Wheat is generally used as a major source of energy in animal diets(Reference Carre, Idi and Maisonnier1). In the European poultry diet, for example, wheat accounts for more than 600 g/kg(Reference Austin, Wiseman and Chesson2). The major components of wheat are starch and proteins, whereas NSP derived from the cell walls account for only 3–8 % of the total mass of wheat grain(Reference Saulnier, Sado and Branlard3). Starch is the major source of energy, whereas NSP are generally considered as anti-nutritional factors because they are non-digestible, highly hydrophilic and may be chelators of minerals such as Ca or Fe(Reference Jamroz, Jakobsen and Bach Knudsen4). About half of NSP in mass is constituted by arabinoxylan (AX) polymers(Reference Saulnier, Sado and Branlard3, Reference Knudsen5). The content and structure of AX polymers show large differences between wheat cultivars, which affect the end-use properties and nutritional quality of the grain. Furthermore, the different degrees of substitution of the xylan chain, composed mainly of arabinose and phenolic compounds, are correlated with the viscosity and digestibility of wheat carbohydrates(Reference Fincher and Stone6, Reference Izydorczyk and Biliaderis7).
Single-stomached animals, especially poultry, lack NSP-degrading enzymes so that the accessibility of α-amylase to wheat starch is limited. Consequently, in order to improve the metabolisable energy of wheat-based poultry diets, the effectiveness of NSP enzymes has been exhaustively studied(Reference McCracken and Quintin8–Reference Choct, Kocher and Waters10), and numerous enzyme preparations and cocktails have been marketed. Besides in vivo tools, which are rather hard to use, in vitro models, such as the TNO gastrointestinal model (TIM)(Reference Minekus, Marteau and Havenaar11), mimicking the gastrointestinal tract, have emerged as efficient tools for investigating carbohydrate digestion, targeting the feed and food sectors. Indeed, it has recently been shown that high repeatability of β-glucan digestibility values could be obtained in TIM-1, and the digestibility values of resistant starch obtained using the same model have been found to be similar to those obtained in ileostomy patients(Reference Haraldsson, Rimsten and Alminger12, Reference Fassler, Arrigoni and Venema13). In addition, using this in vitro model and AX extracted from different wheat cultivars, it has been shown that the AX structure might influence the improvement of digestibility due to supplementation with NSP-degrading enzymes(Reference Maisonnier-Grenier, Clavurier and Saulnier14). Although the main target of NSP enzymes is the soluble AX fraction, it has been demonstrated that the addition of some enzymes also targets the insoluble AX fraction(Reference Choct, Kocher and Waters10). However, the relationships between wheat characteristics and the responses of wheat batches to enzyme addition are not yet fully understood.
In the present study, two wheat cultivars, Caphorn and Isengrain, were chosen, based on their monosaccharide composition, to investigate carbohydrate digestibility in TIM-1. The in vitro model was first compared with caecectomised cockerels, with regard to the apparent digestibility of organic matter. The AD of Caphorn and Isengrain carbohydrates was then determined based on the analysis of organic matter, reducing ends and end products in the TIM-1 compartments: jejunal and ileal dialysates (absorbed fraction), ileal delivery (non-absorbed fraction) and the residues that remained in the TIM-1 compartments after the 6 h digestion trial. Finally, the digestion kinetics of Caphorn and Isengrain carbohydrates in the presence or absence of an enzyme supplement was followed in TIM-1 based on the measurements of the three aforementioned digestibility markers and of total monosaccharides. Using such an integrative approach, the objective was to obtain both an overview of and molecular information on the enzymatic digestion of wheat carbohydrates and on the benefits to be expected regarding supplementation with commercial enzymes.
Materials and methods
Materials
The enzyme preparation (Rovabio™ Excel LC) was obtained from Adisseo SAS (Commentry, France). It consists of a Penicillium funiculosum secretome containing more than nineteen glycolytic and two proteolytic activities. Endo-β(1,4)-xylanase, endo-β-(1,3)-glucanase, cellobiohydrolase and endo-β(1,4)-glucanase account for the main glycolytic activities(Reference Karboune, Geraert and Kermasha15, Reference Karboune, L'Hocine and Anthoni16), whereas proteomic analysis revealed the existence of fifty distinct proteins in this secretome(Reference Guais, Borderies and Pichereaux17). The wheat cultivars Aztec, Tapidor, Oratorio, Apache, Caphorn and Isengrain were purchased from Euronutrition (St Symphorien, France). Trypsin from bovine pancreas, pepsin from porcine gastric mucosa and porcine bile extract were purchased from Sigma (St-Quentin-Fallavier, France), whereas Rhizopus lipase was purchased from Amano Enzyme Europe, and the pancreatin solution was from Paines & Byrne (Greenford, Middlesex, UK).
Digestion medium
The digestion medium designed for the digestion trials in TIM-1 was based on a previous report(Reference Maisonnier-Grenier, Clavurier and Saulnier14), with some modifications. It was composed of 45 g (dry basis) ground wheat (grinding screen size 3 mm), 85 g of gastric salt solution (NaCl 1 g/l, KCl 1·1 g/l and CaCl2 dihydrate 0·15 g/l), 5 g pepsin and lipase solution (90 000 and 11 200 U/mg, respectively) and 170 g of water. (One unit of pancreatic lipase is the quantity of enzyme that catalyses the formation of 1·0 μmol fatty acid from TAG in 1 h at 37°C and pH 7·7 using olive oil, and one unit of porcine pepsin corresponds to an increase of absorbance (at 280 nm) of 0·001 unit per min measured at 37°C and pH 2·0 using Hb as substrate.) When added, Rovabio was at 0·2 μl/g of wheat.
In vivo digestibility assays
The in vivo experimental procedure involving animals was approved (C-03-159-4) by the Departmental Direction of the Veterinary Services (Allier, France). Apparent organic matter digestibility (AOMd) was determined for caecectomised cockerels using the method based on wet force-feeding and total excreta collection(Reference Lessire18). A total of twelve roosters (age 13 months and weight 3·45 (sd 0·15) kg) per diet were randomly distributed in individual cages, which were placed in a temperature-controlled room. After 24 h of fasting, the cockerels were force-fed with 110 g of meal (96 % wheat and 4 % mineral–vitamin mixture). The excreta were collected at 24 and 48 h after force-feeding, and pooled, weighed, freeze-dried and stored until analysis of the DM and ash contents. Analysis of the DM and ash contents was also performed for diet ingesta.
Wheat digestion assays in TNO gastrointestinal model-1
A schematic diagram of TIM-1 (installed at the Centre of Expertise Research and Nutrition, Adisseo, Commentry, France) is shown in Fig. 1. The in vitro model allows the control of digestive secretions, pH, temperature and peristaltism. The protocol of Minekus et al. (Reference Minekus, Marteau and Havenaar11), which is adapted for pigs, was utilised along with a few modifications that targeted conditions of poultry digestion. In this in vitro model, gastric and ileal deliveries (f) are described by the equation ![]() , where t 1/2 is the half-time of delivery and β accounts for the shape of the time-course delivery curve. In the present study, for gastric delivery, t 1/2 and β were set at 120 min and 1, respectively, whereas for small-intestinal delivery, t 1/2 and β were set at 400 min and 2·17, respectively. The pH set for the gastric compartment was 6·5, 5·0, 4·0, 3·0, 2·5 and 2·0 at 0, 60, 120, 180, 240 and 300 min, respectively. The pH for the duodenal, jejunal and ileal compartments was 6·5, 6·8 and 7·2, respectively. Before starting the digestibility trials, the duodenal compartment was flushed with 1 ml of trypsin solution (2 mg/ml), 7·5 ml of pancreatin solution (7 %, w/w), 15 ml of bile extract solution and 32 ml of small-intestinal salt solution (NaCl 5 g/l, KCl 0·6 g/l and CaCl2 dihydrate 0·3 g/l). The jejunal and ileal compartments were filled with 130 ml of the small-intestinal salt solution. The absorption was mimicked using haemodialyser HG-400 membranes with a cut-off of 5–10 kDa (Hospal Cobe, Lyon, France). The dialysis fluid was pumped at 10 ml/min and collected in the intervals 0–60, 60–120, 120–180, 180–240 and 240–360 min. The samples were weighed and stored at − 20°C until analysis. The ileal effluents were collected between 0 and 120, 120 and 180, 180 and 240 and 240 and 360 min, weighed and stored at − 20°C. The residues in the gastroduodenal and jejunoileal compartments were separately collected at the end of the experiment. The digestion assays in TIM-1 were carried out in duplicate.
, where t 1/2 is the half-time of delivery and β accounts for the shape of the time-course delivery curve. In the present study, for gastric delivery, t 1/2 and β were set at 120 min and 1, respectively, whereas for small-intestinal delivery, t 1/2 and β were set at 400 min and 2·17, respectively. The pH set for the gastric compartment was 6·5, 5·0, 4·0, 3·0, 2·5 and 2·0 at 0, 60, 120, 180, 240 and 300 min, respectively. The pH for the duodenal, jejunal and ileal compartments was 6·5, 6·8 and 7·2, respectively. Before starting the digestibility trials, the duodenal compartment was flushed with 1 ml of trypsin solution (2 mg/ml), 7·5 ml of pancreatin solution (7 %, w/w), 15 ml of bile extract solution and 32 ml of small-intestinal salt solution (NaCl 5 g/l, KCl 0·6 g/l and CaCl2 dihydrate 0·3 g/l). The jejunal and ileal compartments were filled with 130 ml of the small-intestinal salt solution. The absorption was mimicked using haemodialyser HG-400 membranes with a cut-off of 5–10 kDa (Hospal Cobe, Lyon, France). The dialysis fluid was pumped at 10 ml/min and collected in the intervals 0–60, 60–120, 120–180, 180–240 and 240–360 min. The samples were weighed and stored at − 20°C until analysis. The ileal effluents were collected between 0 and 120, 120 and 180, 180 and 240 and 240 and 360 min, weighed and stored at − 20°C. The residues in the gastroduodenal and jejunoileal compartments were separately collected at the end of the experiment. The digestion assays in TIM-1 were carried out in duplicate.

Fig. 1 Schematic diagram of the gastrointestinal digestion model (TNO gastrointestinal model-1).
Analysis
The dialysed samples were used without any treatment, whereas the suspensions from the ileal deliveries and the residues were rapidly defrosted and centrifuged at 14 000 g for 10 min, and the pellets and the supernatants were stored at − 20°C until analysis. The supernatants and the dialysed samples were used for the measurement of the reducing ends and end products. Both the supernatants and the pellets were used for GC analysis, whereas the organic matter fraction was determined without centrifugation. Analysis of the DM and inorganic matter was performed by Institut Louise Blanquet (Clermont-Ferrand, France), based on a reference method(Reference Andrews19).
Total monosaccharides
The monosaccharide composition of the wheat cultivars Aztec, Tapidor, Oratorio, Apache, Caphorn and Isengrain and that of the supernatants and the pellets recovered from the TIM-1 experiments with Caphorn and Isengrain were determined by GC after acid hydrolysis(Reference Dervilly, Saulnier and Roger20). Briefly, the liquid fractions were hydrolysed with 4 m-H2SO4 in the presence of inositol as the internal standard (5 g/l) for 2 h at 100°C, whereas the pellets were pre-hydrolysed with 26 N-H2SO4 for 30 min at 25°C before inositol was added. After cooling, the mixture was neutralised with an ammoniac solution (25 %, v/v) and then reduced (1 h at 40°C) with 3 m-NaBH4. Acetylation was performed at room temperature using acetic anhydride in the presence of N-methylimidazole. Finally, the acetylated samples were extracted with dichloromethane and injected in the GLC system (Perkin-Elmer Autosystem, Covina, CA, USA) using a 25 × 0·32 mm silica column (BP-225; SGE, Ringwood, VIC, Australia) and a flame ionisation detector. The AX content was calculated as the sum of arabinose and xylose. Each determination was performed in triplicate.
Reducing ends
The liberated reducing ends were determined according to the dinitrosalicylic acid method(Reference Bailey, Biely and Poutanen21), using ninety-six-well microplates and a KRL microplate spectrophotometer (Kirial International, Couternon, France). The blank contained water instead of the sample. The data are expressed as absorbance units, since the extinction coefficients varied strongly depending on the nature of the reducing product (data not shown).
End products
The end products were analysed using a high-performance anion exchange chromatography coupled with a pulsed amperometric detection (HPAEC-PAD) system equipped with a Carbo-Pac PA-100 (250 × 4 mm) column, an ED40 electrochemical detector (Dionex Corporation, Sunnyvale, CA, USA) and an AS3500 autosampler (Thermoelectron, Courtaboeuf, France). Appropriate sample dilution was performed before injection (typically 20 μl) onto the HPAEC system. Elution (1 ml/min) was carried out using a 20 min linear gradient program from 100 % A (5 mm-NaOAc and 80 mm-NaOH) to 30 % A and 70 % B (500 mm-NaOAc and 80 mm-NaOH). The peaks of the HPAEC-PAD chromatograms were identified based on the standard curves of xylo- and malto-oligosaccharides, containing up to six units of xylose and glucose (Sigma-Aldrich), respectively, with 3 % of maximal deviations in the retention times. Chromeleon® software (Dionex) was used for data acquisition and processing. Each determination was performed in triplicate.
Calculation of digestibility and statistical analysis
AOMd from caecectomised cockerels was calculated as percentage of intake:
For the in vitro model TIM-1, the digestibilities of organic matter, total monosaccharides and end products are expressed as follows:
where x is the digestibility marker and i is the time of cumulative recovery (at 60, 120, 180, 240 and 360 min). The residues were the remaining part of the meal in the TIM-1 compartments after digestion experiments.
Statistical analysis of repeated-measures data was performed using the mixed procedure of SAS 9.1.3 (SAS Institute, Inc., Cary, NC, USA). As the values were cumulated over time, the covariance structure was specified as ‘Auto Regressive type 1’. The restricted maximum-likelihood method was used to estimate covariance parameters, and the tests of fixed effects (in model, contrasts and least-squares means) were performed using the residual df (using the ‘ddfm = RESIDUAL’ option). Time was specified as the factor of repeated-measures ANOVA determinations. Focusing on each compartment (jejunum and ileum) and their sum, the following multifactor statistical model was used to study the enzyme effects on the polysaccharide digestion processes:
where α is the meaning effect; β, χ, δ, η, π and μ are the adjusted coefficients of the fixed effects in the model; ɛ is the random error associated with the jth treatment in experiment k assigned to the ith wheat at time l; subscripts i, j, k and l are the df of each factor: two wheat cultivars, two treatments, two experiments, five times.
Regarding the effects of enzyme preparation on each cultivar, as the interaction ‘treatment × wheat × cumulative time’ was significant, a SLICE option was added to the LSMEANS statement in order to compute the treatment effect at each time for both cultivars separately. Moreover, contrasts were computed (using the ESTIMATE statement) to compare the difference between cultivar controls at each time. All statistical analyses were considered to be significant at P < 0·05.
Results and discussion
Carbohydrate composition of Caphorn and Isengrain wheat cultivars
Among the six wheat cultivars tested, Caphorn and Isengrain exhibited the largest difference regarding the monosaccharide composition and the degree of AX ramification and were thus chosen for in vivo and in vitro studies. The individual monosaccharide content, the AX content and the arabinose:xylose molar ratio in the whole grain and in the soluble fraction of both Caphorn and Isengrain wheat cultivars are shown in Table 1. The individual monosaccharide content was found to be similar in the whole grain for both cultivars. The total monosaccharide content was also found to be similar in the whole grain (83·2 % in Caphorn v. 82·7 % in Isengrain). Glucose accounted for 89·6 % of the total monosaccharides in Caphorn and for 91·0 % of Isengrain total monosaccharides. Previous studies have shown that glucose from starch represents about 85 % of wheat carbohydrates(Reference Saulnier, Sado and Branlard3, Reference Knudsen5), which suggests that NSP glucose amounts to 5 % of the total wheat monosaccharides. Interestingly, the total monosaccharide content was three times higher in the Caphorn than in the Isengrain soluble fraction, and the AX content was also higher. The soluble fraction, although accounting for only a few mass percentage units, should play an important role, as a positive correlation has previously been observed between the content of soluble AX and the relative viscosity that has an impact on the rheological behaviour of the chyme(Reference Maisonnier-Grenier, Clavurier and Saulnier14). Moreover, the lower A:X ratio in the Caphorn than in the Isengrain soluble fraction predicts different enzyme susceptibilities for the two water-soluble fractions, as the arabinose content in AX affects the xylanase activity(Reference Bonnin, Daviet and Sorensen22).
Table 1 Carbohydrate composition* and arabinoxylan ramification of Caphorn and Isengrain wheat cultivars†
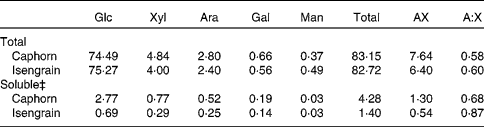
Glc, glucose; Xyl, xylose; Ara, arabinose; Gal, galactose; Man, mannose; AX, arabinoxylan; A:X, arabinose:xylose molar ratio.
* Expressed in g/100 g of dry mass.
† All samples were analysed in triplicate.
‡ Monosaccharides of the soluble fraction extracted from 1 g of ground wheat grain in 4 ml of water.
In vitro v. in vivo apparent organic matter digestibility for Caphorn and Isengrain
The organic matter digestibility of both wheat cultivars did not differ between the caecectomised cockerels and the TIM-1 model: 72·0 (sd 2·6) % in vivo v. 70·6 (sd 0·6) % in TIM-1 for Caphorn (P = 0·580) and 73·0 (sd 2·3) % in vivo v. 71·1 (sd 1·9) % in TIM-1 for Isengrain (P = 0·252). Thus, TIM-1 is able to predict the digestibility of organic matter in poultry, and significant time may consequently be saved.
Caphorn and Isengrain digestion in the TNO gastrointestinal model-1 compartments
Digestibility markers (organic matter, reducing ends and end products) measured in the TIM-1 compartments after the 6 h digestion trial are shown in Table 2. These digestibility markers account for the transit of whole organic solids, the hydrolysis of osidic linkages and the released products, especially monomers and dimers. This allows an overview on the digestion of carbohydrates to be obtained. Overall, these markers were distributed as follows: 41·4–58·9 % in the jejunal dialysate; 18·3–27·1 % in the ileal dialysate; 8·7–21·1 % in the ileal delivery; 3·3–14·6 % in the digestive residues. In the digestible fraction (jejunal+ileal dialysates) for both Caphorn and Isengrain, the value of the reducing ends is higher than that of the organic matter (P = 0·032), while the opposite is observed for the ileal delivery (P = 0·004) and residues (P = 0·026). This means that the organic matter of the ileal delivery and that of the residues were depleted in reducing end-containing carbohydrates, due to the hydrolysis performed by α-amylase and other glycosidases in the proximal parts of TIM-1. The liberated mono-, di- and oligomers were able to cross the TIM-1 membranes ( < 10 kDa). In addition, in the jejunal dialysate, whatever the wheat cultivar, the value of the end products was lower than that of the organic matter and reducing ends. The ratios of the end-product value over that of the organic matter or that of the reducing ends should be indicative of the stage of the digestion of wheat carbohydrates throughout TIM-1. Finally, in the jejunal dialysates, a tendency of a higher amount of the end products liberated from Caphorn than from Isengrain was observed. This may be linked to the higher soluble carbohydrate fraction in Caphorn compared with Isengrain (Table 1), allowing more rapid digestion of Caphorn compared with Isengrain.
Table 2 Repartition (%)* of organic matter, reducing ends and end products from Isengrain and Caphorn wheat cultivars in the TNO-gastrointestinal model-1 compartments
(Mean values and standard deviations)
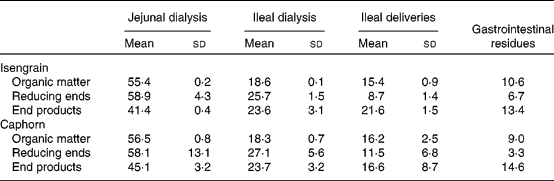
* For each digestibility marker, 100 % represents the sum of the cumulative values at 360 min of all the TNO gastrointestinal model-1 compartments.
Kinetic effects of an enzyme supplement on Caphorn and Isengrain digestibilities in TNO-gastrointestinal model-1
Organic matter
The time courses of AOMd in TIM-1 for Caphorn and Isengrain in the presence or absence of the enzyme supplement are shown in Fig. 2. In the absence of the enzyme, the AOMd for Isengrain was found to be higher than that for Caphorn, both in the jejunal dialysate (3·3–3·9 %) at all the time intervals (P = 0·0075 at 180 min, P = 0·0014 at 240 min and P = 0·0203 at 360 min) and in the ileal dialysate (3·4 % (P = 0·0125) at 180 min and 3·4 % (P = 0·0058) at 240 min). For both cultivars, the dialysis of organic matter took place mainly in the jejunal compartment. The enzyme supplement was found to have a positive effect on the jejunal AOMd, especially at 360 min of digestion (6·9 %, P < 0·0001), but no marked effect was observed on the ileal AOMd (no significant variation, except at 360 min ( − 2·7 %, P = 0·003)). The effect of the enzyme supplement on the global apparent digestibility (jejunal+ileal TIM-1 compartments) was 5·2 % at 180 min (P < 0·0001), 6·9 % at 240 min (P < 0·0001) and 4·2 % at 360 min (P = 0·003; data not shown). For Isengrain, the two curves with or without the enzyme supplement are not clearly distinct from each other, both for the jejunal and ileal dialysates, although the global effect at 360 min was positive (2·9 %, P = 0·027). It therefore seems that the supplemented glycosidases have more rapid access to their substrates in Caphorn than in Isengrain, in line with the content of the soluble carbohydrate fraction and the degree of AX ramification.

Fig. 2 Cumulative time course of the TNO gastrointestinal model-1 digestibility of organic matter from (a) Caphorn and (b) Isengrain in the presence (—) or absence (- - -) of Rovabio™ Excel in the jejunal (■) and ileal (▲) dialysates. Each determination was performed in triplicate.
Overall, the net cumulative effect of the enzyme supplement at 360 min on AOMd reached 4 % for Caphorn and 3 % for Isengrain. This is consistent with previous studies, which have shown a 3–5 % digestibility improvement in metabolisable energy with the same enzyme supplement in growing chickens(Reference Maisonnier-Grenier, Clavurier and Saulnier14), which notably highlights the usefulness of the in vitro model as a predictor of carbohydrate digestion in poultry.
Reducing ends
The cumulative appearance of the reducing ends from supplemented and unsupplemented wheat in TIM-1 is shown in Fig. 3. A clear positive cumulative effect of the enzyme supplement on the liberated reducing ends was observed for Caphorn in the jejunal compartment (70·6 % at 360 min, P < 0·0001) but not in the ileal one, although there was a tendency for a positive effect at 180 min (28·7 %, P = 0·079). Conversely, for Isengrain, the enzyme supplement significantly improved the liberation of the reducing ends in the ileal compartment but not in the jejunal one: 30·3 % (P < 0·0001) and 7·8 % (P = 0·089), respectively, at the end of the digestion. The cumulative effect of the enzyme supplement on the liberation of the reducing ends in TIM-1 was 47·2 % (P < 0·0001) for Caphorn and 14·2 % for Isengrain (P = 0·0004; data not shown). These positive effects were essentially localised in the jejunal compartment for Caphorn and in the ileal one for Isengrain. The effect of the enzyme supplement on the liberation of the reducing ends is more pronounced than that on organic matter digestibility (Fig. 2). This suggests that large parts of the broken osidic bonds did not lead to reducing end-containing products that would cross the TIM-1 dialysis membranes.

Fig. 3 Cumulative time-course appearance of the reducing ends from (a) Caphorn and (b) Isengrain in the presence (—) or absence (- - -) of Rovabio™ Excel in the jejunal (■) and ileal (▲) dialysates. AU, absorbance unit reflecting the content of the reducing ends recovered in the dialysates (see text for details). Each determination was performed in triplicate.
Total monosaccharides
The time courses of glucose- and xylose-containing carbohydrate liberation in TIM-1 digestible fractions from Caphorn and Isengrain, with and without the enzyme supplement, are shown in Fig. 4. The graphs for glucose were similar to those for organic matter (Fig. 2), i.e. (1) a clear positive effect of the enzyme supplement on Caphorn digestibility in the jejunal compartment varying from 3·6 % (P = 0·009) at 120 min up to 9·7 % (P < 0·0001) at 360 min, (2) no clear effect on Caphorn digestibility in the ileal compartment and (3) less pronounced effects of the enzyme supplement on Isengrain digestibility – global effect (jejunal+ileal compartments) of 3·1 % (P = 0·003) at 180 min, 3·8 % (P = 0·009) at 240 min and 3·1 % (P = 0·031) at 360 min. The similarity between the plots for organic matter and those for total glucose was well expected, since glucose largely accounts for the organic matter in mass. As regarding total xylose, the positive effect of the enzyme supplement was significant for Caphorn (an increase from 2·8 up to 11·1 %, P = 0·0003) but not for Isengrain (an increase from 2·7 up to 5·5 %, P = 0·165). The dramatic increase in xylose liberation from Caphorn is the result of the hydrolytic activity displayed by the glycosidases of the enzyme supplement, which are mainly of the xylanolytic type(Reference Karboune, Geraert and Kermasha15–Reference Guais, Borderies and Pichereaux17). These glycosidases seem to have more difficult access to NSP in Isengrain than in Caphorn, which may be again linked to the soluble carbohydrate content and the AX ramification. It is also possible that Isengrain contains more xylanase inhibitors than Caphorn(Reference Bonnin, Daviet and Gebruers23).

Fig. 4 Time-course appearance of glucose- (up) and xylose-containing carbohydrates (down) from Caphorn (left) and Isengrain (right) in the presence (—) or absence (- - -) of Rovabio™ in the jejunal (■) and ileal (▲) dialysates. The contents of glucose and xylose, among other monosaccharides, in the dialysed samples were determined using GC and are expressed as the percentage of total glucose and total xylose, respectively. Each determination was performed in triplicate.
End products
Fig. 5 shows the time courses for end-product appearance in the jejunal and ileal dialysates from supplemented or not supplemented Caphorn and Isengrain wheat cultivars. The effect of the enzyme supplement on the digestibility of the two wheat cultivars is similar to that observed using organic matter (Fig. 2) or total glucose (Fig. 4) as digestibility markers. For both Caphorn and Isengrain, the effect on the global digestibility (jejunal+ileal dialysates) increased during digestion, culminating at 240 min: 3·0 % (P = 0·033) at 120 min, 7·2 % (P < 0·0001) at 240 min and 3·8 % (P = 0·008) at 360 min for Caphorn, and 3·9 % (P = 0·007) at 120 min, 4·5 % (P = 0·002) at 240 min and − 0·8 % (P = 0·561) at 360 min for Isengrain. The mitigation effect of the enzyme supplement at the end of the run was probably due to the depletion of the NSP enzyme amount and/or to the decrease in the amount of the substrate potentially hydrolysed.

Fig. 5 Time course of the cumulative appearance of end products from (a) Caphorn and (b) Isengrain in the presence (—) or absence (- - -) of Rovabio™ in the jejunal (■) and ileal (▲) dialysates. The end products glucose, maltose, maltotriose and xylobiose were identified in the high-performance anion exchange chromatography coupled with a pulsed amperometric detection chromatograms (see text for details), and their peak areas, along with the areas of minor non-identified peaks, were cumulated over the 6 h digestion trial in all the fractions (jejunal and ileal dialysates, ileal delivery and residues). The percentage values are relative to the total peak area. Each determination was performed in triplicate.
The chronological effect of the enzyme supplement on the appearance of glucose, maltose and xylobiose is shown in Fig. 6. The enzyme preparation improved the digestion of Caphorn especially in the first fractions of the jejunal and ileal dialysates and improved that of Isengrain especially in the first fractions of the ileal dialysates. The positive effect of the enzyme supplement on the release of maltose results from better α-amylase access to starch, as maltose is the main product of starch hydrolysis by α-amylase(Reference Robyt and French24). Its positive effect on glucose liberation accounts for the more efficient hydrolysis of both starch and NSP, while that on xylobiose liberation is representative of only AX hydrolysis. The magnitude of the improvement of Caphorn and Isengrain digestibilities by the enzyme supplement decreased with time, which means that supplemented glycosidases, while they specifically degraded Caphorn and Isengrain NSP, were less and less active as digestion progressed. This reduction in activity may be due either to their degradation by the proteases available in TIM-1 or to a limitation in substrates.
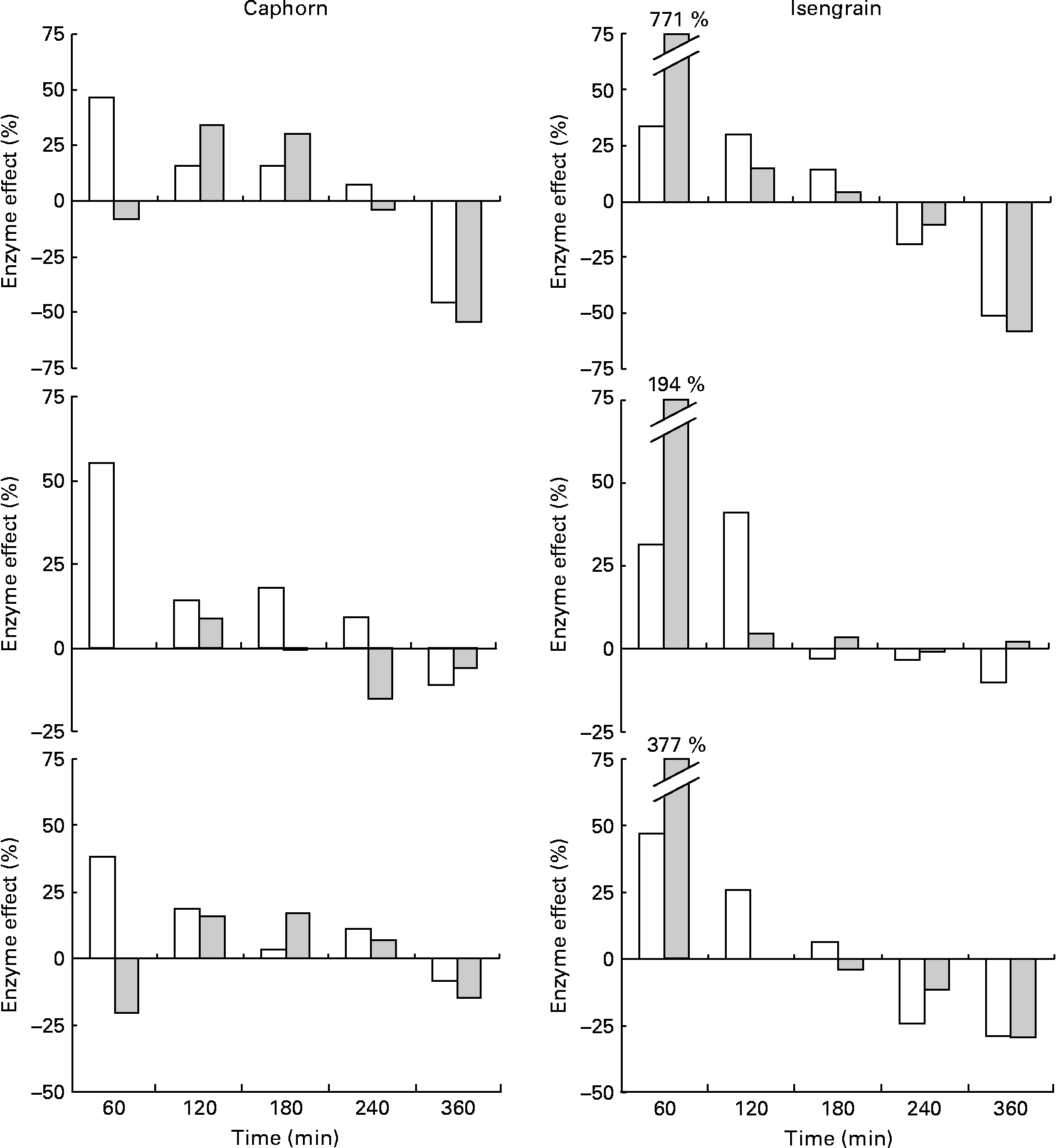
Fig. 6 Time-course effect ((peak area with the enzyme supplement/peak area without the enzyme supplement) × 100) of the enzyme supplement on the liberation of glucose (upper), maltose (medium) and xylobiose (lower) from Caphorn (left) and Isengrain (right) in the TNO gastrointestinal model-1 jejunal (□) and ileal (![]() ) dialysates. The experimental deviations are not shown for clarity purposes.
) dialysates. The experimental deviations are not shown for clarity purposes.
Conclusion
In the present study, we used an integrative approach based on several digestibility markers – organic matter, reducing ends and end products – of wheat carbohydrates using TIM-1. The in vitro digestion model was found to be able to predict the digestibility of wheat organic matter in caecectomised cockerels, supporting it as a valuable prediction tool for the gastrointestinal digestion of food and feed. The integrative approach allowed an overview covering the global transit of organic matter, the degree of rupture of osidic links along with the released end products. The total monosaccharides, as determined in all TIM-1 compartments, are complementary to the three other chronological digestibility markers.
Acknowledgements
The present study was supported by Adisseo SAS Antony. The authors are very grateful to Estelle Devillard for helpful discussions and to Friedrich Rouffineau for assistance in statistical treatments. The authors' contributions were as follows: M. L. was involved in the study design and data interpretation, and wrote the manuscript; B. B. was involved in the study design and statistical analysis, and wrote the manuscript; S. E. performed the GC measurements; E. B. was responsible for the GC measurements and was involved in the data interpretation; E. H. C. was involved in the data interpretation and wrote the manuscript; P.-A. G. was the local coordinator of the TIM-1 studies; T. G. was involved in the study design and data interpretation, and edited the manuscript; E. H. A. was the coordinator of the study design and was involved in the data interpretation and in writing and editing the manuscript. The authors declare no conflict of interest.










