Cu deficiency in ruminants is a problem worldwide, often due to the presence of high levels of a Cu antagonist in the diet(Reference Underwood and Suttle1). In beef cattle, Cu deficiency may present in the form of depressed growth, hair depigmentation and anaemia. These signs can be explained by the reduced activity of cuproenzymes such as cytochrome c oxidase, tyrosinase and caeruloplasmin, which are important in energy production, melanin production and Fe metabolism, respectively(2). Several researchers have investigated the effect of Cu deficiency on growth in beef cattle, with varying results(Reference Spears, Ward and Gengelbach3). Suttle & Angus(Reference Suttle and Angus4) induced Cu deficiency in young calves through supplementation of 1·5 mg Mo/kg DM and reported that deficient calves weighed approximately 30 % less than their supplemented (7 mg Cu/kg DM) counterparts after 24 weeks on experimental diets. More recently, Ward et al. (Reference Ward, Spears and Kegley5) experimentally induced Cu deficiency in calves through Mo and S supplementation for 98 d and reported minimal negative effects on growth. Alternately, Gengelbach et al. (Reference Gengelbach, Ward and Spears6) noted that Cu deficiency induced through Mo supplementation resulted in depressed growth of calves compared with calves supplemented with Cu. It is possible that the effect of Cu deficiency on the growth of cattle is dependent on the duration and severity of Cu deficiency. The age of the animal also appears to play a role in the susceptibility to Cu deficiency. Poole & Rogers(Reference Poole, Rogers, Anke, Schneider and Bruckner7) suggested that the young calf is most vulnerable to Cu deficiency and that even when the deficiency is treated, long-term impacts on growth are observed. To our knowledge, no work has examined the effects of a life-long, severe Cu deficiency in beef cattle.
Cu-deficient soils are a problem worldwide, and are of particular concern in locations such as the British Isles which also have elevated soil concentrations of the Cu antagonist Mo(Reference Suttle8, Reference Thornton9). Mn is another potential Cu antagonist, and excessive dietary Mn from feedstuffs, industrial contamination or soil ingestion may have negative impacts on Cu absorption. Mn concentrations in some forages may be greater than 100 mg/kg DM, while the Cu content of forages is typically low(Reference Grace10). Limited studies examining the antagonistic effects of Mn on Cu have been conducted; however, research with rats has demonstrated a complicated interaction between the effects of dietary Mn (10 or 50 mg/kg DM) and Cu ( < 1 or 6 mg/kg DM) on indices of Cu and Fe status(Reference Reeves, Ralston, Idso and Lukaski11). These authors reported that 50 mg Mn/kg DM reduced duodenal tissue Cu concentrations in rats receiving 6 mg Cu/kg DM, but had no effect on rats consuming the low-Cu diet. It was also observed that increasing dietary Mn reduced serum Cu concentrations when rats were fed Cu-adequate diets but had no effect when rats were fed Cu-deficient diets(Reference Reeves, Ralston, Idso and Lukaski11). It has recently been suggested that Cu and Mn may share a common pathway for intestinal absorption, via a protein known as divalent metal transporter 1 (DMT1)(Reference Arredondo, Munoz, Mura and Nunez12). Competition for this transporter may explain decreased duodenal and serum Cu concentrations observed when high Mn was fed to rats(Reference Reeves, Ralston, Idso and Lukaski11). Additionally, changes in a Cu-specific importer known as Cu transporter 1 in response to a severely Cu-deficient diet may have counteracted the impact of high Mn in Cu-deficient rats. Clearly a complicated relationship exists between Mn and Cu absorption.
Few data on the effect of high dietary Mn on Cu absorption have been reported in ruminants. Ivan & Grieve(Reference Ivan and Grieve13) found that the addition of 50 mg Mn/kg DM to a diet containing 12 mg Mn/kg DM resulted in decreased net Cu absorption throughout the gastrointestinal tract of young Holstein calves; however, the dynamics of this antagonism in ruminants are not understood.
Manipulation of planes of nutrition has been shown to alter liver gene expression in cattle(Reference Loor, Dann, Janovick-Guretzky, Everts, Oliveira, Green, Litherland, Rodriguez-Zas, Lewin and Drackley14). For example, restricting prepartum energy intake in Holstein cows was associated with the up-regulation of genes involved in liver fatty acid oxidation, gluconeogenesis and the synthesis of cholesterol. Dietary manipulation of essential minerals such as Cu and Mn may also induce a transcriptional response in these animals, affecting genes associated with oxidative stress and energy status. Cytochrome c oxidase subunit 1 (COX1) and superoxide dismutase 1 (SOD1) are proteins which play a role in oxidative phosphorylation and oxidant protection, respectively, and require Cu for proper enzymic function. The effect of dietary Cu and Mn concentrations on the expression of genes encoding these important proteins has not been determined in cattle.
Therefore, the objective of the present study was to determine the impacts of a long-term, severe Cu deficiency, in the presence or absence of high dietary Mn, on Cu status, growth and gene expression of beef cattle.
Experimental methods
Animals and experimental design
Experimental procedures were reviewed and approved by the North Carolina State University Animal Care and Use Committee. Twenty-one Angus calves (eleven Angus steers and nine Angus heifers (38·9 (se 2·4) kg body weight at birth)) were used in the present study. Calves were born to cows that were part of a previous study examining the impact of a long-term dietary Cu and Mn imbalance on the biology of brain prion proteins(Reference Legleiter15). By the time calves in the present study were born, dams had been on their respective dietary treatments for at least 410 d. Approximately 33 d before calving, cows were moved into a covered facility with slatted floors and group fed by treatment in pens of two or three cows. Cows calved over a 36 d period, and birth of the calves was considered day 0 of the study, and all days mentioned are based on average calf age on that day. Cows remained in the pens with their calves until weaning when calves averaged age 183 d. After weaning, calves were vaccinated against infectious bovine rhinotracheitis, bovine viral diarrhoea (I and II), parainfluenza-3, bovine respiratory syncytial virus (Titanium 5; AgriLabs, St Joseph, MO, USA), Clostridia (Vision 7; Intervet, Millsboro, DE, USA) and Moraxella bovis (Piliguard Pinkeye-1 Trivalent; Schering-Plough Animal Health, Ltd, Wellington, New Zealand). Calves were also treated for internal and external parasites (Privermectin; First Priority, Inc., Elgin, IL, USA). Following weaning, calves remained in the pens and were bunk fed by treatment for a period of 43 d. On day 226, calves were moved to pens with electronic Calan gate feeders (American Calan, Northwood, NH, USA) and were fed individually for the remainder of the trial.
Treatments included: (1) 10 mg supplemental Cu/kg DM and 20 mg supplemental Mn/kg DM (Cu adequate, +Cu; n 6); (2) no supplemental Cu, 20 mg supplemental Mn/kg DM and 2 mg supplemental Mo/kg DM (Cu deficient, − Cu; n 8); (3) no supplemental Cu, 500 mg supplemental Mn/kg DM and 2 mg Mo/kg DM (Cu deficient plus Mn, − Cu+Mn; n 7). Cu was provided as Cu2(OH)3Cl, Mo as NaMoO4 and Mn as MnSO4.H2O. Calves were fed a maize silage-based diet (analysed 7 mg Cu/kg DM and 59 mg Mn/kg DM) through the 136 d growing phase and a ground maize-based diet (analysed 4 mg Cu/kg DM and 32 mg Mn/kg DM) through the 139 d finishing phase. Diets were formulated to meet or exceed all National Research Council requirements(2), with the exception of Cu. Ingredient compositions of the basal diets are shown in Table 1. Calves were fed once daily, with feed amounts based on consumption in a 24 h period. Individual body weights were taken at birth and on days 73, 114, 183, 217, 241, 269, 297, 325, 353, 416, 459 and 490. Jugular blood samples were collected at birth and on days 114, 183, 241, 297, 422, 459 and 490 for analysis of plasma Cu. Liver biopsy samples were obtained as previously described(Reference Engle and Spears16) on days 114, 183, 297 and 422 for mineral determination.
Table 1 Ingredient composition of the growing and finishing diets
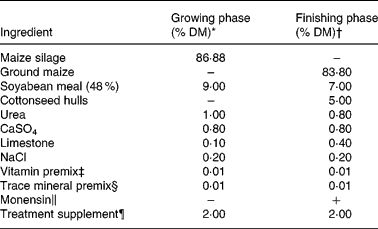
* Analysed 7 mg Cu/kg DM and 59 mg Mn/kg DM.
† Analysed 4 mg Cu/kg DM and 32 mg Mn/kg DM.
‡ Provided (per kg premix): 1·98 g all-trans retinol; 38 mg cholecalciferol; 4·2 g DL-α-tocopheryl acetate.
§ Provided (per kg diet): 30 mg Zn as ZnSO4; 0·5 mg I as Ca(IO3)2(H2O); 0·2 mg Se as Na2SeO3; 0·1 mg Co as CoCO3.
∥ Provided 33 mg monensin/kg DM.
¶ A ground maize supplement provided the following treatments: +Cu (10 mg Cu/kg DM, 20 mg Mn/kg DM); − Cu (20 mg Mn/kg DM, 2 mg Mo/kg DM); − Cu+Mn (500 mg Mn/kg DM, 2 mg Mo/kg DM).
Tissue collection and analytical procedures
On day 492 of the study calves were transported to a commercial abattoir approximately 40 km from our research facility, housed overnight and harvested the following morning. Liver samples were collected for mineral determination and gene expression analysis. Samples were flash frozen in liquid N2 to protect against RNA degradation. Approximately 20–30 min after calves were stunned, intestinal samples were collected in the following manner: a segment of duodenum approximately 25 cm long, extending from just below the pyloric sphincter, was removed from the gastrointestinal tract of the animal. The segment was flushed several times with physiological saline (0·9 % saline, pH 7) and cut open longitudinally. The segment was rinsed briefly with saline once again to remove any remaining digesta. Using a clean glass microscope slide, approximately three scrapings of moderate pressure were taken of the exposed intestinal mucosa. The scrapings were flash frozen in liquid N2, placed into polypropylene tubes and stored on dry ice for transportation back to the laboratory where samples were stored at − 80°C until protein extraction could be performed. Scrapings were analysed for the Cu chaperone protein (CCS) that shuttles Cu through the cytosol to Cu–Zn superoxide dismutase.
Approximately 0·5 g of chilled duodenal tissue was homogenised in 3 ml of a modified Radio Immuno Precipitation Assay buffer (20 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)–HCl, 150 mm-NaCl, 1 % NP-40 (v/v), 0·5 % sodium deoxycholate (w/v), 0·1 % SDS (w/v), 2 mm-EDTA and a protease inhibitor cocktail (Sigma Protease Inhibitor Cocktail P2714; Sigma Aldrich, St Louis, MO, USA)) for CCS determination. After 30 min on ice the 16 % homogenate was centrifuged at 10 000 g for 30 min at 4°C. The clarified supernatant fraction was removed and measured for protein content using the DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). Samples were equilibrated for protein content, sampled into microcentrifuge tubes and stored at − 80°C until analysis.
All electrophoresis and Western blot equipment and supplies were purchased from Invitrogen Corp. (Carlsbad, CA, USA) unless otherwise stated. Before electrophoresis, samples were heated at 70°C for 10 min. PAGE was performed using precast NuPage Novex 4–12 % Bis–Tris gels and the Novex X-Cell Surelock Mini-Cell electrophoresis system. Magic Mark XP Western Protein Standards were used for molecular weight determination.
Approximately 33 μg total protein were loaded into each well. Proteins were separated under reducing and denaturing conditions and transferred onto a polyvinylidene diflouride membrane. Membranes were blocked for 30 min using the blocking solution provided in the Western Breeze Chemiluminescent Kit and probed for 1 h at room temperature with polyclonal rabbit anti-CCS (kindly provided by Dr Joseph Prohaska, University of Minnesota; 1:500 dilution). After incubation with the primary antibody, membranes were washed four times in 0·01 m-PBS (pH 7·1) containing 0·05 % Tween-20 (v/v), incubated for 30 min with the appropriate alkaline phosphatase-linked secondary antibody and then washed four times with the PBS-Tween 20 wash. Membranes were rinsed two times with water and then visualised using Enhanced Chemiluminescent Substrate (Western Breeze Kit; Invitrogen). Images were captured on autoradiography film (CL-XPosure Film; Pierce, Rockford, IL, USA) and band densities were semi-quantified using Image Quant TL software (Amersham Biosciences, Piscataway, NJ, USA). Membranes were stripped with Restore Western Blot Stripping Buffer (Pierce) and reprobed with β-actin (Abcam; 1:5000 dilution for 1 h at room temperature) as a loading control. Results are reported as β-actin-adjusted relative optical intensities in arbitrary units.
Blood was collected in trace mineral-free heparinised vacuum tubes designed for trace mineral analysis (Becton Dickenson, Rutherford, NJ, USA), transferred on ice to the laboratory and centrifuged at 1200 g for 20 min at 20°C. Plasma was removed and stored at − 20°C until analysed for Cu concentration. Plasma was prepared for Cu analysis as previously described(Reference Hansen, Schlegal, Legleiter, Lloyd and Spears17). Feed and liver samples were prepared for analysis by wet ashing using microwave digestion (Mars 5; CEM Corp., Matthews, NC, USA) as described by Gengelbach et al. (Reference Gengelbach, Ward and Spears6). The mineral content of plasma, feed, and liver samples was determined by flame atomic absorption spectroscopy (model AA-6701F; Shimadzu Scientific Instruments, Kyoto, Japan).
RNA isolation and real-time reverse transcriptase polymerase chain reaction
RNA was isolated from liver using the Qiagen RNeasy kit (Qiagen Inc., Valencia, CA, USA) with on-column DNase digestion following the manufacturer's suggested protocol. RNA quantity and purity were examined with a NanoDrop-1000 spectrophotometer. Real-time primers were designed with Beacon Designer software (Premier Biosoft Intl., Palo Alto, CA, USA; Table 2) to be compatible with SYBR Green I by avoiding regions of cross-homology between the genes of interest and the Bos taurus RNA Reference Sequence database. The M-fold program (Premier Biosoft Intl., Palo Alto, CA, USA) predicted template secondary structures that were avoided when designing primers. Because cytochrome c oxidase 1 (cox1) is encoded by the mitochondrial genome and contains no introns, real-time PCR primers were designed within the one exon. Primers for superoxide dismutase 1 (sod1) were designed to amplify across at least one predicted exon–intron boundary so that genomic DNA contamination could be detected. Ribosomal protein S9 (rps9) was selected as the internal housekeeping gene based on findings reported by Janovick-Guretzky et al. (Reference Janovick-Guretzky, Dann, Carlson, Murphy, Loor and Drackley18). Melting curves for each PCR reaction were generated to assess specificity of the reaction and one amplicon generated by each primer pair was sequenced to confirm the identity of the PCR product.
Table 2 Real-time reverse transcriptase polymerase chain reaction primers*
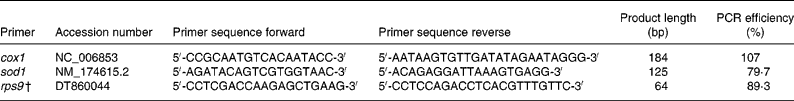
cox1, Cytochrome c oxidase subunit 1; sod1, superoxide dismutase 1; rps9, ribosomal protein S9.
* Primers were designed using Beacon Designer 7 (Premier Biosoft Intl., Palo Alto, CA, USA) unless otherwise stated.
† Reported by Janovick-Guretzky et al. (Reference Janovick-Guretzky, Dann, Carlson, Murphy, Loor and Drackley18).
The High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Inc., Foster City, CA, USA) was used to synthesise cDNA from 1 μg of RNA following the manufacturer's instructions. Real-time PCR was performed in a Bio-Rad iCycler IQ thermocycler (Hercules, CA, USA) with 1X Applied Biosystems Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 300 nm forward and reverse primers, 10 nm-fluorescein, and cDNA. Reactions were heated for 7 min at 95 °C, followed by fifty cycles of 95 °C for 30 s, 51 °C for 30 s, and 72 °C for 30 s and a final extension step at 72 °C for 5 min. Samples were amplified in triplicate and cycle threshold (CT) values were averaged for each sample.
PCR amplification efficiencies for each primer pair were estimated from standard curves of fluorescence intensity on cDNA concentration. Standard curves were generated by amplifying five 1:3 serial dilutions of pooled cDNA in the same plate as samples, all in triplicate. Fluorescence intensity was plotted by dilution series to estimate amplification efficiencies using the iCycler IQ Real-Time PCR Detection System Software v3.1 (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Statistical analysis of performance data was performed by ANOVA for a completely randomised design using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC, USA). All performance and mineral analysis data were tested for the effect of calf sex, which was found to be non-significant, and was subsequently removed from the model. The model for the performance data included the fixed effect of treatment. Age was used as a covariate for body-weight data. When treatment was significant (P ≤ 0·10), differences among means were separated using single df orthogonal contrasts. The comparisons made included: +Cu v. − Cu and − Cu v. − Cu+Mn. Liver Cu and plasma Cu data were log10 transformed to account for heterogeneity of variances. Plasma Cu, liver Cu and liver Mn were analysed as repeated measures with individual animals serving as the experimental unit. The model included the fixed effects of treatment, time and their interaction. When a treatment × time interaction was observed, data were further analysed by sampling day. Interactions that were not significant (P>0·05) for the measurement of interest were removed from the model. The previously stated contrasts were also used for the analysis of these data.
CT values were collected for each reaction. The threshold value for each reaction was set empirically at a value where amplification was proceeding exponentially. Samples expressing high levels of transcript will exceed the threshold value at an earlier PCR cycle than samples expressing low levels of transcript. Therefore, samples with lower CT values were transcribing more transcripts than samples with higher CT values. Before significance testing of RT-PCR data, gene expression was normalised to the housekeeping gene rps9 for each sample as followsn:
where CT T is the CT value for the target gene (gene of interest), CT rps9 is the CT value for the housekeeping gene rps9 and CT N is the normalised CT value. Higher values of CT N have lower gene expression than lower values of CT N. After normalisation, a one-way ANOVA was used to estimate the effect of sex and diet on CT N for each gene. JMP 7 (SAS Institute Inc.) software was used to perform the one-way ANOVA. P values < 0·05 were considered to be statistically significant. Fold-changes for each gene between treatments were calculated by the Pfaffl method(Reference Pfaffl, Horgan and Dempfle19) adjusting values for PCR amplification efficiencies.
Results
Copper status
Plasma Cu averaged across all sampling dates was lower (P = 0·0001) in calves consuming the − Cu diet compared with the +Cu diet, and concentrations were further depressed (P = 0·01) in − Cu+Mn calves compared with − Cu calves (Table 3). Plasma Cu was affected by a time × treatment interaction (P = 0·0001). Plasma Cu concentrations at birth did not differ due to dietary treatment; however, − Cu calves had lower plasma Cu values than +Cu calves on all other dates of the study. From day 114 through to day 490, plasma Cu concentrations in +Cu calves were well within normal ranges. Following birth, plasma Cu concentrations in − Cu calves dropped, with the exception of a slight increase on day 422 during the finishing period. Plasma Cu concentrations in − Cu+Mn calves never rose above the 4·82 μmol/l measured at birth and were lower than − Cu concentrations on days 114, 297, 422 and 459 of the study.
Table 3 Effect of dietary copper and manganese on plasma copper concentration (μmol/l) in growing calves
(Raw mean values and pooled standard errors)
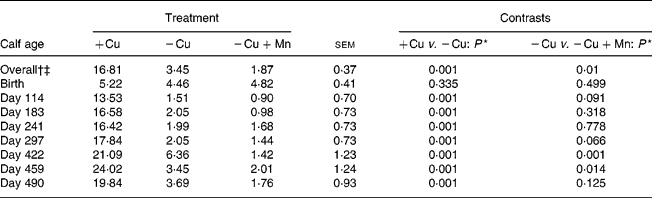
+Cu, Cu adequate; − Cu, Cu deficient; − Cu+Mn, Cu deficient plus high Mn.
* P values shown are based on log10 transformation of data.
† Time effect (P < 0·001).
‡ Time × treatment effect (P < 0·001).
Liver Cu concentrations were greater (P = 0·0001) in +Cu calves compared with − Cu calves and tended (P = 0·088) to be lower in − Cu+Mn calves compared with − Cu calves (Table 4). Cu concentrations in the liver of calves receiving the − Cu and − Cu+Mn diets were well below the threshold for Cu deficiency in cattle (0·31 μmol/g DM; see Underwood(Reference Underwood20)). Liver Cu was affected by a time × treatment interaction (P = 0·001). Liver Cu concentrations in +Cu calves were similar on days 114 and 183, but dipped slightly on day 297 before increasing on days 422 and 493. Concentrations of liver Cu in − Cu calves decreased from days 114 to 297 before increasing slightly during the finishing period (days 422 and 493). Liver Cu concentrations in − Cu+Mn calves remained low with the exception of a mild increase on day 297. In addition, − Cu+Mn calf liver Cu concentrations were lower than − Cu liver Cu concentrations on days 114, 422 and 493 of the trial.
Table 4 Effect of dietary copper and manganese on liver copper concentration (μmol/g DM) in growing calves
(Raw mean values and pooled standard errors)
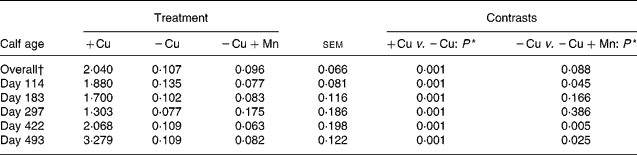
+Cu, Cu adequate; − Cu, Cu deficient; − Cu+Mn, Cu deficient plus high Mn.
* P values shown are based on log10 transformation of data.
† Time × treatment effect (P < 0·001).
Liver Mn concentrations were greater (P = 0·01) in calves receiving 500 mg supplemental Mn/kg DM compared with calves consuming the − Cu diet, and did not differ (P = 0·92) between the +Cu and − Cu treatment groups (Table 5). Liver Mn was affected by a time × treatment interaction (P = 0·001). Liver Mn concentrations in +Cu calves were fairly constant over the course of the study, dipping slightly on days 183 and 422. Similarly, − Cu calves exhibited steady liver Mn concentrations with minor decreases observed on days 114, 183 and 422. Calves receiving excess supplemental Mn exhibited an increase in liver Mn concentrations over the course of the study.
Table 5 Effect of dietary copper and manganese on liver manganese concentration (μmol/g DM) in growing calves
(Raw mean values and pooled standard errors)
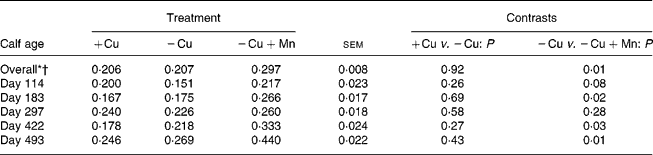
+Cu, Cu adequate; − Cu, Cu deficient; − Cu+Mn, Cu deficient plus high Mn.
* Time effect (P < 0·001).
† Time × treatment effect (P < 0·001).
Duodenal concentrations of CCS were increased (P = 0·03) in − Cu calves compared with +Cu calves and did not differ (P = 0·65) in − Cu+Mn calves compared with − Cu calves (Fig. 1).

Fig. 1 Relative concentrations (relative optical intensities; ROI) of Cu chaperone protein in duodenal mucosal scrapings of beef cattle based on Western blot analysis. Values are means, with standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P = 0·03).
Gene expression analysis
Relative gene expressions of cox1 and sod1 in the liver were investigated in the present study. Rps9 was selected as the housekeeping gene for the present study based on results from Janovick-Guretzky et al. (Reference Janovick-Guretzky, Dann, Carlson, Murphy, Loor and Drackley18). They examined housekeeping gene expression in liver samples acquired from cows in different dietary treatments, physiological states and feed intake amounts. Their results indicated that rps9 was one of the most stably expressed genes they examined across different experimental groups; therefore, this was the housekeeping gene we selected for the present study. Gene expression for the cox1 gene was lower (P < 0·05) in animals that received the − Cu diet compared with those that received either the +Cu diet or the − Cu+Mn diet (Table 6). There was a tendency (P < 0·11) for the sod1 gene to be down-regulated in the animals receiving the − Cu diet relative to those fed the +Cu or − Cu+Mn diets.
Table 6 Gene expression profiles in liver
(Mean values with their standard errors)

+Cu, Cu adequate; − Cu, Cu deficient; − Cu+Mn, Cu deficient plus high Mn; CT, cycle threshold; cox1, cytochrome c oxidase subunit 1; sod1, superoxide dismutase 1; rps9, ribosomal protein S9.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05; Tukey-Kramer mean comparison procedure).
* CT ratios are shown as the target gene:rps9 ratio.
† The mRNA-fold change was calculated relative to the adequate Cu diet values using Relative Expression Software Tool (REST©) adjusting for differences in PCR amplification efficiencies for the target genes and rps9 (Reference Pfaffl, Horgan and Dempfle19).
Growth
Average daily gain, DM intake and gain:feed ratio data are shown in Table 7. Calves born to cows fed − Cu+Mn diets tended (P = 0·13) to be lighter (35·1 (se 2·7 kg)) at birth than calves born to dams fed − Cu diets (40·8 (se 1·8 kg)). Birth weights did not differ between calves born to cows fed − Cu and +Cu diets (40·7 (se 2·7 kg)). Calves receiving the +Cu diet had greater (P = 0·009) average daily gain for the period between birth and weaning than calves of nursing cows in the − Cu treatment. Similarly, the average daily gain of − Cu+Mn calves was also low and did not differ (P = 0·6) from − Cu calves for the period between birth and weaning. However, during the growing phase calves receiving the − Cu diet gained more (P = 0·02) when compared with − Cu+Mn calves. In fact, during the growing phase − Cu calves actually gained at a rate comparable with calves receiving the Cu-adequate diet (P = 0·6). During the finishing phase the average daily gain of calves receiving the − Cu diet increased, with − Cu calves gaining more than the +Cu calves (P = 0·04) and tending (P = 0·12) to gain more than the − Cu+Mn treatment group. Intake did not differ between +Cu and − Cu calves during the growing phase (P = 0·58), but tended (P = 0·08) to be higher in − Cu calves compared with +Cu calves during the finishing period. Supplementation of 500 mg Mn/kg DM to a diet low in Cu depressed (P < 0·01) DM intake during both the growing and finishing phases when compared with the − Cu treatment group.
Table 7 Effect of dietary copper and manganese on growth characteristics of beef cattle
(Raw mean values and pooled standard errors)
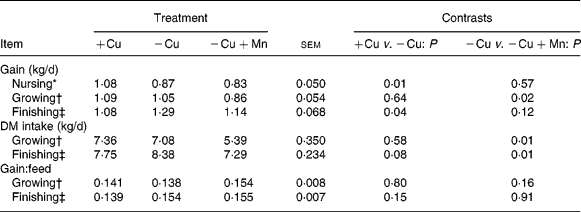
+Cu, Cu adequate; − Cu, Cu deficient; − Cu+Mn, Cu deficient plus high Mn.
* Defined as the period between birth and weaning.
† The growing phase lasted 136 d.
‡ The finishing phase lasted 139 d.
Weight-gain curves for calves in the various treatments are depicted in Fig. 2. Calves receiving the +Cu diet weighed more (P = 0·10) than − Cu calves on days 325 and 353 of the study. Body weights did not differ between +Cu and − Cu calves for the duration of the finishing phase; however, − Cu+Mn calves weighed less (P < 0·05) than − Cu calves from day 422 through to the end of the study. In addition, calves receiving the − Cu+Mn diet weighed 14 % less (P = 0·008; 473 (se 19·9) kg) than calves receiving the − Cu treatment (551 (se 13·4) kg) at harvest, while there was no difference (P = 0·5) between the − Cu and +Cu (560 (se 19·6) kg) groups at this time point.

Fig. 2 Growth curves of growing beef cattle fed diets either adequate in Cu (+Cu; –Δ–), deficient in Cu ( − Cu; –▲–) or deficient in Cu plus high dietary Mn ( − Cu+Mn; –□–) from birth through to harvest. Values are means. * Mean value for the − Cu calves was significantly different from that for the − Cu+Mn calves (P < 0·05). † Mean value for the +Cu calves was marginally significantly different from that for the − Cu calves (P < 0·10).
Discussion
We have previously capitalised on the strong dietary antagonism that exists between Mo and Cu to induce and maintain a deficiency of Cu in cattle via the addition of sodium molybdate to a diet low in Cu(Reference Hansen, Schlegal, Legleiter, Lloyd and Spears17, Reference Legleiter and Spears21). However, the present study is unique in that both the duration and severity of the induced Cu deficiency were very extensive. Calves used in the present study were exposed to their respective treatment from the time of conception through to harvest, creating a distinct opportunity to examine the impact of a lifetime Cu deficiency on growth and performance in beef calves.
Plasma Cu concentrations of calves were low at birth, regardless of Cu content of the diet. This appears to be due to low hepatic production of caeruloplasmin, a protein important in Cu mobilisation, during the first few days of life(Reference Salmenpera, Perheentupa, Pakarinen and Siimes22). Supplementation of Mo to cows fed a diet low in Cu resulted in lower plasma Cu concentrations of both the cows and their offspring(Reference Legleiter15). In addition, low plasma Cu concentrations in − Cu and − Cu+Mn calves were maintained through continued supplementation of Mo to calves following weaning. At all sampling dates after birth, plasma Cu concentrations for +Cu calves were within the normal range, while calves receiving the − Cu diet never exceeded the threshold of Cu deficiency (7·9 μmol/l plasma; see Underwood(Reference Underwood20)). In addition, plasma Cu was even further depressed in those calves receiving the − Cu+Mn diet, at one point dipping as low as 0·90 μmol/l plasma.
Liver Cu values for both Cu-deficient treatments were well below 0·31 μmol Cu/g DM, a threshold below which cattle are considered Cu deficient(Reference Underwood and Suttle1), at all time points measured during the study. With the exception of day 297 for the − Cu+Mn treatment group, liver Cu concentrations of Cu-deficient calves never exceeded 0·16 μmol Cu/g DM, suggesting that Cu deficiency was indeed very severe. As previously discussed by Ward & Spears(Reference Ward and Spears23), liver Cu concentrations below 0·110 μmol Cu/g DM have rarely been observed in our laboratory. Liver Cu concentrations in the present study were as low at 0·063 μmol Cu/g DM, underscoring the severity of Cu deficiency these cattle were experiencing. Once Cu concentrations in the liver have dropped to these levels, it is likely that any measurable Cu is tightly bound in cuproenzymes and is relatively unavailable for mobilisation and use by extrahepatic tissues. In the present study, liver Cu concentrations were significantly (P < 0·05) lower in − Cu+Mn calves compared with − Cu calves on days 114, 422 and 493 of the study. The lower liver Cu concentrations coupled with lower plasma Cu values in − Cu+Mn compared with − Cu calves suggests that supplementation of 500 mg Mn/kg DM may have negative implications on Cu absorption beyond that of Mo supplementation alone.
Intestinal CCS concentrations were measured to determine the effect of Cu deficiency on cellular processes requiring Cu. CCS is required for the intracellular transport of Cu to superoxide dismutase. Investigations in rodents have revealed that tissue CCS concentrations are elevated in response to dietary Cu deficiency, and that this elevation is probably the result of decreased degradation of CCS due to Cu deficiency rather than increased synthesis, as mRNA of CCS was not enhanced in these studies(Reference Prohaska and Gybina24, Reference Bertinato and L'Abbe25). In the present study, an increase in CCS due to Cu deficiency was observed, probably because of low intracellular Cu concentrations and increased demand for Cu transport to apo-superoxide dismutase (SOD lacking Cu). Interestingly, CCS concentrations were not increased further in − Cu+Mn calves compared with − Cu calves.
The present results suggest that supplementation of high dietary Mn to a Cu-deficient diet further exacerbates Cu deficiency. This antagonism may result from competition between Cu and Mn for transport into absorptive enterocytes. Research using rodent models and intestinal cell lines has suggested that intestinal Cu absorption may occur through more than one route. Among the transporters characterised for Cu are Cu transporter 1(Reference Kuo, Gybina, Pyatskowit, Gitschier and Prohaska26) and DMT1(Reference Arredondo and Nunez27), with as much as 50 % of the Cu transported into the enterocyte being contributed to DMT1(Reference Arredondo, Munoz, Mura and Nunez12). It is interesting to note that both Fe and Mn are also transported via DMT1, and, in fact, it has been demonstrated that DMT1 has a rather high affinity for Mn when compared with other divalent metals(Reference Arredondo and Nunez27). Our laboratory has recently demonstrated the presence of DMT1 in bovine duodenal tissue taken from calves in the present study. We observed that DMT1 concentrations did not differ between +Cu and − Cu calves, but that DMT1 levels were decreased in − Cu+Mn calves compared with − Cu calves. This decrease in DMT1 protein is probably due to decreased Cu-dependent ferroxidase activity limiting Fe export from the enterocyte, causing Fe to build up in the cell and feed back on DMT1, resulting in increased degradation of the protein in order to prevent further import of Fe. These results suggest that DMT1 concentrations in the intestine were indirectly reduced as a result of high dietary Mn, resulting in decreased transporter availability for not only Mn and Fe, but possibly Cu as well(Reference Hansen and Spears28).
Visibly, the effects of Cu deficiency in the − Cu and − Cu+Mn treatments were evident both before weaning and during the growing phase of the trial. Depigmentation of the hair coat, particularly around the eyes, ears and muzzle was apparent in these treatments when compared with the Cu-adequate control calves. Mottled skin was also observed when calves were shaved before liver biopsies were performed. Mills et al. (Reference Mills, Dalgarno and Wenham29) reported calves receiving a diet of less than 1 mg Cu/kg DM developed stilted gaits, a knock-kneed appearance and a grey-brown cast to their normally black hair coats. Discolouration of the hair coat is a commonly reported sign of Cu deficiency, arising from the role of Cu in tyrosinase, an enzyme involved in melanin pigment biosynthesis(Reference Seo, Mohanty, Choi and Hwang30).
Visible signs of Cu deficiency were less obvious after calves entered the finishing phase of the present study. Calves in both Cu-deficient treatments, but particularly those receiving the − Cu diet, showed fewer signs of depigmentation. These changes occurred despite the fact that the finishing diet contained approximately half as much Cu as the growing diet (4 v. 7 mg Cu/kg DM). The concentrate-based finishing diet probably resulted in a lower ruminal pH which may have allowed more dietary Cu to remain in solution rather than be bound in insoluble forms. In general, all treatments demonstrated slight increases in plasma Cu concentrations in the finishing phase compared with the growing phase, though both − Cu and − Cu+Mn plasma Cu values remained well below normal throughout this time period. This increase was most noticeable on day 459 in +Cu calves and day 422 in − Cu calves, while minor increases in plasma Cu concentrations in − Cu+Mn calves were observed during the finishing phase (Table 3). Increases in plasma Cu concentrations in +Cu and − Cu calves during the finishing phase may have been due to higher bioavailability of Cu from the finishing diet relative to the growing diet. However, the temporary increase in plasma Cu noted in +Cu and − Cu calves appeared to be negated by the presence of high dietary Mn in − Cu+Mn calves. The molecular mechanism behind this response is unclear at the present time.
To examine the effect of Cu status of the calves on the expression of genes important in cellular metabolism, the expression of cox1 and sod1 genes were examined by RT-PCR. In the present study, expression of the cox1 gene was lower in − Cu calves compared with − Cu+Mn calves. Cytochrome c oxidase, of which COX1 is a subunit, is a cuproenzyme that is a part of the final respiratory complex in the electron transport chain in mitochondria. Numerous studies have reported a decreased activity of liver cytochrome c oxidase in animals consuming low − Cu diets(Reference Johnson, Johnson and Lukaski31, Reference Prohaska32). As a result, Cu deficiency may have a negative impact on growth due to a decrease in cytochrome c oxidase activity and reduced energy production. However, it is unclear whether this was true in the present study, because hepatic cytochrome c oxidase activity was not measured.
Recent work has suggested that decreased cytochrome c oxidase activity during Cu deficiency is not necessarily from low Cu incorporation into the enzyme alone. Zeng et al. (Reference Zeng, Saari and Johnson33) reported protein levels of COX1 were lower in heart-protein extracts from rats fed a Cu-deficient diet compared with rats fed a Cu-adequate diet. These authors speculated that decreased COX1 protein concentrations in this study may have resulted from a combination of increased protein degradation due to an accumulation of apo-COX1 (COX1 lacking Cu) and repressed gene expression due to negative feedback from this protein buildup. This theory would explain why cox1 gene expression was lowered in − Cu calves; however, if expression of the cox1 gene is affected by Cu status, one would expect similar expression levels between calves receiving the − Cu and − Cu+Mn diets. It is unclear why cox1 expression was not changed in − Cu+Mn calves, although Mn is thought to affect thyroid function(Reference Soldin and Aschner34) and thyroid hormone has been shown to regulate gene expression of COX subunits(Reference Sheehan, Kumar and Hood35). Therefore, high Mn levels may affect cox1 gene expression, explaining why cox1 expression was unchanged in the animals fed the − Cu+Mn diet.
There was a tendency (P < 0·11) for the sod1 gene to be down-regulated in the animals receiving the − Cu diet relative to those fed the +Cu or − Cu+Mn diets. The Cu–Zn superoxide dismutase (Cu–Zn SOD) enzyme serves an important role in antioxidant protection for cells by converting the superoxide anion to H2O2 and oxygen. Initially we hypothesised that sod1 expression would be lower in the − Cu+Mn animals than in the − Cu animals. However, Ramesh et al. (Reference Ramesh, Ghosh and Gunasekar36) demonstrated that low-level Mn exposure activates the transcription factor NF-κB, and NF-κB has been reported to transcriptionally regulate sod1 (Reference Rojo, Salinas, Martin, Perona and Cuadrado37). DiSilvestro & Marten(Reference DiSilvestro and Marten38) reported that livers from rats fed Cu-deficient diets demonstrated decreased Cu–Zn SOD activity compared with rats fed Cu-adequate diets; however, the immunoreactive protein concentration of Cu–Zn SOD was not different due to dietary Cu level. Prohaska(Reference Prohaska39) reported similar results, with Cu–Zn SOD activity in liver decreasing due to dietary Cu deficiency.
The growth of calves was negatively affected by severe Cu deficiency in the present study (Table 7). While still nursing their dams, calves on the +Cu treatment out-gained their − Cu counterparts by an average of 0·2 kg/d. The increased rate of gain observed in − Cu calves during the finishing phase may be at least partially explained by the increased plasma Cu concentration observed on day 422 in this treatment, suggesting an increase in available Cu during this time. Alternatively, the increased daily gain may reflect an adaptation of − Cu calves to the low-Cu diet.
The growth curves depicted in Fig. 2 demonstrate that calves receiving the − Cu+Mn diet were generally lighter than calves in the other two treatments throughout the study. In addition, − Cu+Mn calves consumed less feed during both the growing and finishing phases of the trial. It is possible that the high level of MnSO4·H2O affected the palatability of the diet for − Cu+Mn calves, resulting in the observed decrease in feed intake. It should be noted that calves receiving the − Cu+Mn diet consumed approximately 16 % less dietary DM than − Cu calves during the growing phase and 26 % less during the finishing phase. As a result, Cu intake during the growing phase was approximately 49·6 mg in − Cu+Mn calves and 58·7 mg in − Cu calves while Cu intake in the finishing phase averaged 21·6 mg in − Cu+Mn calves and 29·2 mg in − Cu calves. Plasma Cu was also analysed statistically using DM intake as a covariate to determine if this overall reduction in Cu intake by − Cu+Mn calves contributed to the lower plasma Cu concentrations in − Cu+Mn calves compared with − Cu calves. This analysis did not significantly affect interpretation of the data; therefore, it appears that while reduced DM intake by − Cu+Mn calves probably contributed to the slower growth of these calves, it does not appear to fully explain the observed reduction in plasma Cu levels. In a similar study, Legleiter(Reference Legleiter15) also reported that plasma Cu concentrations were lower in − Cu+Mn calves compared with − Cu calves on days 360 and 440 of the study, while no differences in DM intake were reported.
While the 500 mg supplemental Mn/kg DM provided in the present study was below the accepted threshold for Mn toxicosis in adult cattle (1000 mg Mn/kg DM), it is quite possible that the severe Cu deficiency these calves were experiencing made them more susceptible to Mn toxicity(40). Previously reported signs of Mn toxicosis in cattle included decreased growth and feed intake(Reference Cunningham, Wise and Barrick41, Reference Jenkins and Hidiroglou42). Excessive Mn has also been shown to negatively affect Fe metabolism in cattle, resulting in lowered packed cell volume, decreased Hb and decreased total Fe-binding capacity in serum(Reference Cunningham, Wise and Barrick41–Reference Ho, Miller, Gentry, Neathery and Blackmon43). Therefore, the decreased performance in − Cu+Mn calves may be due to toxic effects of Mn, depressed Fe status, or a combination of the two rather than Cu deficiency alone.
The calves used in the present study represent the second set of offspring born to cows receiving the three respective Cu treatments (+Cu, − Cu or − Cu+Mn). The first set of calves were born approximately 60–90 d after cows began receiving dietary treatments and limited liver and plasma Cu data as well as all growth data for the first set of calves have been published(Reference Legleiter and Spears21). Calves used in the present study were born to cows receiving dietary treatments for at least 410 d before calving. Therefore, calves in the present study were exposed to a low Cu environment from the time of conception through to harvest. Liver Cu values for each treatment were similar between the two studies, but plasma Cu concentrations in the present study for − Cu and − Cu+Mn calves were slightly lower than in the study by Legleiter & Spears(Reference Legleiter and Spears21). In addition, plasma levels dropped more rapidly in Cu-deficient calves in the present study.
Legleiter & Spears(Reference Legleiter and Spears21) also reported lower average daily gain during the growing phase in Cu+Mn calves v. − Cu calves; however, the authors noted no differences in performance between treatments (+Cu, − Cu or − Cu+Mn) during the finishing period. It is interesting to note that DM intake did not differ between treatments during either period in the study by Legleiter & Spears(Reference Legleiter and Spears21), suggesting that the addition of 500 mg Mn/kg DM did not affect feed consumption by calves. Collectively, this information suggests that the calves in the present study were experiencing a more severe Cu deficiency and the depressive effects of Cu deficiency on growth of − Cu+Mn calves in the present study may be a result of increased susceptibility to Mn toxicity rather than Cu deficiency alone.
While levels of dietary Mn in excess of 500 mg/kg DM are not commonly found in production settings, many forages often contain levels of Mn much greater than the 40 mg Mn/kg DM recommended by the National Research Council for beef cattle(2). For example, Grace et al. (Reference Grace10) reported that Mn concentrations of forages in New Zealand are often greater than 400 mg/kg DM. In situations of low dietary Cu, such as those found when soil Cu concentrations are low or concentrations of Cu antagonists in feedstuffs are high, increased dietary Mn may further exacerbate Cu deficiency in ruminants. Further research exploring the effects of varying levels of dietary Mn on Cu metabolism in cattle provided with adequate dietary Cu is warranted.
Conclusion
The present results show that feeding a diet high in Mn to beef cattle may result in the depression of Cu status and growth beyond that caused by feeding of the Cu antagonist Mo alone. A reduction in DM intake in the present study probably contributed to the depressed growth of − Cu+Mn calves, but using DM intake in a covariate analysis did not significantly explain differences in plasma Cu status between the Cu-deficient treatments. In both the present study and the experiment conducted by Legleiter & Spears(Reference Legleiter and Spears21) supplementation with high dietary Mn decreased plasma Cu levels in beef calves that were already severely Cu deficient. Therefore, it seems plausible that high dietary Mn could have a depressive effect on the Cu status of animals fed moderately low or even normal Cu concentrations. It is possible that this result is due to competition between Mn and Cu for a common intestinal transporter, DMT1. However, further research is necessary to elucidate the exact mechanism by which excessive dietary Mn negatively affects Cu absorption in the ruminant.
Acknowledgements
Funding for the present study was provided by the North Carolina Agriculture Research Service. The authors declare no conflict of interest. J. W. S. designed the study and assisted with writing the manuscript. M. S. A. designed, conducted and analysed all gene expression work and contributed to the manuscript. S. L. H. conducted laboratory and data analysis and wrote the manuscript. L. R. L., R. S. F. and K. E. L. assisted with sample collection and analysis. The authors are grateful to Greg Shaeffer, Jay Woodlief and Andrew Collicutt for expert care of experimental animals and to Audrey O'Nan, Michael Gonda, Mary E. Drewnoski, Valencia Rillington and Callie P. McAdams for assistance in sample collection and analysis.











