Plant sterols in habitual diets are mainly derived from vegetable oils, nuts, grains, fruit and vegetables, and daily intakes vary from 160 to 360 mg( Reference Ling and Jones 1 ). Avoiding the diet containing plant sterols slightly increases the concentrations of serum LDL-cholesterol( Reference Racette, Lin and Lefevre 2 ). In contrast, increasing the daily intakes of plant sterols by 2 to 3 g using functional foods lowers the concentrations of serum LDL-cholesterol up to 10 %( Reference Plat, Mackay and Baumgartner 3 ). Despite these well-known effects of plant sterol intake on serum LDL-cholesterol levels, there is a lack of detailed understanding about the underlying mechanisms. In vitro and animal studies have suggested that plant sterols affect intestinal cholesterol metabolism at the cellular level( Reference De Smet, Mensink and Plat 4 ). There are multiple suggestions that plant sterols affect specific processes in the intestine. For example, Lee et al. ( Reference Lee, Kim and Kim 5 ) demonstrated that β-sitosterol inhibited the shortening of the colon, lowered macroscopic scores of the disease grade and myeloperoxidase activity, and inhibited the release of pro-inflammatory cytokines in mice with 2,4,6-trinitrobenzene-induced colitis. In addition, β-sitosterol inhibited the growth of colon cancer cells in vitro, suggesting its chemopreventive effects against colon cancer( Reference Baskar, Ignacimuthu and Paulraj 6 ). A study conducted in mice fed plant sterols for 2 weeks has shown increased faecal neutral sterol excretion associated with lipid metabolism, probably via the activation of the transintestinal cholesterol excretion pathway( Reference Brufau, Kuipers and Lin 7 ). Field et al. ( Reference Field, Born and Mathur 8 ) further showed that mRNA expression levels of 3-hydroxy-3-methylglutaryl-CoA reductase were decreased after incubating Caco2 cells with micelles containing β-sitosterol. Finally, it has been postulated that plant sterols and stanols could act as local liver X receptor (LXR) agonists in enterocytes( Reference Plat and Mensink 9 ). However, despite all these interesting observations, data on the effects of plant sterol- or stanol-enriched food consumption on human enterocytes are lacking. Therefore, to better understand the cellular effects in the intestine, we examined the acute changes in the transcriptome of the upper small intestine in healthy individuals after consuming a plant stanol-enriched shake at breakfast after an overnight fast. Pathways involved in sterol metabolism were not altered in both the duodenum and the jejunum. However, immune-related pathways were down-regulated in the jejunum. This extends our earlier in vitro finding that sitostanol may exert immunomodulatory effects in subjects with a disturbed T-cell response( Reference Brüll, Mensink and Steinbusch 10 ). However, the present study population had no known immune disorders. Therefore, the effects of plant stanol and sterol esters on the (intestinal) immune system deserve further attention.
Materials and methods
Study design
The present study was part of a previously published randomised double-blind cross-over trial( Reference De Smet, Mensink and Plat 4 ). In brief, a total of eighteen healthy normolipidaemic volunteers participated in two experimental periods of 4 weeks, separated by a 4-week washout period (Fig. 1). Although intake of plant stanol esters is advised for (slightly) hypercholesterolaemic subjects, reductions and the underlying mechanisms in normolipidaemic subjects are comparable( Reference Gylling, Puska and Vartiainen 11 , Reference Mensink, Ebbing and Lindhout 12 ). During two screening visits, which were separated by at least 3 d, body weight, height and blood pressure of the participants were determined. An Omron M7 (Omron Healthcare Europe B.V.) was used to measure blood pressure in 4-fold in the left arm. The first measurement was discarded and the last three measurements were averaged. Since there are no studies so far that have shown an effect of BMI and sex on the cholesterol-lowering effects of plant stanol esters, men as well as women with a BMI ranging from 20 to 30 kg/m2 were included.

Fig. 1 Schematic overview of the study design. ![]() , Blood sample;
, Blood sample; ![]() , postprandial test (intestinal biopsies were taken).
, postprandial test (intestinal biopsies were taken).
Blood samples were taken for the analysis of serum total cholesterol concentrations. Subjects were excluded from analysis if their mean serum total cholesterol concentration was >7·8 mmol/l.
At 1 week before the start of each experimental period, subjects were instructed to avoid dietary products that were relatively rich in plant sterols and stanols. Immediately after this week, all subjects participated in a postprandial test for the examination of the acute effects of plant stanol esters on the expression profiles of intestinal genes. To further minimise the differences between two postprandial test days, all subjects consumed a standard lasagne meal at dinner, the evening before the test days. Except for water, they were not allowed to consume any other foods or drinks after dinner until the morning of the postprandial test. In the morning, they arrived at our department by public transportation or by car to reduce physical activity as much as possible. After resting for 15 min in a supine position, a fasting blood sample was collected (T0) by inserting an intravenous cannula into the antecubital vein. Next, subjects consumed a slice of white bread with jam and a high-fat shake with or without plant stanol esters (4 g plant stanols). The plant stanol ester mixture was incorporated into margarines as provided by Raisio (Raisio Nutrition Limited, Finland) and was composed of sitostanol (73·2 %), campestanol (22 %), sitosterol (0·9 %), campesterol (1·6 %) and other sterols/stanols (2·3 %). Plant stanols were esterified with food-grade fatty acids based on rapeseed oil. The margarine was incorporated into the shake (Table 1). Volunteers were requested to consume the shake and bread within 10 min, and were not allowed to consume any other foods or drinks except water during the next 5 h. At 5 h after consumption of the shake, intestinal biopsies were taken at the gastroenterology unit. During gastroscopy, no sedatives were given to the subjects. To compare the expression profiles of genes at different parts of the proximal small intestine, four mucosal tissue samples from the duodenum (around the papilla of Vater) and four from the jejunum (20 cm distal from the papilla of Vater) were taken using standard biopsy forceps. The diameter of the biopsies varied from 2·0 to 2·2 mm. After sampling, the biopsies were immediately frozen in liquid N2 for microarray analysis. For histological analysis, the biopsies were first put on a drop of Tissue-Tek in isopentane followed by freezing in liquid N2. All the samples were stored at − 80°C and analysed at the end of the study within the same run. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Medical Ethical Committee of the University Hospital Maastricht. All subjects gave written informed consent before their participation in the study. The trial was registered at ClinicalTrials.gov as NCT01574417.
Table 1 Nutritional composition and ingredients of the shake

Blood sampling
Blood was sampled in serum and NaF-containing vacutainer tubes (Becton Dickinson). To obtain serum, blood samples taken in serum tubes were allowed to clot for 30 min at 21°C, followed by centrifugation at 1300 g for 15 min at 21°C. The NaF-containing tubes were placed on ice directly after sampling and centrifuged at 1300 g for 15 min at 4°C within 60 min. Serum and plasma aliquots were directly snap-frozen in liquid N2 and stored at − 80°C until analysis. All samples obtained from each subject were analysed within the same analytical run.
Lipids
Concentrations of serum total cholesterol (CHOD-PAP method; Roche Diagnostics Systems, Hoffmann-La Roche) and TAG with correction for free glycerol (GPO-Trinder; Sigma Diagnostics) were determined enzymatically in fasting serum samples.
Glucose, insulin and high-sensitivity C-reactive protein concentrations
Plasma glucose (Roche Diagnostic Systems, Hoffmann-La Roche) concentrations were measured. Serum insulin concentrations were determined using a human insulin-specific RIA kit (Linco Research). High-sensitivity C-reactive protein concentration was determined by a highly sensitive immunoturbidimetric assay (Kamiya Biomedical Company).
Microarray processing and data analysis
Total RNA was extracted from frozen mucosal samples obtained from the duodenum or the jejunum using TRIzol reagent (Invitrogen), and purified on columns using the Qiagen RNeasy Micro Kit (Qiagen). Total RNA (35 ng/μl) was labelled by using Whole-Transcript Sense Target Assay and hybridised to human whole-genome Affymetrix Gene 1.1 ST arrays targeting 19 682 unique genes (Affymetrix). Individual genes were defined as ‘changed’ when comparison of the normalised signal intensities showed a P value < 0·05 in a two-tailed paired intensity-based moderated t statistic. Further functional data analysis was performed on the filtered dataset with gene set enrichment analysis, and on the differentially expressed genes with Ingenuity Pathway Analysis software.
Immunohistochemical analysis
Frozen intestinal biopsies were sectioned at 5 μm and fixed in filtered, ice-cold acetone for 15 min at 4°C, as described previously( Reference Wolfs, Buurman and Zoer 13 ). Briefly, endogenous peroxidase activity was blocked with 0·3 % H2O2 in methanol. After washing, slides were blocked with bovine serum albumin for 30 min at room temperature. Subsequently, the slides were incubated overnight at 4°C with appropriate primary antibodies, i.e. a polyclonal rabbit anti-human CD3 (cluster of differentiation number 3) antibody (1:1000, DAKO A0452; DAKO), a monoclonal mouse anti-human CD4 (cluster of differentiation number 4) antibody (1:50, Abcam ab846) or a monoclonal mouse anti-human Foxp3 (forkhead box P3) antibody (1:250, eBioscience 14-7979). After washing, the sections were incubated for 1 h at room temperature with the appropriate secondary antibody, i.e. a biotin-conjugated polyclonal swine anti-rabbit Ig (1:200, DAKO E0353; DAKO), a biotin-conjugated polyclonal goat anti-mouse Ig (1:200, DAKO E0433; DAKO) or a biotin-conjugated goat anti-mouse Ig (1:200, DAKO E0433; DAKO). Immunostaining was enhanced with the Vectastain ABC Peroxidase Elite kit (1:50, PK-6200; Vector Laboratories) followed by nickel sulphate-diaminobenzidine staining. The sections were counterstained with 0·1 % Nuclear Fast Red in 5 % aluminium sulphate for 2 min and dehydrated in ascending ethanol series. Images of the jejunal sections were acquired at 100 × magnification using a Leica DM2000 microscope equipped with a Leica DFC295 digital camera (Leica Microsystems) and Leica Application Suite (LAS) software (Leica LAS version 3.7; Leica Microsystems). As these staining procedures are laborious, analyses were carried out only in three randomly chosen subjects.
Results
Subject characteristics
A total of eighteen healthy participants completed the study. Intestinal samples from four participants were excluded from the microarray analysis as their RNA integrity numbers were too low to expect a successful outcome. The baseline characteristics of the remaining fourteen subjects are presented in Table 2.
Table 2 Baseline characteristics of the fourteen study participants (Mean values and standard deviations)

hs-CRP, high-sensitivity C-reactive protein.
* To convert insulin in μU/ml to pmol/l, multiply by 6.945.
Microarray analysis
From the 19 682 genes present in the microarray data, 9852 were expressed in the duodenum and 10 003 in the jejunum. After consumption of a shake enriched with 4 g plant stanol esters, the expression of 388 genes in the duodenum and of 610 genes in the jejunum was significantly altered. However, the expression of only twenty genes was altered in both the duodenum and jejunum (Fig. 2).
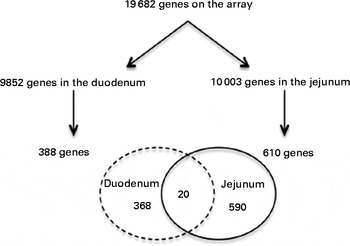
Fig. 2 Venn diagram showing the number of present and regulated genes determined by the microarray analysis of human intestinal biopsies. Probes were assigned to unique gene identifiers (Entrez). Genes were considered to be expressed when the intensity of the signal was >20 on at least eight arrays. Only genes with six or more probes were considered for the analysis. The P value was >0·05 in a two-tailed paired intensity-based moderated t statistic.
Gene set enrichment analysis
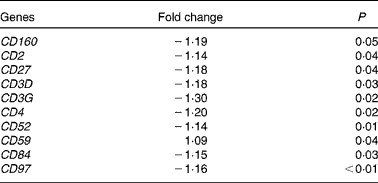
At 5 h after consumption of plant stanol esters, expression profiles of genes present in the pathways involved in sterol metabolism were not found to be altered in biopsies taken from the duodenum or jejunum. Gene sets that were altered after consumption of plant stanol esters are presented in online Supplementary Table S1. A consistent finding in the jejunum was the down-regulation of gene sets in pathways such as T-cell receptor signalling, generation of second messenger molecules, phosphorylation of CD3 and T-cell receptor ζ chains, and downstream T-cell receptor signalling (see online Supplementary Table S1). Part of the genes that were responsible for the observed enrichment in these gene sets were members of the CD family, which are all T-cell-specific surface molecules (Table 3). These effects were not observed in the samples obtained from the duodenum, in which modest changes appeared randomly.
Table 3 Changes in cluster of differentiation (CD) genes in the jejunum after an acute intake of plant stanol esters
Ingenuity Pathway Analysis
The immune-related functions that were affected by differentially regulated genes, as determined by gene set enrichment analysis, were also investigated (Fig. 3). A z score above 2 or below − 2 indicate, respectively, an increase or decrease in the specific function that was changed after consumption of plant stanol esters. An acute intake of plant stanol esters reduced the expression of genes associated with the quantity of double-positive thymocytes, as well as with the interaction, activation, adhesion and chemoattraction of T lymphocytes.

Fig. 3 Analysis of immune-related functions using the Ingenuity Pathway Analysis software in the jejunum after an acute intake of plant stanol esters. A z score above 2 or below − 2 indicates, respectively, an increase or decrease in the specific function that was significantly changed after consumption of plant stanol esters.
Fig. 4 displays heat maps of the gene sets that were associated with either the quantity (Fig. 4(a)) or the functionality (Fig. 4(b)) of T cells, which were regulated after consumption of plant stanol esters according to the Ingenuity Pathway Analysis software. A description of these genes can be found in Supplementary Table S2. These maps showed a highly consistent decrease in the transcript abundance level of genes associated with T-cell immune responses. However, for one of the subjects, the results were found to be highly aberrant, for which we have no explanation.

Fig. 4 Gene sets that were down-regulated in the jejunum after consumption of plant stanol esters, as determined by the Ingenuity Pathway Analysis software. The z score was calculated by subtracting the mean expression value of each transcript from each of the values and then dividing the resulting values by the standard deviation. The colour displayed in the heat maps reflects the relative transcript abundance level of genes, with blue being lower and red higher than the mean transcript abundance value. Immune-related functions were classified into functions associated with (a) the quantity or (b) the functionality of T cells. Subject 1 is denoted by p1, subject 2 by p2, etc. For definition of gene names, see Supplementary Table S2.
Immunohistochemical analysis
To further examine the consistent changes in the expression signatures of genes associated with the presence of T cells in the biopsies from the jejunum, the number of CD3+ cells in the jejunum of three randomly chosen subjects consuming the control and plant stanol-enriched shakes were quantified. CD3 is a T-cell-specific surface glycoprotein. As shown in Fig. 4, the number of CD3+ cells was reduced in the plant stanol ester condition when compared with the control condition. The microarray analysis also showed a decrease in the mRNA expression of T helper lymphocytes and regulatory T cells (Treg). Therefore, expression of CD4 and Foxp3 were analysed by immunohistochemistry in the biopsies. CD4 is a glycoprotein, among others, found on the surface of T helper cells, and Foxp3 is expressed especially in the CD4+ subpopulation of Treg. Compared with the control condition, the number of CD4+ and Foxp3+ cells was reduced in the plant stanol ester condition, which further extends support to the microarray data (Fig. 5).

Fig. 5 Immunohistochemical analysis of CD3 (cluster of differentiation number 3), CD4 (cluster of differentiation number 4) and Foxp3 (forkhead box P3) in human jejunal biopsies 5 h after consumption of a shake enriched with or without plant stanol esters. Compared with the control condition, the number of CD3, CD4 and Foxp3 cells was reduced in the plant stanol ester condition.
Discussion
Numerous in vitro and animal studies have demonstrated the effects of plant sterols and stanols on intestinal function( Reference Chen, Gruber and Pakenham 14 – Reference Dieter, Maher and Cheng 18 ), but there are no human data. Therefore, we evaluated the acute effects of plant stanol esters on the expression profiles of genes in biopsies from the human duodenum and jejunum using microarray analysis. In contrast to our expectations, the expression profiles of genes involved in sterol metabolism were not altered. However, immune-related T-cell-oriented pathways were consistently down-regulated in the jejunum. The expression of villin 1-like protein, an enterocyte-specific actin-regulatory protein involved in the formation of microvilli in enterocytes( Reference Kang and Lee 19 ), was comparable between two test days (data not shown). This suggests that the number of enterocytes present in biopsies during the control and plant stanol ester conditions was comparable. The finding that T-cell-related pathways are not predominantly expressed in enterocytes suggests that at least part of the observed down-regulation of T-cell-related pathways is due to the observed reduction in the number of T cells in the intestinal biopsies.
Knowledge regarding the intestinal kinetics of plant sterols and stanols and their effects on (intestinal) metabolism has rapidly evolved over the past decade. Recently, it was assumed that plant sterols and stanols are inert molecules and hardly absorbed, as their serum concentrations are low. However, it is now well established that concentrations of plant sterols and stanols in enterocytes can be increased up to 7-fold( Reference Igel, Giesa and Lutjohann 20 ) without any significant changes in plasma concentrations. This can be explained by the presence of different sterol transporters in the apical membrane of enterocytes( Reference Repa, Berge and Pomajzl 21 – Reference Repa, Dietschy and Turley 23 ) that regulate the uptake and secretion of plant sterols. Intracellular concentrations of plant sterols and stanols can even reach the levels well above the half maximal effective concentration (EC50) for specific transcription factors( Reference Plat, Bragt and Mensink 17 ). This suggests that plant sterols and stanols might affect gene regulation and modify metabolic pathways in vivo.
It has been reported that plasma concentrations of TAG increase within 120 min after food intake( Reference Sanders, Filippou and Berry 24 ). Thus, dietary lipids enter the enterocytes well before 2 h. Therefore, we reasoned that a 5 h period was long enough to induce changes in gene expression. We further assumed that an acute challenge, when no new steady state was reached, was more powerful to induce changes in gene expression than challenging after a long-term intake. To minimise the effects of resident plant sterols on intestinal sterol metabolism during both test days and to create a contrast in plant sterol and stanol intake as large as possible, subjects were instructed to avoid the consumption of products relatively high in plant sterols and stanols 1 week before the test day. Yet, we could not exclude the fact that with another protocol with other time points or by measuring in steady-state conditions, changes in genes associated with lipid sterol metabolism could have been observed.
However, it is unlikely that this can be explained by a lack of absorption within the 5 h time frame of the study. Igel et al. ( Reference Igel, Giesa and Lutjohann 20 ) already showed an increase in plant sterol concentrations after 15 min in the enterocytes of male C57BL/6OlaHsd mice. In agreement with this finding, we found an increase in plant stanol concentrations in the scraped enterocytes of C57Bl/6J mice 15 min after an oral administration (E De Smet, RP Mensink, M Konings, G Brufau, AK Groen, R Havinga, M Schonewille, A Kerksiek, D Lütjohann and J Plat, unpublished results). Also, in the present set-up, expression profiles of genes changed within 5 h after consumption of plant stanol esters. It might be possible that a 5 h follow-up period was too short to observe changes in the expression profiles of genes involved in sterol metabolism.
In animal studies, gene regulation in the intestine was observed within 12 h after the administration of a phytosterol-derived LXR agonist (YT-32)( Reference Kaneko, Matsuda and Yamada 25 ). No measurements were taken before this time point. A limited number of studies have also shown that in human subjects, expression profiles of intestinal genes involved in sterol metabolism can be changed during the postprandial phase( Reference Lally, Tan and Owens 26 , Reference Phillips, Mullan and Owens 27 ). The expression profiles of genes involved in the adaptive T-cell immune response were down-regulated. We have already suggested that plant sterols and stanols influence immune function, when the immune response is disturbed. Brüll et al. ( Reference Brüll, Mensink and Steinbusch 10 ) showed that plant stanols induced a shift towards T helper 1 cells (Th1) in isolated human peripheral blood mononuclear cells from asthma patients. Furthermore, the number of Treg cells, which are important for maintaining the Th1/Th2 (T helper 2 cell) balance, tended to increase, whereas the activity of Treg cells increased significantly. These ex vivo changes may be specific for a situation of skewed T-cell behaviour, as they were not observed in peripheral blood mononuclear cells from healthy subjects characterised by a balanced Th1/Th2 response( Reference Brüll, Mensink and Steinbusch 10 ). In vivo, Calpe-Berdiel et al. ( Reference Calpe-Berdiel, Escola-Gil and Benitez 28 ) showed that administration of 2 % phytosterols increased the Th1:Th2 ratio in mice treated with turpentine in order to induce an acute, aseptic inflammation model. The studies described so far have suggested that the plant stanol/sterol-induced Th1 or Th2 response depends on the direction of the disturbed Th1/Th2 balance. Restoration of this imbalance is probably the result of alterations in the number and activity of Treg cells( Reference Brüll, Mensink and Steinbusch 10 ). In other words, when Th2 cells are overactivated, the activation of Treg cells restores the balance by stimulating the activity of Th1 cells, which via feedback mechanisms results in the dampening of Th2 cells( Reference Mosmann and Coffman 29 ). However, in the present study, the changes in expression profiles suggested a dampening of the intestinal T-cell-mediated immune potential, in general, in healthy volunteers without an a priori skewed response. The physiological and functional consequences of these effects remain to be elucidated.
An intriguing question is how these effects on T cells can be explained. One may speculate that plant stanol esters induce changes in the composition of the gut microbiota, which may be associated with immunological processes( Reference Koboziev, Reinoso Webb and Furr 30 ). Recently, Martinez et al. ( Reference Martinez, Perdicaro and Brown 31 ) showed that the faecal microbiota of hamsters was significantly altered after feeding a plant sterol-enriched diet for 4 weeks. However, it is not very likely that the gut microbiota has changed within the 5 h time frame of the present study. Another explanation is based on the observations of Bensinger et al. ( Reference Bensinger, Bradley and Joseph 32 ), who showed that the transcriptional regulation of intracellular cholesterol homeostasis is linked to cell proliferation and acquired immune responses. Ligand activation of LXR, thereby increasing the expression of ABCG1 (ATP-binding cassette transporter G1), prevented effective proliferative responses. In contrast, activation of T cells by an antigen was accompanied by an up-regulation of the SREBP pathway, resulting in an increased availability of sterols needed for membrane biogenesis. Furthermore, the activities of the oxysterol-metabolising enzyme sulfotransferase family cytosolic 2B member 1 (SULT2B1) and multi-drug resistance protein 1 (MRP1/ABCC1) were increased, thereby eliminating oxysterol ligands of LXR and leading to T-cell expansion( Reference Bensinger, Bradley and Joseph 32 , Reference Glass and Saijo 33 ). Based on these findings, it can be speculated that plant stanols reduced the cellular oxycholesterol content of intestinal T cells, mimicking the effect of LXR activation and resulting in the dampening of T-cell expansion. However, further research is needed to elucidate this possible association. Unfortunately, the amount of biopsy material that we collected was too small to analyse the levels of oxysterol. In this context, it is interesting to investigate whether the effects observed are specific to plant stanols or whether similar effects could have been achieved by changing the dietary intake of cholesterol. In our earlier in vitro ( Reference Brüll, Mensink and Steinbusch 10 ) and ex vivo ( Reference Brull, Mensink and van den Hurk 34 ) experiments, we have used identical concentrations of cholesterol and plant stanols/sterols to evaluate their impact on the changes in the behaviour of T-cells. These studies have suggested that effects are plant stanol and sterol specific. However, further studies are needed to examine whether these effects can be extrapolated to the results of the present study.
In summary, an acute intake of plant stanol esters did not change the expression profiles of genes involved in sterol metabolism 5 h postprandially. However, the pathways involved in T-cell functions were consistently down-regulated in the jejunum. The physiological and functional consequences of plant stanol ester-induced immunomodulatory effects deserve further investigation.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451400350X
Acknowledgements
The authors thank all the study participants for their time and effort. They also thank Maud Beckers and Maurice Konings for their technical support and our dietitian, Nina Wystyrk, for nutritional counselling throughout the study. The authors thank Elhaseen Elamin, Nico Kloosterboer and Melanie Schnijderberg for the immunohistochemical analysis.
The present study was financially supported by Raisio Life Sciences, Finland. Raisio had no role in the design or analysis of the study, or in the writing of this article.
The authors' contributions are as follows: E. D. S., R. P. M. and J. P. designed the research; E. D. S. conducted the research; R. d. R. was responsible for collecting the biopsy samples; E. D. S., R. P. M. M. V. B. and J. P. analysed the data; E. D. S., R. P. M., M. V. B., T. G. A. M. W., W. T. V. G. R. d. R. and J. P. wrote the paper; J. P. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors have no conflict of interest to declare.











