Barrett’s oesophagus (BE) is a metaplastic transformation from the normal squamous mucosa of the oesophagus to a columnar lining and it is a known precursor for oesophageal adenocarcinoma (EAC)( Reference Shaheen and Richter 1 , Reference Schneider and Corley 2 ). Erosive oesophagitis (E) is not considered a precancerous lesion, but, like BE, is associated with gastro-oesophageal reflux disease (GERD), a spectrum of hiatal hernia, gastro-oesophageal reflux and symptoms like heartburn and regurgitation( Reference Koek, Sifrim and Lerut 3 ). Nevertheless, only a small fraction of patients with typical GERD symptoms have endoscopic evidence of BE or E( Reference Koek, Sifrim and Lerut 3 , Reference Ronkainen, Aro and Storskrubb 4 ), although these disorders have also, in common, other potentially modifiable risk factors such as cigarette smoking and overweight( Reference Schneider and Corley 2 , Reference Koek, Sifrim and Lerut 3 , Reference Lee, Lee and Kim 5 ). Alcohol, in particular heavy alcohol consumption, has been associated with increased risk of oesophageal squamous cancer, but knowledge about the association between alcohol and reflux E, BE and EAC is conflicting( Reference Bagnardi, Rota and Botteri 6 ). So far, most evidence supports no association between alcohol intake and BE risk( Reference Thrift, Cook and Vaughan 7 – Reference Thrift, Kramer and Richardson 9 ). On the other hand, an inverse correlation between wine intake and BE risk has been suggested( Reference Kubo, Levin and Block 10 ). A recent meta-analysis of twenty observational studies found no overall association between alcohol consumption and BE, whereas in a subgroup analysis an increased risk of BE was found for overall alcohol consumption in men (+35 %) and for liquor consumption (16 %)( Reference Xu, Guo and Shi 11 ).
In this study we aimed to evaluate the role of specific alcoholic beverages in BE and E occurrence, compared with a group of control (C) subjects undergoing upper endoscopy, but with no BE or E.
Methods
A multicentre case–control study was carried out in twelve endoscopic units situated in different Italian areas (five in northern, two in central and five in southern Italy). Three groups of patients who were willing to answer a questionnaire were consecutively selected from those referred for upper gastro-intestinal endoscopy: BE patients, E patients and C subjects without BE or E. E and C subjects were recruited from among the patients undergoing upper endoscopy in the same centres as BE patients and at the time the BE patients were identified.
For this study, BE was defined as a 15-mm upward displacement of the squamocolumnar junction (Z-line) from the gastro-oesophageal junction at endoscopy, with histological confirmation of specialised intestinal metaplasia with ‘goblet’ cells( Reference Wang and Sampliner 12 ). Interobserver variability in evaluating the length of columnar-lined oesophagus has been reported when BE segments <1 cm are considered. In addition, intestinal metaplasia of the gastric cardia may be misclassified as short-segment BE. Therefore, we used a 15-mm cut-off value to increase the accuracy of the BE diagnosis. At endoscopy, the Prague C & M criteria were considered to define BE length( Reference Sharma, Dent and Armstrong 13 ). Multiple biopsies of BE were taken, according to the Seattle Protocol( Reference Reid, Blount and Feng 14 ).
The E group was identified among patients with an endoscopic diagnosis of reflux E characterised by mucosal breaks. Grades A and B of the Los Angeles classification were considered( Reference Lundell, Dent and Bennett 15 ). Patients in the E group underwent four biopsies: two at the Z-line and two at 2 cm above it.
Cases were consecutively recruited from eligible patients with a new diagnosis of BE or E from March 2009 to October 2012. The control group was consecutively recruited in the same units as the cases, through a non-random selection among eligible patients with no BE or E, undergoing upper endoscopy for any reason in the same period as the cases, both in presence or absence of GERD. Diagnosis of GERD was based on the presence of typical symptoms: heartburn or pyrosis (defined as retrosternal burning sensation, starting from the epigastric region and radiating up to the neck), regurgitation (as an acid or bitter taste in the mouth) and dysphagia. We defined as GERD positive those subjects reporting at least weekly heartburn and/or acid regurgitation 1 year before diagnosis( Reference Edelstein, Bronner and Rosen 16 ). Biopsies were interpreted in every centre by experienced gastro-intestinal pathologists.
In all cases, eligible patients were men or women, aged 18 years or older, able to give informed consent and agreeing to participate in a questionnaire, all without history of previous cancer or serious chronic diseases. The study was approved by the Ethical Committee of each centre and informed consent was obtained from all participating patients.
Questionnaire
The interviewers were centrally trained. The same questionnaire and coding manual were used for all subjects. Questions referred to symptoms or habits before the diagnosis of BE or E or before endoscopy for controls.
The questionnaire included questions on individual characteristics (education, occupation, weight and height), lifestyle habits (diet, tea, coffee and other types of beverage consumption, and smoking habit), past medical history, use of drugs, presence and duration of GERD symptoms (as the sum of the duration of at least weekly heartburn or regurgitation symptoms) and family history of cancer.
Each subject was asked to report about lifetime consumption of all alcoholic beverages: red and white wine, beer and liquors. To measure consumption of liquors three items were used: aperitifs and digestifs, containing up to 35 % alcohol by volume, and spirits with more than 35 %.
For all beverages, subjects’ entire drinking history was recalled in detail according to his/her drinking status, namely non-drinker, former drinker (who had quit at least 1 year before enrolment) and current drinker. Subjects were considered ever drinkers if they had consumed beverages at least monthly for 6 months or more. Questions were asked about the frequency of consumption, years of duration, age at initiation and, for former drinkers, years since cessation. One unit was equivalent to one glass of red or white wine (about 125 ml), one glass of lager or stout beer (one can or bottle, 330 ml) and one shot/glass of liquor (80 ml for aperitifs and 40 ml for digestifs or spirits).
Statistical methods
To estimate the effect of alcohol habit on the three-level health outcome (i.e. C group, E patients and BE patients) a multinomial logistic regression (MLR) modelling was applied( Reference Hosmer and Lemeshow 17 ). MLR can be considered as an extension of the more widely used logistic regression modelling for binary outcome (i.e. ill cases v. healthy controls) in that it allows to assess the statistical association between health status and study exposure (i.e. alcohol-related characteristics) performing simultaneously two binary comparisons: E patients v. C subjects and BE patients v. C subjects. Within each comparison, OR point estimate, along with corresponding 95 % CI, is computed and considered as an index of association between each binary outcome (E v. C or BE v. C) and each potential risk factor.
Alcohol habit is represented by several quantitative characteristics (frequency of consumption, number of units consumed, years of duration, age at initiation and years since cessation) each of which should be carefully considered and properly analysed in order to evaluate their distinct effect on individual health outcome. Such characteristics are generally well correlated and this may seriously impede a joint assessment through a regression modelling and, accordingly, prevent from controlling for the reciprocal confounding effect( Reference Leffondré, Abrahamowicz and Siemiatycki 18 ). For these reasons, the following regression strategy was applied. Data were stratified according to drinking status (former and current drinkers) and in each stratum a MLR analysis was performed using non-drinkers as a reference category. Only one quantitative drinking variable (main predictor) at a time entered the regression equation after categorisation based on specific thresholds (percentiles) a priori defined on the distribution of the C group. The remaining quantitative characteristics, appropriately transformed (centred), entered the equation as continuous variables (covariates)( Reference Leffondré, Abrahamowicz and Siemiatycki 18 ). In addition to alcohol-related variables, all MLR included age at interview, sex, BMI, smoking habit, years of schooling, duration of GERD and categorical terms for collaborative centres.
The statistical significance (two-tailed P<0·05) was assessed using the likelihood-based χ 2 test for linear trend (TLT)( Reference Hosmer and Lemeshow 17 ). All statistical analyses were performed using STATA software (Release 13.1, 2013; StataCorp LP).
Results
Baseline characteristics
Characteristics of BE patients (n 339), E patients (n 462) and C subjects (n 619) are given in Table 1. A total of 190 BE cases had also E with mean age 56·2 (sd 15·2) years for BE, 52·6 (sd 14·7) years for E and 53·7 (sd 14·1) years for C. Controls had a higher percentage of females and a lower BMI. C also had a lower percentage of smokers and a higher education qualification compared with the other groups. Reasons for endoscopy among C were mostly epigastric pain (38 %), regurgitation (25 %), dyspepsia (24 %), pyrosis or dysphagia (9 %), gastric or duodenal ulcer (3 %) and anaemia (1 %). According to our definition, GERD symptoms were present in 78·5 % of BE, 80·3 % of E and in 54·8 % of C. Among GERD-positive subjects, 80·8 % of BE patients had suffered from symptoms for more than 3 years v. 50·4 % of E and 40·7 % of C.
Table 1 Characteristics of Barrett’s oesophagus (BE), oesophagitis (E) and controls (n 1420) (Numbers and percentages)

GERD, gastro-oesophageal reflux disease.
Red and white wine consumption
Red wine was consumed by 72 % of BE, 69·7 % of E and 68·7 % of C. With respect to C, BE patients were significantly more likely to be current drinkers (54 v. 46·8 %, P=0·041), drank more (≥6 glasses/week: 35·4 v. 27·3 %, P=0·012) and for more time (>35 years: 33·1 v. 24·9 %, P=0·0092). In addition, BE started drinking at earlier age (<25 years: 26·5 v. 17·4 %, P=0·010) and quitted drinking red wine later than C (time since cessation ≤3 years: 4·4 v. 1·5 % C, P=0·011). A slightly higher percentage of current drinkers was present also among E patients (52·8 %, P=0·053) who also drank more (≥6 glasses/week: 37·2 %, P=0·001) and started drinking at earlier age (<25 years: 25·3 %, P=0·002) than C. Instead, no differences were observed as for duration (>35 years: 26 %) and time since cessation (≤3 years: 2·2 %).
Tables 2 and 3 show the results of MLR modelling reporting the risk of BE and E in former and current red wine drinkers, respectively, using non-wine drinkers as reference category. In current drinkers, main quantitative predictors were a priori categorised according to quartile values of alcohol consumption among C subjects. In former drinkers, given the small number of subjects (n 66), categorisation was based on median values.
Table 2 Relative risk of Barrett’s oesophagus (BE) and oesophagitis (E) according to red wine drinking habit estimated through multinomial logistic regression modelling among former drinkers using never drinkers as a referenceFootnote * (Odds ratios and 95 % confidence intervals)

C, control; Ref., referent values; GERD, gastro-oesophageal reflux disease; TLT, test for linear trend. P of the likelihood-based χ 2 test for linear trend.
* OR (relative risk) point estimate, adjusted for age at interview, sex, BMI, smoking habit, years of schooling, duration of GERD and collaborative centre.
Table 3 Relative risk of Barrett’s oesophagus (BE) and oesophagitis (E) according to red wine drinking habit estimated through multinomial logistic regression modelling among current drinkers using never drinkers as a referenceFootnote * (Odds ratios and 95 % confidence intervals)

C, control; Ref., referent values; GERD, gastro-oesophageal reflux disease; TLT, test for linear trend. P of the likelihood-based χ 2 test for linear trend.
* OR (relative risk) point estimate, adjusted for age at interview, sex, BMI, smoking habit, years of schooling, duration of GERD and collaborative centre.
Overall, no clear, monotonic (increasing or decreasing) and statistically significant dose–response relationship was pointed out in both former and current drinkers. However, generalised U-shaped (non-linear) trend of E/BE relative risk due to red wine consumption is noteworthy, particularly among current drinkers (Table 3; E v. C: models 2 and 3; BE v. C: all models), although such a tendency can be observed to some extent also in former drinkers (Table 2).
White wine was consumed by 54·3 % of BE, 56·9 % of E and 52·3 % of C. E patients were significantly more likely to be current drinkers than C (41·8 v. 34·9 %, P=0·025), whereas there was no difference between C and BE (39·8 %). The study subjects were similar with regard to frequency (≥6 glasses/week: BE 23·9 %, E 18·4 % and C 20·2 %). Subjects who had drunk for more time (>35 years) were 18·4 % in C, 23·9 % in BE (P=0·055) and 21 % in E (P=0·180). Both BE and E started drinking at younger age (≤24 years=35·1 and 34·9 v. 28·1 %, P=0·030 and 0·021, respectively). Only a few subjects (n 44) were former drinkers (5·1 % in BE, 2·1 % in E and 2·8 % in C) and this circumstance prevented us from performing further analyses on this subgroup. Table 4 shows the results of MLR analysis. Alcohol consumption variables were categorised according to quartiles (glasses/week and years of duration) and tertiles (age at initiation). No evidence of upward or downward trend in E/BE risk was highlighted, but, as already seen for red wine consumption, a noteworthy U-shaped dose–response relationship was observed in at least four out of six cases (Table 4; E v. C: models 1 and 3; BE v. C: all models).
Table 4 Relative risk of Barrett’s oesophagus (BE) and oesophagitis (E) according to white wine drinking habit estimated through multinomial logistic regression modelling among current drinkers using never drinkers as a referenceFootnote * (Odds ratios and 95 % confidence intervals)

C, control; Ref., referent values; GERD, gastro-oesophageal reflux disease; TLT, test for linear trend. P of the likelihood-based χ 2 test for linear trend.
* OR (relative risk) point estimate, adjusted for age at interview, sex, BMI, smoking habit, years of schooling, duration of GERD and collaborative centre.
Liquors and spirits consumption
Overall, liquors/spirits were consumed by 42·5 % of BE, 46·8 % of E and 38·6 % of C, with a significant difference between E and C (P=0·012). Percentage of current drinkers was lower among C (8·9 %) with respect to E (17·3 %, P<0·001) and BE (15·6 %, P=0·0027). Both BE and E consumed these beverages for a longer time than C (≥15 years: 17·7 and 12·1 v. 7·6 %, P<0·001 and P=0·017, respectively). Furthermore, BE and E started consuming spirits at an earlier age (<20 years: 8·6 % BE, 6·1 % E v. 3·1 % E, P<0·001 and P=0·026, respectively). With regard to the type of beverages, only a few subjects declared to drink aperitifs (18 % BE, 20·1 % E and 16·8 % C). Percentage of subjects consuming digestifs was similar between BE (28·6 %) and C (24·7 %), whereas there was a higher percentage of E with respect to C (31·6 %, P=0·015). Spirits consumption was reported by 30·1 % of BE, 26·8 % of E and 22·1 % of C, with a significant difference between BE and C (P=0·009). Among these subjects a consumption frequency ≥1 glass/week was reported by 15·9 % BE, 16·3 % E and 11·5 % C, with a significant difference between E and C (P=0·030). Table 5 shows the results of MLR analysis performed on current drinkers solely because of the limited information available on former drinkers. In this context, MLR failed to point out any apparent linear trend in risk by alcohol consumption. However, higher alcohol consumption levels (>1 glass/week, >15 years of duration and <30 years of age at initiation) seemed to bring about some risk excesses, for both adverse health outcomes, ranging from 1·14 to 2·30, although statistically non-significant.
Table 5 Relative risk of Barrett’s oesophagus and oesophagitis according to heavy alcohol consumption habit estimated through multinomial logistic regression modelling among current drinkers using never drinkers as a referenceFootnote * (Odds ratios and 95 % confidence intervals)

C, control; Ref., referent values; GERD, gastro-oesophageal reflux disease; TLT, test for linear trend. P of the likelihood-based χ 2 test for linear trend.
* OR (relative risk) point estimate, adjusted for age at interview, sex, BMI, smoking habit, years of schooling, duration of GERD and collaborative centre.
Beer consumption
Beer was currently consumed by 52·8 % of BE, 58·7 % of E and 50·7 % of C, with a significant difference between C and E (P=0·011). A higher consumption (≥6 glasses/week) was reported by 22·7 % of BE, 24·5 % of E and 21·2 % of C. E patients differed significantly from C also with regard to duration of intake (>20 years: 18·4 v. 11·8 %, P<0·010), whereas there was no difference between C and BE (15·7 %). Moreover, in this case, given the lack of information about former drinkers, regression analysis was restricted to current drinkers (Table 6). Noteworthy and statistically significant decreasing dose–response relationships were found in BE patients for frequency (model 1, TLT P=0·002) and duration of beer consumption (model 2; TLT P=0·009). Similar but less clear downward tendencies were also found for E patients (model 1, TLT P=0·801; model 2, TLT P=0·078).
Table 6 Relative risk of Barrett’s oesophagus (BE) and oesophagitis (E) according to beer drinking habit estimated through multinomial logistic regression modelling among current drinkers using never drinkers as a referenceFootnote * (Odds ratios and 95 % confidence intervals)
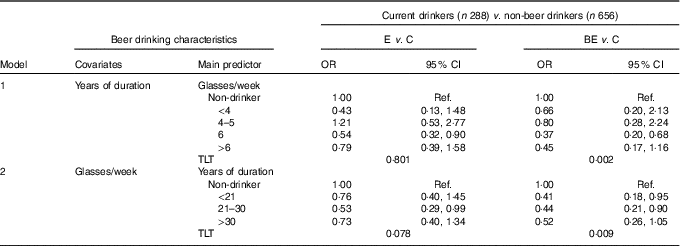
C, control; Ref., referent values; GERD, gastro-oesophageal reflux disease; TLT, test for linear trend. P of the likelihood-based χ 2 test for linear trend.
* OR (relative risk) point estimate, adjusted for age at interview, sex, BMI, smoking habit, years of schooling, duration of GERD and collaborative centre.
Discussion
In this study we evaluated the association between consumption of alcoholic beverages (wine, beer, liquors) and the presence of BE or reflux E, compared with a control group of non-neoplastic patients undergoing endoscopy for any reason, but with no BE or E.
We observed some risk excesses for both pathologies with a higher intake of any type of liquors/spirits, nevertheless, no apparent linear trends by alcohol consumption categories were found.
Interestingly, no evident monotonic dose–response relationship was found with wine consumption. In particular, using restricted cubic splines( Reference Harrell 19 ) generalised U-shaped (non-linear) trends of E/BE risk by consumption of both red and white wine were pointed out: a beneficial effect seems to occur with moderate intake or shorter duration, whereas worse outcomes resulted for non-wine drinkers and heavy/longer drinking habit (Fig. 1).

Fig. 1 U-shaped dose–response relationships between risk of oesophagitis (E)/Barrett’s oesophagus (BE) and level of red and white wine consumption in current drinkers compared with non-wine drinkers. Smoothed OR based on three/four-knot restricted cubic splines. C, control.
In addition, beer consumers were found to be at lower risk of BE and, to a lesser degree, of E, irrespective of frequency or duration. It is noteworthy that a similar response was shown in the majority of surveys examining the relationship between alcohol consumption and multiple cardiovascular outcomes( Reference Ronksley, Brien and Turner 20 – Reference Wakabayashi 22 ). According to a study, there was a U-shaped relationship between beer, wine and spirits intake, and heart failure incidence, with a nadir at low-to-moderate intake. In this case, wine and spirits no longer appeared protective above 7 drinks/week, whereas beer appeared potentially protective for 7–14 drinks/week( Reference Dorans, Mostofsky and Levitan 23 ). On the other hand, occasional or chronic ethanol intake at high levels increases the risk for myocardial infarction and stroke( Reference Krenz and Korthuis 24 ).
Alcohol is an established risk factor for oesophageal squamous cell carcinoma( Reference Bagnardi, Rota and Botteri 6 ), but reports about the association between alcohol and reflux E and BE, as well as EAC are still inconsistent. Alcohol consumption may increase gastro-oesophageal reflux symptoms, cause damage to the oesophageal mucosa and/or promote carcinogenesis( Reference Keshavarzian, Rizk and Urban 25 ). Several studies have demonstrated an association between alcohol intake and increased GERD symptoms( Reference Kawanishi 26 – Reference Locke, Talley and Fett 29 ), nevertheless, these results are not corroborated by some authors( Reference Pandeya, Green and Whiteman 30 ) or an inverse association was observed( Reference Dore, Maragkoudakis and Fraley 31 ). A higher total alcohol intake was found among EAC patients compared with controls, although the risk of tumour was quite lower than the risk of squamous cell carcinoma( Reference Vaughan, Davis and Kristal 32 – Reference Garidou, Tzonou and Lipworth 34 ). On the contrary, other authors reported an inverse association with moderate intake( Reference Gammon, Schoenberg and Ahsan 35 – Reference Freedman, Murray and Kamangar 37 ), whereas no effect was found in other studies( Reference Veugelers, Porter and Guernsey 38 – Reference Yates, Cheong and Luben 44 ). Little is known about the real effect of alcohol on BE, particularly with regard to alcohol types. Again, a certain number of studies found no relationship( Reference Koek, Sifrim and Lerut 3 , Reference Steevens, Schouten and Driessen 8 , Reference Thrift, Kramer and Richardson 9 , Reference Edelstein, Bronner and Rosen 16 , Reference Anderson, Cantwell and Watson 43 , Reference Conio, Filiberti and Blanchi 45 – Reference Johansson, Hakansson and Mellblom 47 ), but lifetime alcohol intake was associated with lower BE risk according to some reports( Reference Thrift, Kramer and Richardson 9 , Reference Yates, Cheong and Luben 44 ). Thrift et al.( Reference Thrift, Pandeya and Smith 48 ) found inverse associations with intermediate or higher levels of lifetime total alcohol consumption, for comparisons with both population and inflammation controls. Conversely, alcohol was found to increase the risk only when comparing cases with GERD controls according to Kubo et al.( Reference Kubo, Levin and Block 10 ). In a recent pooled analysis of data from five case–control studies, a borderline significant inverse association between BE and any alcohol consumption was found when cases were compared with population controls, although the risk did not decrease in a linear manner. In this contest, subjects consuming 3 to <5 drinks/d had about half the risk of BE compared with non-drinkers, but there were no statistically significant associations with lower or higher levels of alcohol. No association with alcohol was found when cases were compared with GERD controls( Reference Thrift, Cook and Vaughan 7 ).
Considering alcohol type, a modest intake of red wine has been associated with a reduced risk of EAC in some reports( Reference Gammon, Schoenberg and Ahsan 35 , Reference Pandeya, Williams and Green 49 , Reference Mayne and Navarro 50 ), but not in a cohort study( Reference Freedman, Abnet and Leitzmann 42 ). An inverse association was found also with BE( Reference Conio, Filiberti and Blanchi 45 , Reference Hirota, Loughney and Lazas 46 ), even when there was no association with total alcohol use( Reference Kubo, Levin and Block 10 ).
The role of beer consumption is contradictory, too. The highest intake of beer at younger age was inversely related with BE, but the association was no more present when considering consumption 5 years before the interview according to Anderson et al.( Reference Anderson, Cantwell and Watson 43 ). Thrift et al.( Reference Thrift, Pandeya and Smith 48 ) found an inverse linear trend with beer consumption using both population and inflammation controls, whereas BE patients were up 2-fold more likely to drink beer when compared with GERD controls in another survey( Reference Kubo, Levin and Block 10 ).
A few studies reported an adverse effect of liquor consumption. Thrift et al.( Reference Thrift, Pandeya and Smith 48 ) observed a significant linear trend for increasing risk of dysplastic BE with rising liquor consumption by comparing BE cases with inflammation controls. A high liquor consumption (≥40 drinks/month) was also associated with an increased risk of both GERD and BE when patients were compared with asymptomatic individuals. In this case, the risk of BE increased 3-fold( Reference Veugelers, Porter and Guernsey 38 ).
Most reports on the relationship between alcohol consumption and E are from Asian areas where consumption of different beverages could not be representative of that in Western countries. Alcohol consumption was positively correlated with both reflux E and non-erosive reflux disease in a large Japanese cohort of subjects who underwent upper gastro-intestinal endoscopy, compared with GERD-free subjects( Reference Minatsuki, Yamamichi and Shimamoto 51 ). Total alcohol consumption did not seem to be a risk factor of E according to a number of Western authors( Reference Koek, Sifrim and Lerut 3 , Reference Avidan, Sonnenberg and Schnell 52 , Reference Ryan, Hetzel and Shearman 53 ), nevertheless, Anderson et al.( Reference Anderson, Cantwell and Watson 43 ) found that alcohol consumption at least once per month in early adulthood may increase more than 2-fold the risk of developing E. In this case, nevertheless, there was an inverse association when the alcohol intake increased. In addition, a regular alcohol intake was found to increase about 70 % of the risk of any grade of E in a prospective cohort study on patients with heartburn( Reference Labenz, Jaspersen and Kulig 54 ).
When considering alcohol type, the risk of E appeared to increase up to 2-fold with a high liquor intake before diagnosis( Reference Anderson, Cantwell and Watson 43 , Reference Conio, Filiberti and Blanchi 45 ), whereas subjects with higher wine intake had half the risk of the disease compared with non-drinkers( Reference Anderson, Cantwell and Watson 43 ).
The data on the association between E and beer are very scarce. Anderson et al.( Reference Anderson, Cantwell and Watson 43 ) showed that the above-reported results seen for the overall alcohol intake in early adulthood could be explained by beer consumption and that there was no association when beer was consumed 5 years before the interview date.
To date, no clear hypotheses exist on the association between alcoholic beverages and BE or E; nevertheless, some mechanisms may support the hypothesis of a negative or protective effect. Polyphenols, in particular resveratrol, present in red grape skin may reduce the oxidative damage caused by GERD, thereby decreasing the risk of E and BE, as well as of EAC( Reference Rivero-Perez, Muniz and Gonzalez-Sanjose 55 – Reference Bianchini and Vainio 58 ). Antioxidants are also present to a lesser extent in beer( Reference Gerhauser 59 ) and this could explain the benefits of beer consumption as observed in some reports( Reference Thrift, Pandeya and Smith 48 ). It has also been suggested that the protective effects of ethanol consumption may also arise from reductions in insulin resistance or increased levels of lipoproteins( Reference Vasdev, Gill and Singal 60 ), but it could be that the apparent health benefits of moderate alcohol consumption and wine drinking may be due to other protective unmeasured or unknown lifestyle habits of moderate drinkers v. abstainers or to favourable risk profiles in moderate drinkers( Reference Barefoot, Gronbaek and Feaganes 61 ). On the other hand, as emphasised by other authors, unlike wine drinkers, liquor drinkers are less likely to consume these beverages with food, thereby increasing the possibility of irritation and damage to the oesophageal tissues( Reference Kubo, Levin and Block 10 ).
The divergent results among studies might derive from heterogeneity of the cases or reference groups examined in many surveys (incident or prevalent cases, population controls without endoscopy, GERD controls) that might have influenced the individual behavioural patterns( Reference Edelstein, Bronner and Rosen 16 ). The lack of information on the presence of GERD( Reference Steevens, Schouten and Driessen 8 ) and the use of population controls may not provide sufficient number of GERD patients to estimate the effect of a risk factor independent of GERD symptoms( Reference Kubo, Levin and Block 10 ). In addition, controls sampled from the general population might have an undiagnosed BE or GERD, even if BE is not frequent in endoscopy series of healthy volunteers and has been diagnosed in <10 % of patients with severe reflux undergoing endoscopy( Reference El-Serag, Petersen and Carter 62 , Reference Nandurkar, Locke and Murray 63 ).
Also, contrasting results may be obtained when adjustment is made for possible confounders or with different temporalities of the associations; that is, a lifetime or recent beverage consumption and timing of the intake in relationship to diagnosis (cases or GERD controls may have consumed more alcohol in early life, reducing their intake later because of symptoms or diagnosis of oesophageal abnormality)( Reference Kubo, Levin and Block 10 , Reference Anderson, Cantwell and Watson 43 , Reference Thrift, Pandeya and Smith 48 , Reference El-Serag and Lagergren 64 ).
Method of ascertaining alcohol consumption and definition of drinking (i.e. frequency of servings, quantity, ethanol content) may also be a limitation in comparing studies( Reference Thrift, Kramer and Richardson 9 , Reference Anderson, Cantwell and Watson 43 ).
One of the strengths of present survey is the fact that, to the best of our knowledge, this is the first study analysing the association between BE or E and drinking habits in a pool of Italian areas. In this study, controls did not seem to differ from the Italian population in terms of their alcohol consumption and it is worth mentioning that it is possible that the type and amount of alcohol-containing beverages as consumed by Italians differ from those in other studies. Furthermore, both cases and controls were endoscopically documented, cases had no prior diagnosis of BE or E and we had the possibility of controlling for the presence and the length of GERD duration. On the other hand, information on symptoms was self-reported. The recording of exposure may vary depending on the investigator’s knowledge of an individual’s disease status, but in this case interviewers knew that patients (both cases and controls) might have had GERD symptoms, but were unaware of the subjects diagnosis.
A potential bias is the fact that subjects with symptoms or suspicion of BE or E may have avoided alcohol because it could exacerbate their symptoms. These subjects are more likely to be diagnosed with BE or E having a more health-seeking behaviour, nevertheless, in our series the number of former drinkers was quite low and also controls had endoscopy because of gastric or oesophageal problems. Generally, a recall bias might have influenced individuals’ drinking history, with high consumers of alcohol reporting a lower intake.
Another limitation is the use of the analyses of non-drinkers instead of abstainers as the comparison group. Nevertheless, results did not substantially change when using abstainers as the reference category because of the quite low number in this group (for a total of ninety subjects). In addition, we did not report results for total alcohol intake for subjects drinking both red and white wine as a clear overestimate of alcohol consumption was obtained.
Selection of controls was non-random; nevertheless, we think there was no non-response bias or a different reporting of exposure between cases and controls, as it is generally recognised that some dietary habits (and alcohol consumption) may be risk factors for digestive diseases. On the other hand, controls were younger than cases: this aspect and the fact that they did not represent an asymptomatic population may have resulted in an underestimation of the strength of the association between exposure (alcohol) and outcome.
Although we had information on dietary habits of both cases and controls, we did not consider them in this analysis as diet is an extremely complex mix of several characteristics (foods, servings, macro- and micro-nutrients, dietary patterns, etc.), which can show different effects on human health. Hence, even if we had taken into consideration one or more dietary patterns as potential confounding factors it would have not ruled out residual confounding factors anyway.
Actually, different components of diet or dietary habits can modulate the association with alcohol intake and oesophageal diseases, both leading to an increased risk or acting as protective factors( Reference Shivappa, Hébert and Rosato 65 ). Moreover, as outlined by some authors, the frequency of general alcohol consumption and type of beverage are related to many factors; as wine drinkers may have different (and more probably healthier) lifestyles and dietary habits compared with beer and liquor drinkers( Reference Kubo, Levin and Block 10 , Reference Gronbaek 66 ). Nevertheless, adjusting for fruit and vegetable intake and for most of the factors that were associated with alcohol preference did not attenuate the inverse association for wine drinkers compared with non-drinkers in a study from Kubo et al.( Reference Kubo, Levin and Block 10 ).
In conclusion, consistent with findings from other studies, our results did not support a causative role of alcohol in aetiogenesis of BE or E. Although our results were often not statistically significant, data suggested a reduced risk of BE and E with a low intake of wine and with beer consumption. A non-significant increased risk for both BE and E was observed with a higher intake of any type of high-alcoholic beverage, but in this case no conclusion can be drawn because of the high percentages of non-spirit drinkers in all study groups and the small number of drinkers at higher levels.
Acknowledgements
The authors thank the study participants for their confidence and collaboration.
This work was supported by Bracco Spa. Bracco Spa contributed to the study design and had no role in analysis or writing of this article.
The authors’ contributions are as follows: M. C. was the principal investigator and contributed to the study design; R. A. F. contributed to the study design and wrote the manuscript; V. F. carried out data analyses and wrote the manuscript; E. G. contributed to the study design; A. D. C., S. B., D. D. C., T. L., M. D. M., O. I., R. C., M. F., F. L., V. D and G. I. contributed to subject briefings and data collection; A. R. carried out data analyses. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.










