Dietary portfolios that combine plant-based foods such as soya, viscous fibres, plant sterols and nuts have proven to be highly efficient nutritional strategies for the modification of CVD risk factors(Reference Jenkins, Kendall and Marchie1). Under metabolically controlled conditions, such dietary portfolios induced marked reductions in LDL-cholesterol (LDL-C) concentrations that were comparable in magnitude with those achieved by a first-generation statin(Reference Jenkins, Kendall and Marchie1–Reference Jenkins, Kendall and Marchie3).
However, beneficial effects of dietary portfolios on HDL have not been well characterised to date(Reference Jenkins, Kendall and Faulkner4). Low-fat/high-carbohydrate (CHO) diets lower plasma HDL-cholesterol (HDL-C) concentrations, a consequence that may counteract their positive LDL-C-lowering effect on CVD risk(Reference Mensink, Zock and Kester5). Studies have actually shown that consumption of diets enriched with MUFA increases plasma HDL-C concentrations compared with low-fat/high-CHO diets in healthy(Reference Jansen, Lopez-Miranda and Castro6–Reference Desroches, Paradis and Perusse8) and hypercholesterolaemic(Reference Mattson and Grundy9–Reference Grundy, Florentin and Nix11) subjects. Our group has recently shown that consumption of a dietary portfolio of cholesterol-lowering foods with either a high or a low MUFA content similarly reduced plasma LDL-C and apoB concentrations(Reference Jenkins, Chiavaroli and Wong12). However, the addition of MUFA to the dietary portfolio significantly increased HDL-C and apoAI concentrations compared with a conventional low-MUFA dietary portfolio(Reference Jenkins, Chiavaroli and Wong12).
Studies of intravascular lipoprotein kinetics have provided an in-depth understanding of mechanisms that determine plasma lipoprotein concentrations at steady state, and provided insights related to the efficacy of dietary interventions for CVD prevention. The primary objective of the present study was to assess the impact of adding MUFA to a dietary portfolio of cholesterol-lowering foods on the intravascular kinetics of apoAI. We hypothesised that compared with a conventional low-MUFA dietary portfolio, a MUFA-rich dietary portfolio increases the production rate (PR) and decreases the fractional catabolic rate (FCR) of apoAI. We also investigated how adding MUFA to a dietary portfolio of cholesterol-lowering foods modifies the intravascular kinetics of apoB-containing lipoproteins.
Methods
Subjects
The study was conducted between August 2007 and April 2009 as described previously(Reference Jenkins, Chiavaroli and Wong12). Men and postmenopausal women with previously recorded LDL-C concentrations above 4·1 mmol/l were recruited from the Clinical Nutrition and Risk Factor Modification Centre at St Michael's Hospital, Toronto, Ontario, Canada and through newspaper advertisements. Exclusion criteria were a personal history of CVD, the presence of untreated hypertension (blood pressure >140/90 mmHg), diabetes mellitus, renal or liver disease and the use of medications known to interfere with lipid metabolism, apart from stable doses of thyroxine. Participants were asked to maintain their intake of medications and supplements constant throughout the study, and to inform the research team if any alterations were made. Fe supplementation (ferrous gluconate 7 mg three times daily) was also provided to participants whose pre-study ferritin level was below 50 μg/l.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Toronto Ethics Board, the ethics committee of St Michael's Hospital and the Natural Health Products Directorate of Health Canada. Written informed consent was obtained from all subjects. No financial compensation was offered for participation in the study. The study was registered at ClinicalTrials.gov (no. NCT00430430).
Study design and diet composition
The present study used a randomised parallel design with two balanced (1:1) 4-week dietary treatments preceded by a 4-week run-in stabilisation period. The study design and the diet composition have been described in detail previously(Reference Jenkins, Chiavaroli and Wong12). Briefly, the stabilisation period consisted of a metabolically controlled diet very low in SFA based on the National Cholesterol Education Program (NCEP) – Adult Treatment Panel III guidelines(13) (Table 1). The run-in stabilisation diet included skimmed milk, fat-free cheese and yogurt, egg substitute and liquid egg-white, whole-grain breakfast cereals and whole-grain bread. Values measured after this run-in period will be referred to as the baseline, pre-portfolio diet values.
Table 1 Macronutrient composition of the study diets as consumed by the sixteen participants
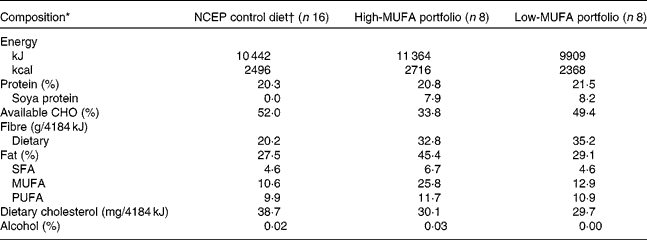
NCEP, National Cholesterol Education Program; CHO, carbohydrate.
* Values in percentage represent the mean daily percentage of energy. Nutritional data for the entire study group (n 24) have been reported in a previous publication(Reference Jenkins, Chiavaroli and Wong12).
† One-month run-in stabilisation period.
Just before the end of the stabilisation feeding period, participants were randomly assigned to one of two treatment arms, low- or high-MUFA dietary portfolios, as described previously(Reference Jenkins, Chiavaroli and Wong12). The aim of the background dietary portfolio was to provide 1·0 g of plant sterols per 4184 kJ (1000 kcal) in a plant sterol ester-enriched margarine (Flora Pro-Activ; Unilever) with a minimum of 2 g/d and a maximum of 3 g/d of plant sterols; 10·3 g of viscous fibres per 4184 kJ from oats, barley, psyllium, eggplant and okra; 20 g of soya protein per 4184 kJ as soya milk, tofu and soya meat analogues and 21·5 g of whole almonds per 4184 kJ, as described previously(Reference Jenkins, Chiavaroli and Wong12). MUFA replaced 13·0 % of total energy from CHO in the high-MUFA dietary portfolio and were provided in the form of a sunflower oil containing 80 % MUFA, with the option for a partial exchange with avocado. All diets were vegetarian. The Harris–Benedict equation, including an activity factor, was used to estimate participants' energy requirements for the 8-week study period.
All meals were provided to participants according to a 7 d cycle menu with foods delivered by courier at weekly intervals. During the diet periods, participants completed menu checklists at weekly intervals, along with 7 d exercise records. Participants were blinded to dietary assignments as food items included in the high-MUFA and low-MUFA dietary portfolios were essentially identical. As described previously(Reference Jenkins, Chiavaroli and Wong12), study coordinators were not blinded to the diets but the laboratory staff responsible for the biochemical analyses were.
Anthropometric and clinical measures
As previously described(Reference Jenkins, Chiavaroli and Wong12), blood samples were obtained at 2-week intervals after 12 h overnight fasts. Blood pressure was also measured at that time and body weight was recorded. Serum lipids were analysed as described previously(Reference Jenkins, Chiavaroli and Wong12).
Kinetic study protocol
Kinetic studies were performed in the Metabolic Test Centre of the Toronto General Hospital twice in each individual, i.e. after the 4-week metabolically controlled run-in stabilisation diet and after the 4-week dietary portfolio period, using a primed, constant infusion of L-[5,5,5-2H3]leucine. The kinetic study protocol has been described in detail previously(Reference Tremblay, Lamarche and Ruel14). Briefly, after a 12 h fast, subjects were fed small meals every 30 min for 15 h. Each half-hourly portion represented one-thirtieth of their estimated daily energy requirements. The nutritional composition of the small meals reflected the mean macronutrient profile of the experimental diet that the participants had just completed (NCEP control diet v. dietary portfolio either high or low in MUFA). At 3 h after their first half-hourly meal, subjects received an intravenous bolus dose of L-[5,5,5-2H3]leucine (10 μmol/kg body weight), which was subsequently followed by a continuous infusion at 10 μmol/kg per h over a 12 h period. Blood samples were obtained throughout the infusion at pre-specified time points through a second intravenous line and collected into Vacutainer tubes containing EDTA. This fed-state kinetic protocol allows study participants to achieve a steady state rapidly, with plasma TAG concentrations being maintained throughout the infusion period(Reference Tremblay, Lamarche and Ruel14).
Isolation of apolipoproteins
VLDL (d< 1·006 g/ml), intermediate density lipoprotein (IDL; d= 1·006–1·019 g/ml), LDL (d= 1·019–1·063 g/ml) and apoAI (d< 1·25 g/ml) fractions were isolated from fresh plasma by sequential ultracentrifugation(Reference Mauger, Couture and Paradis15, Reference Havel, Eder and Bragdon16). Concentrations of apoB100 in VLDL, IDL and LDL and of apoAI were determined by non-competitive ELISA using immuno-purified polyclonal antibodies (Alerchek, Inc.) to calculate their pool sizes (PS). The CV for the apoB assays varied between 6 and 10 %, depending upon the region of the standard curve. ApoB48 and apoB100 were separated by SDS–PAGE according to standardised procedures(Reference Kotite, Bergeron and Havel17). ApoB48 concentrations in the d< 1·006 g/ml plasma fraction were determined relative to the apoB100 concentrations using their respective proportion on the SDS gel, assuming that both have the same chromogenicity(Reference Zilversmit and Shea18). Concentrations were determined for each lipoprotein fractions by averaging values from three different time points during the kinetic study.
ApoAI from the d< 1·25 g/ml plasma fraction was dialysed overnight, incubated with cysteamine for 4 h at 37°C and delipidated using acetone–ethanol and diethyl ether as described previously(Reference Batal, Tremblay and Krimbou19). Apolipoproteins were then separated using preparative isoelectric focusing on polyacrylamide–urea gels (pH gradient 4–7).
Isotopic enrichment determination
ApoAI, apoB100 and apoB48 bands were excised from gels, and the bands were hydrolysed in 6 m-HCl at 110°C for 24 h(Reference Welty, Lichtenstein and Barrett20). Trifluoroacetic acid and trifluoroacetic anhydride (1:1) were used as derivatisation reagents for the amino acids before analysis on a Hewlett-Packard 6890/5973 gas chromatograph/mass spectrometer(Reference Dwyer, Barrett and Chan21). Isotope enrichment (%) and the tracer:tracee ratio (%) were calculated from the observed ion current ratios using standardised formulae(Reference Cobelli, Toffolo and Bier22). Isotopic enrichment curves of VLDL, IDL and LDL apoB100 and of apoB48 in the high-MUFA and low-MUFA dietary portfolios are shown in Supplementary Appendix 1 (available online).
Multicompartmental modelling
Kinetic data were generated through multicompartmental modelling of the tracer enrichment data in each fraction as described previously(Reference Tremblay, Lamarche and Ruel14, Reference Mauger, Couture and Paradis15, Reference Tremblay, Lamarche and Hogue23). Kinetics of apoAI and apoB48 were derived from mono-exponential functions. The VLDL apoB100 tracer:tracee ratio data at plateau were used as the precursor forcing function in the apoAI model(Reference Mauger, Couture and Paradis15). The apoB48 tracer:tracee ratio data at plateau were used as the precursor function in the apoB48 model(Reference Welty, Lichtenstein and Barrett20).
Kinetics of apoB100 in VLDL, IDL and LDL fractions were modelled simultaneously as described previously(Reference Tremblay, Lamarche and Hogue23, Reference Welty, Lichtenstein and Barrett24), with each compartment representing a group of kinetically homogeneous particles. Briefly, a plasma amino acid pool was used as a forcing function based on the VLDL apoB100 enrichment values at plateau. The model also included an intra-hepatic delay compartment, three compartments representing slowly turning-over VLDL as part of a classical delipidation cascade, and one compartment representing rapidly turning-over VLDL particles. The rate constants between removal from the three compartments of slowly turning-over VLDL were set as being equal. The model assumed that apoB entered plasma after a delay exclusively as rapidly turning-over VLDL particles. Thus, transport rates into the compartment of rapidly turning-over VLDL particles corresponded to total VLDL apoB100 production. IDL and LDL were represented as single compartments. The SAAM II program (SAAM Institute) was used to fit the model to the observed tracer data, while taking into account apoB100 fraction masses in VLDL, IDL and LDL. VLDL and IDL apoB100 masses were defined as adjustable parameters in the model while the LDL apoB100 mass was fixed(Reference Welty, Lichtenstein and Barrett24).
Under steady-state conditions, the FCR is equivalent to the fractional synthetic rate. ApoAI, apoB100 in VLDL, IDL and LDL fractions and apoB48 PR were determined by the formula PR (mg/kg per· d) = (FCR (pools/d) × apo concentration (mg/l) × plasma volume (litres))/body weight (kg)(Reference Cohn, Wagner and Cohn25). Plasma volume was estimated as 4·5 % of body weight.
Statistical analyses
Data analyses were performed with the SAS statistical software package version 9.2 (SAS Institute, Inc.). Because many study outcomes were not normally distributed and because of the small sample size, non-parametric Wilcoxon matched-pairs signed-rank tests were used to assess the within-diet impact of the high-MUFA and low-MUFA dietary portfolios on study outcomes (delta scores calculated as post-diet minus pre-diet values). Differences in study outcomes between the high-MUFA and low-MUFA dietary portfolios were assessed using Wilcoxon rank-sum tests (between-diet differences in delta scores). Results are therefore presented as medians and interquartile ranges, unless stated otherwise. Participants' characteristics at the end of the run-in stabilisation diet, i.e. when they were randomised to the high-MUFA or low-MUFA dietary portfolio, were considered as the baseline characteristics and were compared using the Wilcoxon rank-sum test. Differences were considered as significant at P< 0·05 (two-sided).
Power calculation
Within- and between-diet differences in the PR and FCR of apoAI and VLDL apoB100 were the a priori-defined primary outcomes of the present study. Within- and between-diet differences in the PS of apoAI and VLDL apoB100, in kinetic parameters of IDL apoB100, LDL apoB100 and apoB48, as well as in conversion rates of VLDL apoB100 to different lipoprotein subfractions were considered as secondary outcomes. Sample size calculations were originally performed based on the premise that we would use parametric statistical tests and pre-defined values of P< 0·05 and β < 0·20. These calculations were also based on data from a previous kinetic study by our group(Reference Desroches, Paradis and Perusse8), according to which we estimated that the standard deviation associated with the main treatment effect would be approximately equal in magnitude to the main effect per se. However, considering that eight subjects (n 4 per group) had to be excluded from the present analyses (see the Results section) and that post- minus pre-diet changes in three of the four primary outcomes were not normally distributed, we have revised these calculations using non-parametric statistical tests with a final sample of eight subjects per group (total n 16). Our calculations indicated that this sample size allowed us to detect the following within-diet differences: apoAI PR 40 %; apoAI FCR 32 %; VLDL apoB100 PR 83 %; VLDL apoB100 FCR 81 %. Minimal detectable between-diet differences were estimated to be: apoAI PR 31 %; apoAI FCR 30 %; VLDL apoB100 PR 97 %; VLDL apoB100 FCR 85 %.
Results
Fig. 1 shows the flow of participants throughout the study. Of the twenty-five subjects enrolled in the study, a total of twenty-four (seventeen men and seven women) completed the run-in stabilisation diet and were randomised to either the high- or low-MUFA dietary portfolio (n 12 in each diet). All twenty-four subjects completed the dietary portfolio feeding period (no dropout). However, eight subjects were excluded from the present kinetic study analyses due to incomplete kinetic data (pre- or post-dietary portfolio; Fig. 1). The final kinetic study group included an equal number of subjects (n 8) on each dietary portfolio. As shown in Table 2, the characteristics of the kinetic study participants after the run-in stabilisation diet, i.e. when they were randomised to the high- or low-MUFA dietary portfolio, were comparable between the groups. Overall, participants were overweight and slightly dyslipidaemic but otherwise healthy. The characteristics of the kinetic study subgroup (n 16) did not differ from the characteristics of the whole study population (n 24; P≥ 0·18; not shown).

Fig. 1 Flow of the participants throughout the study. F, female; M, male; R, randomisation. * Of the twelve participants who chose not to participate, four had a medical issue, two wanted to lose weight, one was not willing to stop statin therapy, one was not willing to stop vitamin supplements, one was not willing to give blood samples for analysis, one was not willing to undergo kinetics testing, one had a family issue and one was not interested. Part of the diagram has been published previously(Reference Jenkins, Chiavaroli and Wong12).
Table 2 Characteristics of the participants at the end of the run-in stabilisation diet, when randomised to the high-MUFA or low-MUFA dietary portfolio (Median values and interquartile ranges (IQR))

C, cholesterol; MET, metabolic equivalent of tasks; ASA, acetylsalicylic acid.
* There was no significant difference in characteristics between the groups randomised to the high-MUFA or low-MUFA dietary portfolio, as assessed by the Wilcoxon rank-sum test.
† Data obtained at study onset only, i.e. at the beginning of the run-in stabilisation diet.
‡ Index expressing energy cost of physical activities, as multiples of RMR.
§ Provided as part of the study for those with a ferritin level < 50 μg/l at the time of screening.
Changes in plasma lipid concentrations between the two dietary portfolios in the kinetic study subgroup of sixteen subjects were generally similar in magnitude to changes observed between the two diets within the entire study group(Reference Jenkins, Chiavaroli and Wong12), with the exception of total cholesterol and LDL-C, which tended to be reduced to a greater extent among the kinetic study participants who consumed the high-MUFA v. low-MUFA dietary portfolio (P= 0·053 and P= 0·10, respectively; Table 3). As reported previously(Reference Jenkins, Chiavaroli and Wong12), the high-MUFA dietary portfolio significantly increased HDL-C concentrations compared with the low-MUFA dietary portfolio (between-treatment difference 12·5 %, P= 0·003) when considering all participants. The difference in plasma HDL-C concentrations between the treatments in the kinetic study subgroup of sixteen subjects was similar in magnitude (14 %) but did not reach statistical significance (P= 0·33; Table 3).
Table 3 Effects of the dietary portfolios high and low in MUFA on plasma lipid and lipoprotein concentrations (Median values and interquartile ranges (IQR))

* Data for the entire study group (n 12 per group) have been presented in a previous publication(Reference Jenkins, Chiavaroli and Wong12).
† Pre-diet values correspond to the end of the 4-week metabolically controlled run-in stabilisation diet designed according to the National Cholesterol Education Program guidelines.
‡ P values for within-diet effects, as determined by the Wilcoxon matched-pairs signed-rank test.
§ P values for between-diet effects, as determined by the Wilcoxon rank-sum test performed on post- v. pre-diet variations.
On the other hand, diet-induced changes in apoAI concentrations were significantly different between the high-MUFA (6·3 %, within-diet P= 0·07) and low-MUFA dietary portfolios ( − 5·0 %, within-diet P= 0·48, between-diet P= 0·04). Diet-induced changes in apoAI PS were also significantly different between the two diets (P= 0·03; Fig. 2(a)). Consumption of the high-MUFA dietary portfolio resulted in a significant 5·6 % reduction in apoAI FCR (P= 0·02; Fig. 2(c)), with no significant change in apoAI PR (1·7 %, P= 0·11; Fig. 2(b)). Consumption of the low-MUFA diet tended to reduce apoAI PS ( − 6·5 %, P= 0·055; Fig. 2(a)), with no significant change in apoAI FCR ( − 8·5 %, P= 0·20; Fig. 2(c)) or PR ( − 5·7 %, P= 0·20; Fig. 2(b)).

Fig. 2 Effects of the dietary portfolios high and low in MUFA on (a) apoAI pool size (PS), (b) production rate (PR) and (c) fractional catabolic rate (FCR) in the kinetic study subgroup (n 16). Baseline, pre-dietary portfolio (![]() ) values measured after the 4-week metabolically controlled run-in stabilisation diet designed according to the National Cholesterol Education Program guidelines. ■, Post-dietary portfolio values. Values are expressed as medians and interquartile ranges in parentheses inside the bars. P values for between-diet effects (high-MUFA v. low-MUFA dietary portfolio) were determined by the Wilcoxon rank-sum test performed on post- v. pre-diet variations. P values for within-diet effects were determined by the Wilcoxon matched-pairs signed-rank test.
) values measured after the 4-week metabolically controlled run-in stabilisation diet designed according to the National Cholesterol Education Program guidelines. ■, Post-dietary portfolio values. Values are expressed as medians and interquartile ranges in parentheses inside the bars. P values for between-diet effects (high-MUFA v. low-MUFA dietary portfolio) were determined by the Wilcoxon rank-sum test performed on post- v. pre-diet variations. P values for within-diet effects were determined by the Wilcoxon matched-pairs signed-rank test.
Consumption of the high-MUFA dietary portfolio tended to reduce apoB48 PS and concentrations (within-diet differences of − 29·9 and − 45·2 %, respectively, P= 0·08, not shown). There was, however, no significant change in apoB48 PR ( − 11·0 %, P= 0·94) or FCR ( − 4·5 %, P= 0·55) with the high-MUFA dietary portfolio (not shown). Consumption of the low-MUFA dietary portfolio had no impact on apoB48 concentrations, PS, PR and FCR (all P≥ 0·58, not shown). Diet-induced changes in apoB48 kinetic parameters were not significantly different between the two dietary portfolios (all P≥ 0·22).
There was no significant within- or between-diet difference in VLDL apoB100 concentrations, PS, PR and FCR (all P>0·11; Table 4). However, the high-MUFA dietary portfolio induced a 169 % increase in the conversion rate of VLDL apoB100 directly to LDL (within-diet P= 0·02). Despite this large increase, the proportion of VLDL apoB100 directly transferred to LDL (16 %) after the high-MUFA dietary portfolio remained small compared with the proportion of VLDL apoB100 directly cleared from the circulation (72 %). Although not significant, the low-MUFA dietary portfolio also tended to increase the conversion rate of VLDL apoB100 to LDL (89·5 %, P= 0·15). Changes in VLDL apoB100 conversion rates to different lipoprotein subfractions were not significantly different between the two dietary portfolios (all P≥ 0·21).
Table 4 Effects of the dietary portfolios high and low in MUFA on VLDL, intermediate density lipoprotein and LDL apoB100 kinetic parameters (Median values and interquartile ranges (IQR))

PS, pool size; PR, production rate; FCR, fractional catabolic rate; IDL, intermediate density lipoprotein.
* Pre-diet values correspond to the end of the 4-week metabolically controlled run-in stabilisation diet designed according to the National Cholesterol Education Program guidelines.
† P values for within-diet effects, as determined by the Wilcoxon matched-pairs signed-rank test.
‡ P values for between-diet effects, as determined by the Wilcoxon rank-sum test performed on post- v. pre-diet variations.
There was no significant between-diet difference in IDL apoB100 kinetic parameters (all P≥ 0·15; Table 4). However, both the high-MUFA and low-MUFA dietary portfolios significantly reduced the conversion rate of IDL apoB100 to LDL (within-diet reductions of 65·0 and 45·4 %, respectively, P≤ 0·02 for both).
LDL apoB100 concentrations and PS were significantly reduced after the high-MUFA dietary portfolio compared with pre-diet values (within-diet P= 0·008 for both; Table 4). These changes occurred in parallel to a strong trend towards an increase in LDL apoB100 FCR (22·9 %, within-diet P= 0·055). Consumption of the low-MUFA dietary portfolio had no significant impact on LDL apoB100 kinetic parameters (all P≥ 0·25). The high-MUFA dietary portfolio was associated with a greater increase in LDL apoB100 FCR compared with the low-MUFA dietary portfolio (between-diet difference 23·2 %, P= 0·04).
Discussion
We have previously shown that adding MUFA to a dietary portfolio of cholesterol-lowering foods significantly increases HDL-C and apoAI concentrations compared with a conventional low-MUFA portfolio diet(Reference Jenkins, Chiavaroli and Wong12). The objective of the present study was to investigate the mechanisms underlying these changes. To the best of our knowledge, this is the first study assessing the kinetics of apoAI but also of apoB100 and apoB48 following the consumption of the dietary portfolio as an example of a highly effective cholesterol-lowering diet.
The present results first showed that compared with a conventional dietary portfolio, consumption of a dietary portfolio enriched in MUFA, replacing 13·0 % of total energy from CHO, resulted in higher apoAI PS most probably through a reduction in apoAI clearance rate. To the best of our knowledge, only one study has documented the impact of a MUFA-rich diet (40·1 % energy from fat; 22·5 % MUFA) on apoAI kinetics(Reference Desroches, Paradis and Perusse8). In contrast with results from the present study, consumption of the high-MUFA diet for 6–7 weeks had no significant impact on apoAI FCR (9·6 %, within-diet P= 0·44)(Reference Desroches, Paradis and Perusse8). By design, the ad libitum feeding conditions in that earlier study led to significant weight loss that may have confounded to some extent the impact of MUFA on apoAI kinetics(Reference Desroches, Paradis and Perusse8).
MUFA have been shown to inhibit cholesteryl ester transfer protein activity, which may in turn favour accumulation of HDL particles that are relatively enriched in cholesterol esters and relatively depleted in TAG(Reference Abbey and Nestel26). Such cholesteryl ester-enriched HDL particles are catabolised less rapidly than TAG-enriched HDL(Reference Lamarche, Uffelman and Carpentier27). Partial inhibition of cholesteryl ester transfer protein activity may therefore represent one of the mechanisms underlying the reduction in apoAI FCR seen after the high-MUFA diet in the present study.
The observed trend towards an increase in apoAI PR with the high-MUFA dietary portfolio may represent another possible mechanism responsible for the increased apoAI PS after the high-MUFA dietary portfolio compared with the low-MUFA dietary portfolio. MUFA have been shown to up-regulate apoAI synthesis(Reference Osada, Fernandezsanchez and Diazmorillo28), and this may have counteracted the decline in apoAI PR usually seen with low-fat, cholesterol-lowering diets(Reference Desroches, Paradis and Perusse8, Reference Brinton, Eisenberg and Breslow29, Reference Velez-Carrasco, Lichtenstein and Welty30). Consistent with this, we have previously shown that consumption of a high-MUFA diet had no significant impact on apoAI PR, while consumption of a low-fat/high-CHO diet significantly reduced apoAI production(Reference Desroches, Paradis and Perusse8). We cannot exclude the possibility that components of the dietary portfolio of cholesterol-lowering foods other than MUFA may also have affected apoAI synthesis since there was no change in this parameter after consumption of the low-MUFA dietary portfolio, despite important cholesterol lowering.
The present results also revealed that a high-MUFA dietary portfolio had no significant impact on VLDL and IDL apoB100 PS, PR or FCR compared with a low-MUFA dietary portfolio. Similarly, the study by Desroches et al. (Reference Desroches, Paradis and Perusse8) showed no significant within- or between-diet differences in VLDL apoB100 PS, PR or FCR when comparing a low-fat/high-CHO diet with a high-MUFA diet. These data are also consistent with those from a study by Gill et al. (Reference Gill, Brown and Caslake31), which showed no significant difference between a low-MUFA diet (7·8 % of energy) and a high-MUFA diet (13·7 % of energy) on VLDL1 and VLDL2 kinetics.
Interestingly, the high-MUFA dietary portfolio increased the conversion rate of apoB100 from VLDL directly to LDL. A trend towards an increase in the conversion of apoB100 from VLDL to LDL was also observed after the low-MUFA dietary portfolio, thus suggesting that consumption of a dietary portfolio of cholesterol-lowering foods, irrespective of its MUFA content, may be associated with an increased proportion of apoB100 in VLDL being channelled rapidly to the LDL pool. On the other hand, this had little impact downstream on other apoB100-containing lipoprotein fractions as the majority of apoB100 in VLDL was directly cleared from the circulation, irrespective of the MUFA content of the dietary portfolios.
LDL-C concentrations and LDL apoB100 PS tended to be reduced to a greater extent after the high-MUFA compared with the low-MUFA dietary portfolio in this subgroup of sixteen subjects. Our data suggested that this was most probably attributable to a greater increase in LDL apoB100 clearance rate with the high-MUFA dietary portfolio compared with the low-MUFA diet. This is consistent with observations from Gill et al. (Reference Gill, Brown and Caslake31), who suggested that increasing the MUFA content of the diet may accelerate LDL clearance through an up-regulation of the LDL receptor activity. Studies performed in animal and in vitro models have also shown that MUFA may prevent the suppression or even increase the activity of the LDL receptor(Reference Daumerie, Woollett and Dietschy32–Reference Kurushima, Hayashi and Toyota35), as previously highlighted by Gill et al. (Reference Gill, Brown and Caslake31).
High levels of intestinally derived lipoprotein particles have previously been associated with increased CVD risk(Reference Kolovou, Anagnostopoulou and Daskalopoulou36). Consumption of a dietary portfolio enriched in MUFA tended to reduce apoB48 concentrations and PS compared with an NCEP prudent diet (run-in diet), while no such changes were observed after the low-MUFA dietary portfolio. While this may represent another potential benefit of adding MUFA to the dietary portfolio, this needs to be more thoroughly investigated in the future.
Since experimental diets in the present study were of short duration and consumed under metabolically controlled feeding conditions, the effect and applicability of the dietary portfolio in longer-term real-world conditions may be questioned. Recent data from our group indicated that 6-month adherence to a conventional dietary portfolio in free-living conditions was associated with clinically meaningful reductions in plasma LDL-C concentrations(Reference Jenkins, Jones and Lamarche37). Although overall adherence to components of the dietary portfolio was below 50 % in average, the degree of adherence significantly predicted the magnitude of the change in plasma LDL-C concentrations (r − 0·34, P< 0·001). There was no change in plasma HDL-C concentrations over 6 months with the conventional dietary portfolio(Reference Jenkins, Jones and Lamarche37), which is consistent with the data from the present study. The longer-term effect of a dietary portfolio enriched in MUFA on plasma lipids, including HDL-C, however, remains to be demonstrated.
Strengths and limitations inherent to the present study need to be pointed out. First, the small number of subjects in each diet in the final analysis limited our ability to detect changes that otherwise may have been significant with larger sample sizes. However, the dietary portfolios were consumed by participants under metabolically controlled conditions and were preceded by a 4-week run-in stabilisation diet also metabolically controlled, eliminating bias pertaining to background food intake. We cannot exclude the possibility that the increase in HDL-C and apoAI after the high-MUFA dietary portfolio(Reference Jenkins, Chiavaroli and Wong12) may in part be attributable to the higher content of SFA in that diet (1·43-fold) compared with the low-MUFA dietary portfolio. However, it must be stressed that the SFA content of the high-MUFA diet in the present study was still very low, representing less than 7 % of energy. Finally, it is known that sex-specific differences exist in the kinetics of non-fasting TAG-rich lipoprotein, IDL and LDL apoB100(Reference Matthan, Jalbert and Barrett38). The small number of subjects did not allow us to dissect out the impact of the experimental diets on apolipoprotein kinetic parameters according to sex, and this topic warrants further investigation in future studies.
In conclusion, our data suggest that substituting MUFA for CHO in a dietary portfolio of cholesterol-lowering foods provides the added advantage of raising HDL-C primarily through a reduction in HDL clearance rate. Replacing CHO with MUFA in a dietary portfolio may also lead to reductions in LDL apoB100 concentrations by increasing LDL clearance rate, thus potentiating further the well-known cholesterol-lowering effect of this diet.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114.51200534X
Acknowledgements
We thank the metabolic kitchen staff from the Clinical Nutrition and Risk Factor Modification Centre of St Michael's Hospital, Toronto, for their excellent work during the study. We are grateful to the nurses and the laboratory staff for their technical assistance and for the expert care provided to the participants. We also express our gratitude to the participants, without whom the study would not have been possible.
The authors' contributions were as follows: D. J. A. J., G. F. L., C. W. C. K. and B. L. were responsible for study concept and design; D. J. A. J., L. C., J. M. W. W., C. W. C. K., G. F. L. and B. L. supervised the study and were responsible for acquisition of data; M.-E. L. performed statistical analyses, interpreted the data, and wrote the manuscript; J.-C. H., P. C. and B. L. participated to analysis and interpretation of the kinetic data. B. L. has primary responsibility for the final content of the paper. All authors critically reviewed the manuscript and approved its final version.
The present study was supported by the Canadian Institutes of Health Research (CIHR), the Canada Research Chair Program and Loblaws. D. J. A. J., G. F. L. and B. L. were funded as Canada Research Chairs by the Government of Canada. J. M. W. W. received a Doctoral Research Award and a Fellowship Award from the CIHR. M.-E. L. is recipient of doctoral scholarships from the CIHR and the Fonds de la recherche en santé du Québec. None of the funding organisations or sponsors played any role in the design and conduct of the study; in the collection, management, analysis and interpretation of the data; or in the preparation, review or approval of the manuscript.
D. J. A. J. declared that he holds grants from Solae, Unilever, Loblaws Supermarkets, Barilla, Haine Celestial, the Sanitarium Company, BENEO-Orafti, the Almond Board of California, the CIHR, the Canada Foundation for Innovation, Advanced Foods and Materials Network (AFMNet), the International Tree Nut Council Nutrition Research & Education Foundation, the Peanut Institute, Pulse Canada, Saskatchewan Pulse Growers, the Calorie Control Council, the Kellogg Company, Quaker Oats, the Coca Cola Company, the Pepsi Company, the Canola and Flax Councils of Canada, and the California Strawberry Commission. He has received honoraria from Herbalife International, Nutritional Fundamentals for Health, Pacific Health Laboratories, Metagenics/Metaproteomics, Bayer Consumer Care, BENEO-Orafti, the Science Advisory Committee of Agriculture and Agri-Food Canada, the Canadian Agriculture Policy Institute, Solae, Oldways Preservation Trust, Unilever, Procter and Gamble Technical Centre Limited, Loblaws Supermarkets, Barilla, the Canola and Flax Councils of Canada, the Soy Advisory Board – Dean Foods, the California Strawberry Commission, NuVal System (Griffin Hospital, New Haven), Abbot Laboratories, Haine Celestial, the Sanitarium Company, the Almond Board of California, AFMNet, the International Tree Nut Council Nutrition Research & Education Foundation, the Peanut Institute, Pulse Canada, Saskatchewan Pulse Growers, Alpro Soy Foundation, the Calorie Control Council, the Kellogg Company, Quaker Oats, the Coca Cola Company, and the Pepsi Company. He also has stock options for Pacific Health Laboratories Inc. and he has received consulting fees from Herbalife International, Nutritional Fundamentals for Health, Pacific Health Laboratories, Metagenics/Metaproteomics, Bayer Consumer Care, BENEO-Orafti, the Science Advisory Committee of Agriculture and Agri-Food Canada, the Canadian Agriculture Policy Institute, Solae, Oldways Preservation Trust, Unilever, Procter and Gamble Technical Centre Limited, Loblaws Supermarkets, Barilla, the Canola and Flax Councils of Canada, the Soy Advisory Board – Dean Foods, the California Strawberry Commission, NuVal System (Griffin Hospital, New Haven, CT), Abbot Laboratories, Haine Celestial, the Sanitarium Company, the Almond Board of California, AFMNet, the International Tree Nut Council Nutrition Research & Education Foundation, the Peanut Institute, Pulse Canada, Saskatchewan Pulse Growers, Alpro Soy Foundation, the Calorie Control Council, the Kellogg Company, Quaker Oats, the Coca Cola Company, and the Pepsi Company. D. J. A. J.'s wife is a director of Glycemic Index Laboratories, Toronto, Ontario.
C. W. C. K. has received research grants from the CIHR, the Almond Board of California, the International Tree Nut Council Nutrition Research & Education Foundation, Barilla, Solae, Unilever, Saskatchewan Pulse Growers, Pulse Canada, and the Coca Cola Company and travel support from the Almond Board of California, the International Tree Nut Council Nutrition Research & Education Foundation, Saskatchewan Pulse Growers, the Kellogg Company, Danone, Solae, and Oldways Preservation Trust. He also has received honoraria from the Almond Board of California, the Kellogg Company, Danone, and Solae.
P. C. and B. L. have received research grants from the Dairy Farmers of Canada, Dairy Australia and the Canola Council of Canada.
B. L. has received research funding from the Danone Institute and Atrium Innovations, and honoraria from Unilever, Danone, and the Dairy Farmers of Canada. B. L. is Chair in Nutrition and Cardiovascular Health, supported in part by Provigo/Loblaws.
M.-E. L., G. F. L., L. C., J. M. W. W. and J.-C. H. had no conflict of interest.








