The majority of advanced cancer patients experience accelerated involuntary loss of muscle with or without adipose tissue loss during the course of their disease trajectory(Reference Vigano, Bruera and Jhangri1). Weight loss, a defining feature of cachexia, is associated with poorer treatment response and decreased survival in several cancer diagnoses including pancreatic cancer(Reference Bachmann, Heiligensetzer and Krakowski-Roosen2). Despite the importance of the interaction between body composition and survival, few studies have characterised specific body composition changes (fat and muscle) using precise measures of muscle and fat such as computed tomography (CT) image analysis. Thus, the relationship between these changes and clinical and physical characteristics is unclear.
A recent international consensus highlighted the importance of identifying key biological predictors of cachexia, such as insulin resistance(Reference Fearon, Strasser and Anker3). Body composition is related to a variety of these parameters, and thus, these markers may be potential determinants of muscle and adipose tissue losses. Pancreatic cancer patients experience some of the most severe symptoms of cachexia(Reference Wigmore, Plester and Richardson4); therefore, the relationships between changes in body composition and physical and clinical characteristics may be more pronounced in this population. Insulin resistance, for example, has been associated with increased muscle wasting(Reference Wang, Hu and Hu5), suggesting that diabetics may exhibit accelerated rates of muscle wasting when concurrently diagnosed with pancreatic cancer. Anaemia has also been used to diagnose cancer cachexia(Reference Evans, Morely and Argiles6), while surgery has been associated with accelerated tissue loss(Reference Liedman, Andersson and Bosaeus7). Thus, these parameters are of particular interest. The potential relationships between body composition and clinical parameters that are documented during routine care, such as inflammatory markers, markers of tumour burden and secondary disease states, have not been evaluated.
Weight and crude measures of fat and lean mass, derived from bioelectrical impedance analysis, are methods commonly used to examine body composition. For example, Bachmann et al. (Reference Bachmann, Heiligensetzer and Krakowski-Roosen2) demonstrated that weight loss is associated with poor survival outcomes in pancreatic cancer patients; however, weight loss measurements do not allow examination of the specific and independent influences of muscle and each of the three major adipose tissue depots (i.e. visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT) and intramuscular adipose tissue (IMAT)).
CT image analysis provides a precise and highly reproducible method to quantify changes in individual fat and muscle tissue depots. In one of the few studies using CT image analysis, Tan et al. (Reference Tan, Birdsell and Martin8) suggested that the proportion of muscle and fat that a patient carried at diagnosis may significantly influence survival. They further noted that muscle and adipose tissue each had distinct patterns of tissue loss; thus, these findings suggest value in examining the independent relationship of muscle and adipose tissue with survival.
Each adipose tissue depot possesses unique metabolic properties; however, VAT may have independent influences on survival and other clinical outcomes. Excessive accumulation of VAT, the most metabolically active adipose tissue(Reference Despres and Lemieux9), is associated with the metabolic syndrome, a risk factor for pancreatic cancer(Reference Johansen, Stocks and Jonsson10). VAT accumulation at cancer diagnosis may also decrease survival time specifically in pancreatic cancer patients(Reference Balentine, Enriquez and Fisher11), suggesting that VAT measurements may be particularly important in understanding survival and other clinical parameters.
The objectives of the present study were to precisely quantify body composition at diagnosis and during the pancreatic cancer trajectory. Subsequently, changes in body composition were related to clinical markers acquired during routine follow-up and clinical outcomes, including survival, to identify associated features of cachexia. Identification of these features is essential for the early detection of cachexia and the attenuation of complications associated with cachexia. We hypothesised that patients would lose significant amounts of muscle and adipose tissue over the disease trajectory. Second, patients with the largest losses and the most accelerated rates of muscle and adipose tissue loss would be associated with the poorest survival outcomes and clinical parameters.
Materials and methods
Patient selection
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the University of Waterloo Research Ethics Board and the Hamilton Health Sciences Research Ethics Board. Patients diagnosed with pancreatic cancer and who had died between July 2003 and February 2010 were identified at the Juravinski Cancer Centre in Hamilton, ON, Canada, which includes a catchment of approximately 1·2 million people. We classified patients based on stage (I, II, III and IV), progression and treatment. Patients were included if they: (1) died from pancreatic cancer, (2) were diagnosed with either a carcinoma or adenocarcinoma, (3) had at least two abdominal CT scans performed during their disease trajectory and (4) CT scans were performed up to 2 years before their time of death. Patients surviving for less than 4 months were excluded from analysis, as these patients were in the terminal stage of their disease and changes in body composition would have been distinct from patients at earlier stages of the disease(Reference Fearon, Strasser and Anker3); these patients would also be less likely to positively respond to neoplastic and nutritional therapies. At this stage of disease, the associated risk of nutritional therapy probably outweighs any potential benefit(Reference Fearon, Strasser and Anker3). CT scans that were performed 3 months or more before the date of diagnosis were not included in the present analysis, as they are not representative of body composition during disease presentation. Any two consecutive scans were at least 1 month apart and no longer than 1 year apart (Fig. 1).

Fig. 1 Representative timeline of the disease trajectory with the average timing of computed tomography (CT) scan.
Physical and clinical characteristics
Anthropometric measurements at diagnosis including height and weight were recorded from patient records and used to calculate BMI (kg/m2). Stage of disease at diagnosis was determined using the tumour node metastasis (TNM) classification of malignant tumours system. Survival, stage of tumour, histology and presence of diabetes were recorded at diagnosis only. The presence of anaemia (Hb < 120 g/l for females, Hb < 130 g/l for males)(12), CA19-9 values as well as whether patients received nutritional consults (i.e. patients who met with a registered dietitian regardless of whether nutritional supplements were prescribed) or dietary supplementation (i.e. patients who consumed some form of nutritional supplement in addition to their habitual diet independent of a dietetic consult) were recorded throughout the disease trajectory. Hb values and CA19-9 values within 1 month of the first CT scan were considered representative of diagnostic values. All patients had Hb values within that time frame, while only twenty-eight patients had CA19-9 values within that time. These blood parameters were recorded and then related to body composition.
Computed tomography image analysis
Skeletal muscle and adipose tissue cross-sectional areas (CSA) were quantified using single-slice CT scans at the third lumbar vertebrae (L3). Tissue CSA at this location are strongly correlated with whole-body skeletal muscle and adipose tissue mass distribution(Reference Shen, Punyanitya and Wang13–Reference Mourtzakis, Prado and Lieffers15). Skeletal muscle tissue included psoas, erector spinae, quadratus lumborum, transverse abdominal, internal and external obliques, and rectus abdominus. We also specifically measured VAT, as it is the most metabolically active fat tissue(Reference Despres and Lemieux9). Importantly, VAT is associated with the metabolic syndrome(Reference Alberti, Zimmet and Shaw16) and pancreatic cancer(Reference Johansen, Stocks and Jonsson10), and it has been associated with decreased survival time in pancreatic cancer patients(Reference Balentine, Enriquez and Fisher11). Thus, VAT may influence clinical outcomes in pancreatic cancer more than other adipose tissue depots.
Slice-O-Matic® version 4.3 (Tomovision) allows the precise quantification of specific tissue using Hounsfield units. Hounsfield unit threshold values were defined at − 29 to 150 for skeletal muscle(Reference Mitsiopoulos, Baumgartner and Heymsfield17), − 150 to − 50 for VAT(Reference Miller, Jones and Yanovski18), and − 190 to − 30 for both SAT and IMAT(Reference Mitsiopoulos, Baumgartner and Heymsfield17). CSA (cm2) were computed for each tissue by summing tissue pixels and multiplying by the pixel surface area. All CT images were analysed by a single trained analyst who was blinded to clinical markers and patient outcomes. To calculate the intra-analyst CV, five CT images were analysed twice on two separate days 6 months apart. The mean CV for skeletal muscle CSA was 0·322 and 0·130 % for adipose tissue CSA. To avoid bias, two additional trained analysts (who were blinded to both the objectives of the study and the patient characteristics of the CT scans) quantified muscle and adipose tissue for all scans and resulted in a mean CV < 1·0 % for skeletal muscle CSA and approximately 3·0 % for adipose tissue CSA. Thus, all data presented are from the primary analyst to ensure consistency.
Calculations for estimating tissue mass, net tissue change and rate of tissue change
There is a strong relationship between L3 skeletal muscle and whole-body lean tissue in cancer patients (r 0·83)(Reference Shen, Punyanitya and Wang13); however, equations to predict whole-body skeletal muscle mass from L3 CT images do not exist for this specific population. Therefore, an algorithm derived from a healthy population was used to estimate whole-body skeletal muscle volume from the CSA at L3(Reference Shen, Punyanitya and Wang13):
where L4 and L5 are the fourth and fifth lumbar vertebrae, respectively.
A density of 1·04 g/cm3 was used to estimate skeletal muscle mass (g) from volume(Reference Snyder, Cooke and Manssett19).
Sarcopenia was defined based on cut-off points described in a cohort of advanced cancer patients and defined as ≤ 38·9 cm2/m2 for females and ≤ 55·4 cm2/m2 for males(Reference Mourtzakis, Prado and Lieffers15). To identify whether a patient was below (indicating sarcopenia) or above (indicating non-sarcopenia) these cut-off points, skeletal muscle CSA at L3 (cm2) was divided by height squared (m2).
Whole-body VAT was estimated from VAT CSA with an algorithm from a healthy population(Reference Shen, Punyanitya and Wang14). Similar to skeletal muscle, predictive equations have not been developed for cancer populations; however, there is a strong relationship (r0·88) between VAT CSA at L3 and whole-body VAT volume in healthy humans(Reference Shen, Punyanitya and Wang14).
A density of 0·96 g/cm3 was used to convert VAT volume into mass (g)(Reference Snyder, Cooke and Manssett19).
There were patients who had portions of SAT that were not visible in the viewing field of the CT (n 22); for those patients for whom SAT could be viewed and calculated in its entirety (n 28), total adipose tissue (TAT) CSA was used to estimate whole-body adipose tissue. TAT was calculated as the sum of SAT, VAT and IMAT. Regression equations from a cohort of advanced cancer patients were used to estimate whole-body TAT from the surface area using the following equation(Reference Mourtzakis, Prado and Lieffers15):
Net tissue change analysis for both skeletal muscle and VAT was based on the absolute difference between the first and last scans available for each patient (average 286 (sd 151) d; Fig. 1). Multiple scans were performed between the first and last scans for each patient; these scans were analysed and tissue change between consecutive scans was averaged over 100 d intervals from the time of death (i.e. 0–100 d before death, 101–200 d before death, etc.) to create a disease trajectory. The time intervals examined included approximately 70, 150, 250 and 350 d from death. There were no significant differences in the rate of change or total tissue change between each consecutive time interval, thus net tissue changes were representative of the longitudinal changes during the disease trajectory (data not shown). Absolute changes in CSA were converted to mass using the aforementioned algorithms for skeletal muscle and VAT.
Absolute changes between the first and last scans were then calculated as a rate of change per 100 d to provide a more realistic time frame for assessing the rate of tissue loss. This also provided a standardised unit to allow comparisons between patients as the timing of CT images was unique for each individual.
Statistical analysis
Statistical analysis was performed using SigmaPlot® version 11.2 (Systat Software, Inc.). All values are presented as means and standard deviations, significance was identified at P= 0·05 and trends were identified as P= 0·120. Power was set at 0·80 for all tests. Non-parametric statistics were performed on this dataset, as it was not normally distributed, while mathematical transformation could not correct the distribution. Comparisons between the two groups were performed by Mann–Whitney rank-sum tests and comparisons between multiple groups (tertile analysis) were performed by Kruskal–Wallis non-parametric one-way ANOVA with Dunn's method for multiple comparisons. Kaplan–Meier survival curves were constructed using the log-rank method for a comparison between multiple groups. The χ2 test was used to determine the relationships between cancer stage at diagnosis and secondary disease states (sarcopenia, diabetes and anaemia).
Results
Patient characteristics
A total of fifty pancreatic cancer patients met the study criteria. Patients were 66 (sd 12) years old with an equal distribution of males (56 %) and females (44 %). Average BMI was 23·4 (sd 3·7) kg/m2, and 58 % of the patients were characterised as normal weight (BMI between 18·5 and 24·9 kg/m2), as defined by the World Health Organization(20). Despite normal BMI at diagnosis, 48 % of the patients were characterised as sarcopenic. Only five of the patients were classified as underweight (BMI < 18·5 kg/m2), indicating that sarcopenia was independent of overall body size (Table 1). Interestingly, sarcopenic patients were more likely to be diagnosed at an earlier stage (IIB) of disease compared with non-sarcopenic patients (stage IV, P= 0·008). However, cancer stage was not significantly related to the other clinical parameters assessed including presence of diabetes at diagnosis (P= 0·778), presence of anaemia at the time of the first CT scan (P= 0·423), nutritional supplementation (P= 0·219) or any other parameters listed in Table 1. In terms of treatment, 62 % of the patients underwent surgery at some point during the treatment trajectory, 46 % received radiation and 88 % received chemotherapy. The heterogeneity of this patient group allows for the identification of global changes in body composition associated with pancreatic cancer and for the identification of any clinical parameters that may be associated with increased muscle and adipose tissue loss. Physical characteristics of patients at diagnosis and treatment parameters are presented in Table 1.
Table 1 Physical diagnostic characteristics and treatment parameters of patients (n 50) (Mean values and standard deviations; number of patients, ranges and percentages)
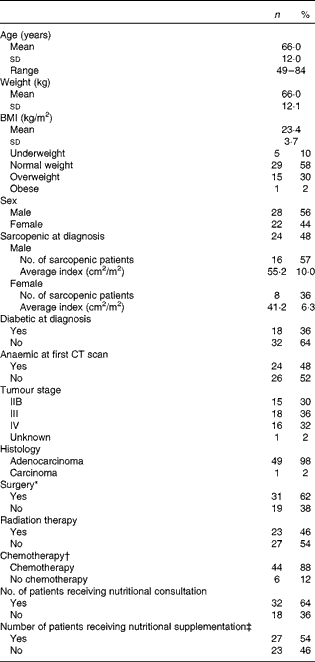
CT, computed tomography.
* Surgery mainly included radical (78 %), palliative or bypass surgeries, and endoscopic retrograde cholangiopancreatography (ERCP) with stent insertion.
† Chemotherapy regimens predominantly included gemcitabine (89 %), 5-flourouricil (infusion or bolus) and capecitabine.
‡ Nutritional supplementations included Ensure, Carnation Breakfast Anytime and protein powder.
Net tissue change analysis
Longitudinal analysis was performed to identify different rates of change in muscle and adipose tissue. There were no significant differences in total tissue change and rates of change between consecutive time intervals (i.e. approximately 70, 150, 250 and 350 d from death) throughout the disease trajectory for muscle or adipose tissue (data not shown). Absolute changes in muscle and VAT were representative of change throughout the disease trajectory; based on these findings, the remainder of the present results are based on net tissue changes. Overall, thirty-eight of the fifty patients (76·0 %) experienced net muscle loss, and 86·0 % (forty-three out of the fifty patients) experienced net VAT loss over the disease trajectory. On average, approximately 1·72 kg (P< 0·001) of muscle was lost at a rate of − 0·55 (sd 1·00) kg/100 d (P< 0·001; Table 2). Patients also lost approximately 1·04 kg of VAT (P< 0·001) between the first and last CT images at a rate of − 0·32 (sd 0·39) kg/100 d (P< 0·001; Table 2). Tissue losses were then ranked based on total loss over the disease trajectory and were subsequently divided into equal tertiles of approximately seventeen patients per group. Patients in tertile 1 lost 4·93 (sd 2·50) kg of muscle, while patients in tertile 3 actually gained a small amount of muscle (1·18 (sd 1·31) kg) over the disease trajectory (P< 0·001; Table 3). VAT showed a similar trend with patients in tertile 1 losing 2·24 (sd 0·67) kg of VAT and patients in tertile 3 showing a relatively little change in VAT (0·04 (sd 0·48) kg) (P< 0·001; Table 3). The rate of change in muscle and VAT followed the same distribution as absolute tissue loss (P< 0·001; Table 3).
Table 2 Tissue characteristics (Mean values and standard deviations)
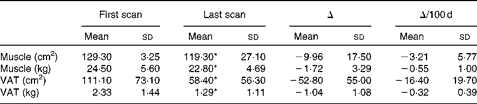
VAT, visceral adipose tissue.
* Mean values were significantly different between the first and last scans (P< 0·05).
Table 3 Tertile analysis (Mean values and standard deviations)

VAT, visceral adipose tissue.
* Mean values were significantly different from those of tertile 1 (P< 0·05).
Interestingly, patients who lost VAT at the most accelerated rate (average − 0·74 (sd 0·36) kg/100 d, tertile 1) demonstrated shorter average survival time (316·8 (sd 26·3) d) compared with patients who lost VAT at the slowest rate (515·1 (sd 62·9) d, average − 0·02 (sd 0·07) kg/100 d, tertile 3), but not patients with moderate VAT loss (423·8 (sd 55·5) d, average − 0·25 (sd 0·09) kg/100 d, P= 0·020; Fig. 2(a)). Also, patients who lost muscle at the most accelerated rate (average − 1·59 (sd 1·00) kg/100 d, tertile 1) tended to demonstrate shorter survival time (333·0 (sd 38·7) d) compared with patients who lost muscle at slower rates (508·4 (sd 57·3) d, average − 0·32 (sd 0·19) kg/100 d, tertile 2) and those who gained muscle (413·8 (sd 56·8) d, average 0·29 (sd 0·32) kg/100 d, tertile 3, P= 0·104; Fig. 2(b)). However, there was no significant difference in survival time between patients who lost the most VAT or skeletal muscle compared with patients who lost smaller absolute amounts of tissue (data not shown, P= 0·876 and P= 0·884, respectively). This suggests that the rate of tissue loss, not the absolute amount of tissue loss, may be specifically important in patient survival.

Fig. 2 Kaplan–Meier survival curves: division of patients based on tertiles of the rate of tissue loss in kg/100 d. (a) Rate of change in visceral adipose tissue (VAT). * Values were significantly different between tertile 1 (![]() ) and tertile 3 (
) and tertile 3 (![]() ) (P< 0·05). Tertile 1: VAT loss > − 0·40 kg/100 d; tertile 2 (
) (P< 0·05). Tertile 1: VAT loss > − 0·40 kg/100 d; tertile 2 (![]() ): VAT loss < − 0·40 and > − 0·10 kg/100 d; tertile 3: VAT loss < − 0·10 kg/100 d. (b) Rate of change in skeletal muscle; tertile 1: rate of muscle loss > − 0·70 kg/100 d; tertile 2: rate of muscle loss < − 0·70 and < − 0·10 kg/100 d; tertile 3: rate of muscle loss < − 0·10 kg/100 d.
): VAT loss < − 0·40 and > − 0·10 kg/100 d; tertile 3: VAT loss < − 0·10 kg/100 d. (b) Rate of change in skeletal muscle; tertile 1: rate of muscle loss > − 0·70 kg/100 d; tertile 2: rate of muscle loss < − 0·70 and < − 0·10 kg/100 d; tertile 3: rate of muscle loss < − 0·10 kg/100 d.
Patients also lost significant amounts of IMAT, SAT and TAT during the course of the disease trajectory. Patients lost − 3·85 cm2 of IMAT at a rate of − 12·0 (sd 17·5) %/100 d (P< 0·001). A subset of twenty-eight patients was used to quantify changes in SAT and TAT, as SAT was cut off from the field of view in twenty-two out of the fifty patients. With the exception of this latter subset having less SAT mass, they were similar to the former subgroup, as there were no differences in any of the descriptive characteristics (data not shown). Patients lost 4·82 (sd 4·44) kg of TAT at a rate of − 1·53 (sd 1·65) kg/100 d over the disease trajectory. TAT loss appears to be accelerated when compared with VAT loss (0·32 (sd 0·39) kg/100 d v. − 1·53 (sd 1·65) kg/100 d); however, TAT accounts for 43·7 (sd 16·7) % of total mass at diagnosis, while VAT accounts for only 3·58 (sd 2·02) %. When looking at a percentage change per 100 d in these tissues, we see that there was no significant difference in the rate of loss for VAT, SAT, IMAT and TAT. VAT was lost at a rate of − 15·5 (sd 17·9) %/100 d, SAT at a rate of − 13·5 (sd 14·1) %/100 d, IMAT at a rate of − 12·0 (sd 17·5) %/100 d and TAT at a rate of − 14·4 (sd 13·2) %/100 d (P= 0·384). These data suggest that changes observed in VAT were similar to the loss of other adipose tissue depots.
Determinants of visceral adipose tissue loss
Physical and clinical characteristics were used to assess determinants of VAT loss. As the rate of VAT loss appears to predict survival, the amount of VAT lost between patients surviving for >1 year and patients surviving < 1 year was examined. Patients surviving for >1 year lost the same amount of VAT as patients who survived for < 1 year ( − 1·03 (sd 1·11) kg v. − 1·05 (sd 1·08) kg, P= 0·755; Table 4); however, patients surviving for < 1 year lost VAT at faster rates than those who survived for >1 year ( − 0·45 (sd 0·48) kg/100 d v. − 0·22 (sd 0·25) kg/100 d, P= 0·041; Table 4). Again, this supports the notion that the rate of VAT loss may be more important in survival than the total amount of tissue lost.
Table 4 Influence of physical diagnostic and clinical characteristics on tissue changes (Mean values and standard deviations)

VAT, visceral adipose tissue.
* Mean values were significantly different between the two groups compared (P< 0·05).
Non-sarcopenic patients tended to lose VAT at an accelerated rate when compared with sarcopenic patients ( − 0·35 (sd 0·32) kg/100 d v. − 0·26 (sd 46) kg/100 d, respectively, P= 0·115; Table 4); however, there were no significant differences in total VAT loss between non-sarcopenic and sarcopenic patients ( − 1·21 (sd 1·03) kg v. − 0·77 (sd 1·13) kg, respectively, P= 0·161; Table 4).
Also, males tended to lose more VAT and at an accelerated rate, when compared with females ( − 1·27 (sd 1·16) kg v. − 0·75 (sd 0·92) kg, P= 0·092, respectively; − 0·42 (sd 0·43) kg/100 d v. − 0·19 (sd 0·29) kg/100 d, P= 0·068, respectively; Table 4). To further investigate this discrepancy between males and females, we examined the amount of VAT lost as a proportion of total body weight at diagnosis. In this case, males presented with greater body weight at diagnosis compared with females (70·1 (sd 8·71) kg v. 60·7 (sd 13·9) kg, respectively), thus a greater VAT loss was proportional to the larger body weight in males. When examining the data from this respect, there were no significant differences between males and females in the proportion of VAT lost relative to body weight ( − 1·77 (sd 1·64) % v. − 1·32 (sd 1·75) %, P= 0·333). In addition, there were no significant differences observed in the amount of VAT lost or the rate of VAT loss relative to age or BMI (Table 4). Chemotherapy was not assessed as a clinical parameter, as 88 % of the patients received chemotherapy at some point during the disease trajectory.
Patients presenting with diabetes at diagnosis lost significantly more VAT when compared with patients who were not diabetic at diagnosis ( − 1·50 (sd 0·99) kg v. − 0·74 (sd 1·06) kg, P= 0·017; Table 4). Diabetic patients also lost VAT at an accelerated rate when compared with non-diabetic patients ( − 0·44 (sd 0·33) kg/100 d v. − 0·20 (sd 0·30) kg/100 d, P= 0·010; Table 4). This also corresponded to a greater proportion of VAT relative to total body weight lost by diabetic patients compared with non-diabetic patients ( − 2·14 (sd 1·49) % v. − 1·17 (sd 1·65) %, respectively, P= 0·033). There were no significant differences in absolute VAT loss and the rate of VAT loss for any of the other clinical parameter listed in Table 4. A description of the characteristics of the subpopulations examined can be found in Table 5.
Table 5 Characteristics of the subpopulations analysed†

* Values were significantly different between the expected proportion of patients in the subpopulation and the actual proportion of patients in the subpopulation (P< 0·05).
† Values represent the number of patients within each subpopulation (columns) with each characteristic examined (rows), except for average BMI and average age.
Determinants of skeletal muscle loss
Muscle tissue was lost at accelerated rates when compared with an ageing population followed over 12 years (age 65·4 (sd 4·2) years at the first evaluation), who typically lose muscle at a rate of approximately 1·4 % per year(Reference Mitsiopoulos, Baumgartner and Heymsfield17), and cross-sectional studies have demonstrated a loss of approximately 1 % of muscle per year (age range 15–83 years)(Reference Miller, Jones and Yanovski18). In the present study, muscle CSA was lost at a rate of approximately 7·48 % per year.
Also, patients who were anaemic at the first CT scan lost more muscle and at an accelerated rate when compared with non-anaemic patients ( − 2·41 (sd 3·17) kg v. − 1·00 (sd 2·78) kg, respectively, P= 0·029; − 0·71 (sd 0·87) kg/100 d v. − 0·29 (sd 0·85) kg/100 d, respectively, P= 0·017; Table 4). Anaemic patients also lost a greater proportion of total initial body weight compared with non-anaemic patients ( − 3·58 (sd 4·76) % v. − 1·07 (sd 3·15) %, respectively, P= 0·018). The presence of diabetes at diagnosis tended to influence the amount of muscle lost, with diabetic patients tending to lose more muscle than non-diabetic patients ( − 2·58 (sd 4·01) kg v. − 1·14 (sd 2·18) kg, respectively, P= 0·110; Table 4).
Also, patients who received nutritional supplementation lost significantly less skeletal muscle and the rate of loss was significantly attenuated when compared with patients who did not receive nutritional supplementation ( − 0·78 (sd 2·44) kg v. − 3·26 (sd 3·39) kg, respectively, P= 0·026; − 0·19 (sd 0·65) kg/100 d v. − 1·16 (sd 1·25) kg/100 d, respectively, P= 0·009; Table 4). Patients receiving nutritional supplementation lost a similar proportion of initial body weight compared with patients not receiving nutritional supplementation ( − 1·77 (sd 4·58) v. − 3·10 (sd 3·60), P= 0·199); however, patients receiving supplementation had a significantly lower initial body weight (62·8 (sd 10·3) kg) compared with patients not receiving supplementation (69·8 (sd 13·3) kg, P= 0·041), which may influence this finding. When examining survival time for patients receiving nutritional supplementation using Kaplan–Meier survival curves, patients who received nutritional supplementation tended to live longer than patients who did not receive nutritional supplementation (median: 468·2 (sd 46·2) d v. 360·2 (sd 37·0) d, respectively, P= 0·098, curves not shown). Nutritional supplementation was associated with dietetic consult (P< 0·001); however, dietetic consult alone was not associated with changes in muscle (P= 0·395; Table 4). There were no significant differences in muscle loss for any of the other characteristics examined (Table 4).
Similar to VAT, males compared with females tended to lose more muscle tissue and at an accelerated rate ( − 2·47 (sd 3·48) kg v. − 0·76 (sd 1·97) kg, P= 0·084; − 0·84 (sd 1·17) kg/100 d v. − 0·19 (sd 0·55) kg/100 d, P= 0·065; Table 4). However, there were no significant differences between the proportion of muscle mass relative to total body weight between males and females ( − 3·24 (sd 4·54) % v. − 1·19 (sd 3·46) %, P= 0·151). Also, patients classified as sarcopenic at the time of the first scan tended to lose less muscle when compared with patients classified as non-sarcopenic at their first scan ( − 0·93 (sd 3·09) kg v. − 2·26 (sd 2·86) kg, respectively, P= 0·054; Table 4). Non-sarcopenic patients lost muscle at an accelerated rate when compared with sarcopenic patients ( − 0·65 (sd 0·80) kg/100 d v. − 0·39 (sd 1·18) kg/100 d, respectively, P= 0·025; Table 4). Sarcopenic patients also tended to live longer than non-sarcopenic patients (456·3 (sd 55·7) d v. 418·8 (sd 64·5) d, P= 0·051), which may be explained by the finding that sarcopenic patients were diagnosed with earlier-stage cancer compared with non-sarcopenic patients. There were no significant influences of any other physical characteristics on changes in skeletal muscle (Table 4). A description of the characteristics of the subpopulations examined can be found in Table 5. CA19-9 was evaluated in the twenty-eight patients with available data and was not related to changes in body composition (data not shown). Multivariate linear regression was performed to evaluate the potential interactions between each parameter. There were no significant interactions identified.
Discussion
The major finding of the present study is that the rate at which pancreatic cancer patients lose VAT and skeletal muscle provides an important indicator of survival compared with the total amount of tissue lost. Despite patients losing similar amounts of VAT over the disease trajectory, patients who survived for >1 year had attenuated rates of VAT loss compared with patients who survived for < 1 year. We demonstrated that muscle tissue was lost at accelerated rates (approximately 7·48 % per year) in this group of cancer patients, which is far more exacerbated than aged individuals (15–83 years old) who typically lose muscle at a rate of 1–1·4 % per year(Reference Frontera, Hughes and Fielding21, Reference Lexell, Taylor and Sjostrom22).
Additionally, this is the first study to identify that the presence of diabetes at diagnosis was associated with significantly more VAT loss and a tendency for greater muscle loss in pancreatic cancer patients. The presence of anaemia at the first CT scan was also associated with significant and accelerated losses of muscle compared with patients with normal Hb levels. These data suggest that anaemic and diabetic patients may be at a greater risk of cachexia when diagnosed with pancreatic cancer. The presence of diabetes at diagnosis or anaemia at the first CT scan, both contributors of muscle wasting and cachexia(Reference Evans, Morely and Argiles6), may serve as potential biomarkers of patients most susceptible to cancer cachexia. We demonstrated that use of nutritional supplementation may decrease the amount of skeletal muscle lost and attenuate the rate of tissue loss, which may improve survival. The present study demonstrated that patients receiving nutritional supplementation tended to live longer than patients not receiving supplementation, which warrants future investigation into this hypothesis. Identification of potential predictors of cachexia may allow early detection in patients who are at a risk of developing cachexia, which may permit the development of interventions near the time of diagnosis of pancreatic cancer patients.
Rate of tissue loss may provide a better indicator of survival than total tissue lost
Pancreatic cancer patients have the highest incidence rates of cancer cachexia and experience the most severe symptoms of cachexia(Reference Wigmore, Plester and Richardson4). While few studies have characterised the specific changes in muscle and adipose tissue, we demonstrated that pancreatic cancer patients lose both muscle and fat during the disease trajectory. The patterns of tissue loss in the present study are different from those observed in other cancer populations such as colorectal cancer(Reference Lieffers, Mourtzakis and Hall23); thus, each cancer type should be considered separately. Despite its metabolic importance, changes in VAT quantitatively represent changes in all adipose tissue depots, as demonstrated by Hallgreen & Hall(Reference Hallgreen and Hall24) and confirmed in the present study. In a recent study by Tan et al.(Reference Tan, Birdsell and Martin8), forty-four pancreatic cancer patients lost − 2·1 (sd 4·1) kg of lean mass and − 4·5 (sd 3·7) kg of TAT mass during the 189 d disease trajectory. The patients in the present study were similar to the Tan et al. (Reference Tan, Birdsell and Martin8) patient cohort in terms of age, BMI and sex, but the patients in the present study survived longer. Thus, when patients were grouped by those surviving < 1 year v. those surviving >1 year, we observed that all pancreatic patients lost similar amounts of total muscle and adipose tissue. However, the rate at which muscle and adipose tissue was lost influenced survival. Patients who lost VAT at the most accelerated rates had the poorest survival outcomes and patients who lost muscle at the fastest rate also tended to have the poorest survival outcomes. Patients with the most accelerated tissue losses may exhibit increased stimulation of the catabolic pathways associated with muscle and adipose wasting, such as increased pro-inflammatory processes, and inhibition of the anabolic pathways(Reference Bruckart, Beca and Urban25, Reference Glass26). Attenuating the rate of muscle and adipose tissue loss through these pathways may improve survival in pancreatic cancer patients.
Determinants of visceral adipose tissue and muscle loss are independent and unique
Pancreatic cancer patients who had been diagnosed with diabetes before their cancer diagnosis lost significantly more VAT and tended to lose more skeletal muscle when compared with non-diabetic patients. The development of glucose intolerance has been documented in advanced cancer patients with cachexia(Reference Tayek27), and is a defining characteristic of diabetes. Pancreatic cancer patients with impaired glucose handling at diagnosis may be predisposed to changes in body composition, as their pre-existing glucose intolerance may exacerbate changes in muscle and adipose tissue during the disease trajectory. Patients who are identified as diabetic at cancer diagnosis are exposed to perturbed glucose and insulin metabolism for a longer duration of time compared with patients who develop diabetes during the disease trajectory. Patients with longer-term diabetes (i.e. diabetic at cancer diagnosis) may have accelerated losses of muscle and adipose tissue for this reason.
The association between diabetes and tissue loss has also been demonstrated in elderly populations with type 2 diabetes(Reference Park, Goodpaster and Lee28, Reference Kim, Park and Yang29). In these studies, elderly individuals with type 2 diabetes display a greater magnitude of sarcopenia, compared with elderly individuals without type 2 diabetes. Also, Wang et al. (Reference Wang, Hu and Hu5) demonstrated that insulin resistance accelerates muscle protein degradation in db/db mice via the ubiquitin–proteasome pathway, the pathway most often implicated in muscle wasting in cancer cachexia(Reference Kahl, Wykes and Russell30). Patients presenting with impaired glucose handling at diagnosis may be at an increased risk of muscle and adipose tissue loss.
The presence of anaemia at the first CT scan also significantly influenced the rate of skeletal muscle loss, with anaemic patients losing significantly more muscle at an accelerated rate when compared with non-anaemic patients. The development of anaemia in conjunction with cachexia has been documented and the presence of anaemia is one of the biochemical markers used to diagnose cachexia(Reference Evans, Morely and Argiles6). This suggests that patients who are anaemic at the first CT scan may be at an increased risk of weight loss and may have poorer survival outcomes. Anaemia affects the ability of erythrocytes to efficiently deliver O2 to peripheral tissues. Impaired O2 delivery may therefore exacerbate the rate of muscle loss by impairing the tissue's ability to oxidise metabolic substrates. Anaemia in chronic disease states is associated with increased inflammation(Reference Weiss and Goodnough31), which, in some diseases such as chronic kidney disease, has been linked to muscle wasting(Reference Rasic-Milutinovic, Perunicic-Pekovic and Ristic-Medic32). It is possible that inflammation may also be mediating the influence of anaemia on muscle wasting in pancreatic cancer patients. Altogether, the interrelationship between diabetes, anaemia and muscle loss suggests that availability of glucose and inflammation may influence the rate of muscle loss, ultimately contributing to cachexia.
Patients who received nutritional supplementation lost significantly less muscle at a slower rate when compared with patients who did not receive any type of nutritional supplementation. Supplemented patients also tended to live longer than non-supplemented patients. This was independent of the stage of cancer at diagnosis. The nutritional supplements varied across patients and included protein powder, Ensure, Boost, Carnation Breakfast Anytime, Glucerna, vitamin and mineral supplementations. Regardless of the type of nutritional supplementation, patients receiving dietary supplements experienced a significantly slower loss of muscle tissue and tended to live longer. Many studies have examined the use of nutritional supplementation to counter the effects of cancer cachexia, for example, by adding ‘calories’(Reference Morley33) or by using n-3 fatty acids(Reference Colomer, Moreno-Nogueira and Garcia-Luna34, Reference Brown, Zelnik and Dobs35); however, results have been inconclusive and may be secondary to the use of crude body composition tools such as weight or bioelectrical impedance analysis. Using CT imaging, we demonstrated that dietary supplement use is associated with attenuated muscle wasting, and this was related to improved survival in pancreatic cancer patients. Similarly, Mantovani et al. (Reference Mantovani, Maccio and Madeddu36) used CT and dual-energy X-ray absorptiometry (DXA) to identify attenuated losses in lean body mass with nutritional supplement. While we found a positive association between dietary supplements and survival in pancreatic cancer patients, the heterogeneity in the type of supplement used, combined with the small number of patients receiving supplement (n 27), prevented further analysis. Thus, future investigations using CT imaging or other precise modalities of body composition to specifically quantify changes in muscle and fat mass are warranted for identifying successful supplements.
Surprisingly, sarcopenic patients lost significantly less muscle tissue at an attenuated rate when compared with non-sarcopenic patients. Sarcopenic patients also tended to lose less VAT and have improved survival outcomes when compared with non-sarcopenic patients. In the present study, sarcopenic patients were diagnosed with earlier-stage cancer when compared with non-sarcopenic patients. The majority of sarcopenic patients (42 %) were diagnosed with stage IIB cancer compared with only 19 % of non-sarcopenic patients (P= 0·008). Involuntary weight loss is often one of the first reported symptoms of pancreatic cancer(Reference Holly, Chaliha and Bracci37); thus, early weight loss may have contributed to their sarcopenia, and may have led to an earlier diagnosis. Patients diagnosed with earlier-stage pancreatic cancer have improved survival outcomes(Reference Wasif, Ko and Farrell38), which supports the findings of the present study.
Similar to the findings of Tan et al. (Reference Tan, Birdsell and Martin8), sarcopenia alone did not predict poorer survival outcome in pancreatic cancer patients. Tan et al. (Reference Tan, Birdsell and Martin8) observed increased hazard ratios in sarcopenic obese patients when compared with other patients (normal-weight non-sarcopenic, obese non-sarcopenic and normal-weight sarcopenic collectively); however, there were no significant differences between sarcopenic patients and non-sarcopenic patients.
Interestingly, we did not observe any significant influence on different treatment types on body composition. Current literature has demonstrated that patients who undergo surgery experience significant tissue loss(Reference Liedman, Andersson and Bosaeus7, Reference Kiyama, Mizutani and Okuda39). However, due to the short time course of the disease, the small sample size and heterogeneity in the type of surgery performed, as well as the variability in the timing of the surgery, surgery itself was not significantly related to muscle and adipose tissue loss. Other treatment modalities such as chemotherapy have been related to lean tissue loss(Reference Awad, Tan and Cui40); however, this could not be evaluated in the present study due to the high proportion (88 %) of patients receiving these treatments.
Limitations of the present study
CT image analysis and patient history reviews provided a feasible method to evaluate body composition and relationships to clinical parameters in pancreatic cancer patients. We identified associations between clinical outcomes and markers, not causality; however, these associations offer essential considerations to build future clinical studies in nutrition and rehabilitative intervention. The present study also demonstrates the importance of using precise methods to measure body composition. CT image analysis presents a highly precise and reproducible method to evaluate changes in body composition. Although it was not possible to control for the timing of CT images or the timing of clinical measurements, body composition analysis was normalised in a representative manner of the disease trajectory. Also, the small sample size of the present study influenced our ability to identify specific nutritional supplements that may attenuate muscle loss and to further examine the influence of nutrition on body composition. The small sample size and the unknown timing of each surgery during the disease time trajectory limited the interpretation of the surgery's potential influence on muscle and adipose tissue. However, we were still able to demonstrate relationships between body composition change and a number of clinical parameters. The description of changes in body composition in pancreatic cancer and the interrelationships between body composition, clinical markers and survival is fundamental to developing future successful interventions that aim to counter muscle and fat loss in cancer cachexia.
Conclusions
In conclusion, patients with pancreatic cancer lost significant amounts of muscle and adipose tissue during the course of the disease trajectory; however, the rate at which patients lost VAT and skeletal muscle was a more important indicator of survival than the total amount of tissue loss. The presence of clinical biomarkers associated with cachexia at cancer diagnosis, such as anaemia and diabetes, may exacerbate muscle and adipose tissue loss. Loss of skeletal muscle may be attenuated by dietary supplementation and may prolong survival; however, the type, quantity and timing of dietary supplementation required future investigation.
Acknowledgements
The authors would also like to acknowledge Roseanna Pressutti, Stephanie Fernandez and Lianne Lin for their technical support. There are no conflicts of interest to disclose. This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. K. M. D. S., K. Z., R. K. W., T. C., D. K., G. R. M. and M. M. have contributed to the design of the study; K. M. D. S., L. Y., K. Z., R. K. W., T. C., M. M. have contributed to the data collection and analysis; all authors have contributed to the writing and reviewing of the manuscript. All authors read and approved the final manuscript.









