased plasma insulin levels with overall 27–29 % greater response (n 7; P < 0·05–0·01; Fig. 10(f)).
Discussion
C. sinensis has been claimed to exhibit anti-diabetic properties(Reference Haidari, Omidian and Rafiei41), but detailed confirmation of this and knowledge of the underlying actions are lacking. The present study was designed to give a full mechanistic insight into the actions of C. sinensis including thorough in vivo studies and evaluation of benefits mediated by improvements of insulin secretion and action, inhibition of DPP-IV enzyme activity, starch digestion, glucose diffusion and protein glycation.
HWCS leaves enhanced insulin secretion in a concentration-dependent manner from clonal β-cells as well as isolated mouse islets. These insulinotropic effects were free from β-cell cytotoxicity at the lower concentrations tested. They were glucose-dependent and mediated primarily through β-cell membrane depolarisation and influx of Ca ions which trigger insulin release. Blockage of secretory effects of C. sinensis by the KATP channel opener, diazoxide(Reference Hannan, Marenah and Ali42), and the voltage-dependent Ca channel blocker, verapamil(Reference Mathews, Flatt and Abdel-Wahab43), implicates direct actions on these ion channels mediated by polar small molecules in the leaf extract. However, insulinotropic effects persisted in cells depolarised by 30 mm KCl or tolbutamide and in the absence of extracellular Ca2+, possibly indicating an ability to activate other pathways such as a direct effect on exocytosis or phosphatidylinositol (PI3) or adenylate cyclase/cAMP(Reference McTaggart, Clark and Ashcroft44).
Insulin primarily acts on skeletal muscle and adipose tissue to regulate post-prandial glucose metabolism(Reference DeFronzo and Tripathy45). An adequate or defective signal reduces the GLUT4 translocation and leads to the development of insulin resistance(Reference Pessin and Saltiel46). Thus, discovery of extracts and active components that can overcome insulin resistance is highly sought after(Reference Fu, Li and Lin13). Encouragingly, C. sinensis leaf extract enhanced glucose uptake by 3T3L1 differentiated adipocyte cells in both the presence and the absence of insulin. Bioactive compounds responsible for this action need to be determined, but previous findings suggest that phytochemicals such as gallic acid(Reference Telapolu, Kalachavedu and Punnoose47), ellagic acid(Reference Telapolu, Kalachavedu and Punnoose47), berberine(Reference Lee, Kim and Kim48) and quercetin(Reference Marín-Aguilar, Pavillard and Giampieri49) activate AMPK pathway and enhance GLUT4 translocation.
Glycosylation of structural proteins is believed to play an important role in diabetes complications. In addition, previous studies have shown that insulin is glycated in the secretory granules during storage in β-cells in diabetes and when secreted exhibits reduced biological activity(Reference Hunter, Boyd and O’Harte50). Therefore, inhibition of the glycation of both structural and functional proteins such as insulin is an additional benefit of anti-diabetic drug therapy. In this study, C. sinensis was found to significantly decrease insulin glycation in a concentration-dependent manner, although less markedly than the established inhibitor, aminoguanidine. We have not studied glycation of structural proteins, but previous studies have reported that C. sinensis polyphenols, in particular epigallocatechin-3-gallate, potentially negatively regulate advanced glycation end products to up-regulate transcription factor, Nuclear factor erythroid-2-related-factor-2 to minimise oxidative damage in T2DM(Reference Fu, Li and Lin13).
Effects of C. sinensis on liberation of glucose from starch were also evaluated using a simple in vitro system and acarbose as a well-known alpha-glucosidase inhibitor. As expected, acarbose inhibited starch digestion by 72–80 %. Although less potent, C. sinensis extract significantly decreased starch digestion in a concentration-dependent manner. Active phytochemicals, such as rutin and isoquercitrin, might be responsible as previous studies have shown that these flavonoids inhibit α-amylase and slow starch digestion(Reference Takahama and Hirota51).
It has been reported that C. sinensis contains high amount of fibre(Reference Adnan, Ahmad and Ahmed52) that may impede gastric emptying(Reference Lattimer and Haub53). Indeed, reduction in intestinal glucose absorption can underlie the anti-hyperglycaemic activity of guar gum which was used clinically as well as many anti-diabetic plants(Reference Edwards, Blackburn and Craigen54). We used an established dialysis-based method to investigate the effects of plant extract on glucose diffusion. C. sinensis extract significantly inhibited glucose passage through dialysis membrane in a concentration-dependent manner.
One of the major contributors to the aetiology of T2DM is obesity, and we employed HFF rats with dietary-induced obesity-diabetes to test the in vivo efficacy of C. sinensis. In acute studies, oral administration of C. sinensis extract together with glucose load significantly improved oral glucose tolerance and plasma insulin responses. This effect was associated with inhibition of circulating activity of DPP-IV enzyme which normally degrades the N-terminal region of the incretin hormones, GLP-1 and GIP, thereby negating their insulinotropic and other actions. In longer-term studies over 9 d, C. sinensis treatment significantly improved in non-fasting blood glucose, glucose tolerance and plasma insulin together with notable decreases in DPP-IV enzyme activity, food intake and body weight. No adverse actions were observed.
GLP-1 plays an important role in post-prandial glucose homoeostasis and mediating satiety after feeding, and as a result stable GLP-1 mimetics and DPP-IV inhibitor drugs have been introduced into clinical practice for treatment of obesity and T2DM(Reference McKillop, Duffy and Lindsay55). The in vivo actions of C. sinensis on enzyme activity were mirrored by concentration-dependent inhibitory action on DPP-IV activity in in vitro, indicating a direct action of phytochemicals on the enzyme. The effects were observed at much higher concentrations than a pure preparation of the DPP-IV inhibitor drug, sitagliptin, but previous research has shown that flavonoids (rutin and isoquercitrin) competitively inhibit DPP-IV activity by directly blocking the DPP-IV binding site(Reference Zhang, Zhang and Yin56). These observations suggest that a component of the enhanced insulin response induced by C. sinensis extract might be due to increased levels of intact GLP-1 and GIP due to inhibition of DPP-IV. Other longer-term effects may be mediated via actions on islet morphology. Consistent with improvement of insulin sensitivity and reduced insulin demand, 9-d treatment of HFF rats with the extract decreased pancreatic insulin content, islet size and areas of islets, β-cells and α-cells area. However, the mechanisms of action underlying these quite rapid changes such as proliferation, apoptosis or trans-differentiation require further study.
As alluded to above, the clear-cut and impressive anti-diabetic actions of the C. sinensis extract beg questions regarding which phytochemicals are responsible for the direct effects observed in vitro. Further studies were performed involving isolation, identification and characterisation of active compounds that possess insulinotropic anti-diabetic activity. The compound isolated from C. sinensis, a yellow amorphous powder, was subjected to HPLC purification, LCMS analysis and structural confirmation by NMR: the negative electrospray ionisation MS gave a quasi-molecular ion peak [M-H] − at m/z 464·2 Da, which was similar to the molecular formula C21H20O12. 1H- and 13C–NMR spectra indicated the presence of a quercetin moiety with a sugar residue that has aglycone parts, similar to quercetin. Other spectroscopic indications show glucose content as a sugar moiety, with spectra suggesting the presence of β-glucopyranose units, ultimately identified as quercetin 3-O-β-glucopyranoside (isoquercitrin)(Reference Francisco57).
The RP-HPLC (Peak 8) fraction was identical to standard rutin. RP-HPLC of fractions labelled Peak 5 to Peak 10 also exhibited significant insulin-releasing activity. Similarly, the Peak 8 (rutin) and Peak 9 (isoquercitrin) induced concentration-dependent insulin release from BRIN-BD11 cells. This effect together with improvement in glucose tolerance and plasma insulin in vivo was recapitulated by commercially available isoquercitrin. Previous studies reported that isoquercitrin possessed potential hypoglycaemic activity in both in vitro and in vivo (Reference Zhang, Zhang and Yin56). These findings indicate that the insulin-releasing effects of C. sinensis were partially due to the presence of polyphenolic compounds such as rutin and isoquercitrin. Further studies are required to evaluate whether these or any of the other phytochemicals identified contribute to the extra pancreatic actions of the C. sinensis.
In conclusion, this study has demonstrated that HWCS leaves exerts significant anti-diabetic effects in a commonly used animal model of obesity-diabetes. Such effects are mediated by a broad spectrum of desirable actions on insulin secretion/action, starch digestion, glucose diffusion, DPP-IV activity and glycation with additional benefits on islet morphology. Evaluation of phytochemicals in C. sinensis extracts identified rutin, isoquercitrin and related small molecules as major contributors to insulinotropic and glucose-lowering activity. These data together with recent demonstration of benefits in humans(Reference Fu, Li and Lin13) support use of C. sinensis leaves as a dietary adjunct for T2DM and the possibility of using its marker compounds for development of new therapeutic agents.
Acknowledgements
The authors would like to thank Ulster University Strategic Research Funding and the award of a Vice Chancellor’s Research Studentship to P. A.
The present study was supported by the Ulster University Strategic Research Funding and award of Vice Chancellor’s research studentship to P. A.
P. R. F., P. H. and Y. H. A. A.-W. were responsible for the conception and design of research and contributed equally to the supervision of the study; P. A. performed the experiments, analysed the data, interpreted the results, prepared the figures and drafted the manuscript with P. R. F.; P. R. F., Y. H. A. A.-W. and P. A. edited the revised manuscript; all authors approved the final version of the manuscript.
The authors declare that there is no duality of interest associated with this paper.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520005085













