Citrus fruits are rich sources of flavanones, a sub-group of the flavonoids, and are widely consumed as part of human diets. Flavanones are biologically active and there has been considerable interest in their antioxidant and other properties that may contribute to suppress inflammation and hence modulate events involved in the initiation and progression of cancer and cardiovascular dysfunction. Early work focused on the ability of citrus flavanones to affect capillary function(Reference Sokoloff, Redd and Dutcher1–Reference Salgado and Green3). More recently, it has been demonstrated that flavanones can modulate cell-signalling pathways that have been implicated in cancer, atherosclerosis and other inflammatory diseases(Reference Agullo, Gamet-Payrastre, Manenti, Viala, Remesy, Chap and Payrastre4–Reference Pollard, Whiteman and Spencer8). There is a considerable body of in vitro data to show that flavanones can interact with a number of enzymes that play key regulatory roles in cellular inflammation processes including receptor binding and cellular activation(Reference Middelton, JA and Vuslig9–Reference Orallo, Camina, Alvarez, Basaran and Lugnier11). These activities may relate to the ability of flavanones to interact with the nucleotide binding sites of regulatory enzymes such as kinases and phosphodiesterases that are involved in controlling cellular activation during inflammation, and to inhibit enzymes of the arachidonic acid metabolic pathway, including cyclo-oxygenases, lipoxygenases and phospholipases(Reference Orallo, Camina, Alvarez, Basaran and Lugnier11, Reference Manthay, Grohmann and Guthrie12). There are reports providing evidence of lipid- and cholesterol-lowering(Reference Santos, Oliveira, Nagem, Pinto and Oliveira13), anti-inflammatory(Reference Crespo, Galvez, Cruz, Ocete and Zarzuelo14), anticarcinogenic(Reference So, Guthrie, Chambers, Moussa and Carroll15–Reference Guthrie and Carroll17) and anti-ageing(Reference Crespo, Galvez, Cruz, Ocete and Zarzuelo14, Reference Kim, Jung, Choi and Chung18) activities. These observations are supported by epidemiological studies indicating that flavanone and citrus consumption is associated with decreased risk for cerebrovascular disease and asthma(Reference Knekt, Kumpulainen, Jarvinen, Rissanen, Heliovaara, Reunanen, Hakulinen and Aromaa19) and cancer at various sites(Reference Lagiou, Samoli, Lagiou, Peterson, Tzonou, Dwyer and Trichopoulos20–Reference Maserejian, Giovannucci, Rosner, Zavras and Joshipura22).
The major flavanone aglycones are hesperetin, naringenin and eriodictyol, which differ in their hydroxyl and methoxyl substitutions in the flavan A- and B-rings (Fig. 1). As with most flavonoids, the natural forms are glycosides. In oranges (Citrus sinensis), the major flavanone glycosides are hesperidin (7-O-rutinoside of hesperetin) and narirutin (7-O-rutinoside of naringenin). The flavanone contents of citrus fruits and juices are high compared with the flavonoid content of many other food products. For example, quercetin, a flavonol for which there are copious literature reports concerned with epidemiological associations, various biological activities in cellular and animal models and bioavailability, reflecting the widespread interest in this compound, the estimated mean daily intake in The Netherlands is about 16 mg/d(Reference Hertog, Hollman, Katan and Kromhout23). In contrast, the reported range of narirutin and hesperedin contents of orange juices are 16–84 mg/l and 200–590 mg/l respectively, and although flavanone intakes have not been estimated in most countries, it has been reported that the most consumed flavonoid in Finland was hesperetin (mean intake >28 mg/d(Reference Kumpulainen, Voutilainen and Salonen24)). Clearly, for those individuals that do consume citrus regularly, the exposure is likely to be relatively high. Conversely, since the distribution of flavanones in plants is limited almost exclusively to citrus fruits, a proportion of the population will consume no flavanones at all.
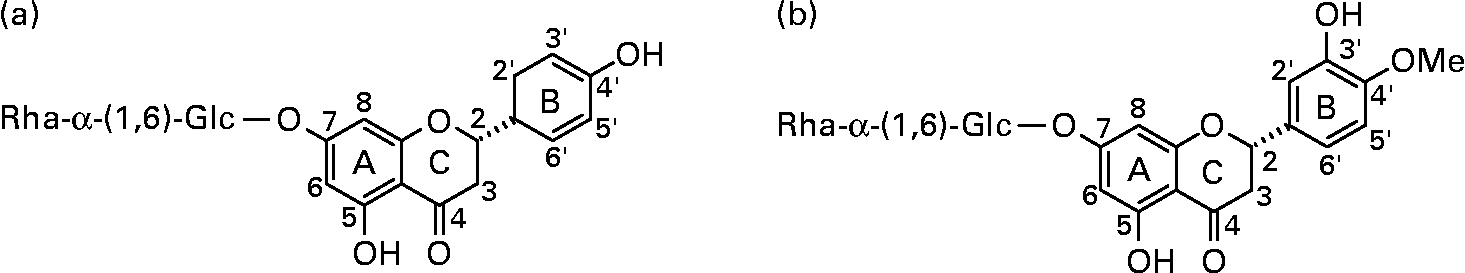
Fig. 1 Structures of the orange flavanones narirutin (a) and hesperidin (b). The carbon at C2 is chiral and in citrus fruits the flavanone glycosides are usually present as the 2S epimer.
Observations that dietary flavanones can affect disease risk beyond the gastrointestinal tract have provided the rationale for research concerned with the oral bioavailability of flavanones. Flavanone absorption, distribution, metabolism and excretion have been studied using a variety of cellular and animal models, and in human feeding studies. In a review of polyphenol human feeding studies, Manach et al. (Reference Manach, Williamson, Morand, Scalbert and Remesy25) described the dose-normalised plasma pharmacokinetic responses and urinary excretion yields for a range of flavonoids and other polyphenols. This indicated that flavanone glycosides such as naringin and hesperidin are reasonably bioavailable, with estimated mean plasma concentrations reaching about 0·50 μm from a 50 mg oral dose and mean urinary yields >8 %. These values are lower than for isoflavone glucosides (for example, daidzein and genistein) and the flavan-3-ol epicatechin, but much higher than for anthocyanins and flavonols (for example, quercetin). To date, a number of studies using relatively small subject numbers have investigated the absorption and excretion of flavanones from orange and grapefruit juices. Typically, the doses used have been high and not typical of what is normally consumed in habitual diets(Reference Erlund, Meririnne, Alfthan and Aro26, Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27). Further, there is little information regarding the effects of the food matrix on bioavailability, and although it has been established that the flavanones are present in plasma and urine as glucuronide, sulfate or sulfo-glucuronide conjugates, the conjugation positions are not known. The importance of establishing the detailed structure of flavonoid human conjugates is clear when one considers the variability of the impact on the biological activity (for a review, see Kroon et al. (Reference Kroon, Clifford, Crozier, Day, Donovan, Manach and Williamson28)).
In the present study, we fed twenty volunteers a single portion of orange fruit or orange juice and quantified the appearance of flavanones in plasma and urine. Using a combination of specific enzyme hydrolysis, HPLC-electrospray ionisation (ESI)-MS and HPLC-ESI-MSn with post-column metal complexation, the conjugation position of four flavanone glucuronides were elucidated and several other sulfates, glucuronides and mixed conjugates identified in plasma and urine samples. The subject exposures to flavanones following a single portion of orange juice or fruit were estimated from the plasma kinetics and from urinary excretion. The absorption of total flavanone, and of the individual flavanones naringenin and hesperetin, were compared between the fruit and juice matrices, and flavanone urinary excretion was measured in a larger group of subjects that was the largest of its kind and facilitated an investigation of the potential impact of age and other subject variables on flavanone bioavailability.
Materials and methods
Chemicals and reagents
HPLC-grade methanol was purchased from Fisher Scientific (Loughborough, UK). Acetonitrile (HPLC grade), trifluoroacetic acid, β-glucuronidase (Helix pomatia type H5), sulfatase (H. promatia type H1), N,O-bis-(trimethylsilyl) trifluoracetamide, rhamnetin, naringenin, hesperetin, perillic acid, ethylbenzoic and propylbenzoic acids, cobalt(II) bromide and 4,7-diphenyl-1,10-phenanthroline (4,7-dpphen) were purchased from Sigma-Aldrich (Poole, Dorset, UK). Pelargonidin-3-glucoside and galangin were obtained from Extrasynthèse (Genay, France).
Sampling of orange fruits and juices
Orange fruits and juices were purchased locally from a variety of outlets including major supermarkets and smaller retailers. The fruits were of the Maroc, Shamouti, Navel, Navelina, Salustiana, Moro and Lane Late varieties. Juices were a mixture of branded, economy and supermarket own-branded products. For the interventions, fresh oranges (Delta seedless variety, South Africa) were obtained in bulk from a local supermarket and stored at 4°C until consumption. All volunteers (with the exception of three) consumed oranges from the same batch. Fresh oranges were prepared by mixing together the segments (eight per fruit) of three whole oranges and portioning a 150 g representative sample for consumption and another 100 g sample for analysis. The orange juice was a commercially available supermarket own-branded product made from concentrate.
Subjects and study design
Twenty apparently healthy volunteers (ten men and ten women) aged between 20 and 65 years were recruited to participate in this study. All study participants were assessed for eligibility on the basis of a health questionnaire and the results of clinical laboratory tests. The following exclusion criteria applied: smokers; long-term medical conditions such as asthma (unless untreated within the past 2 years), heart disease, gastrointestinal disease, diabetes, cancer; regular prescribed medication (except hormone replacement therapy and oral contraceptives); supplement (unless judged not to affect study outcome) or antibiotic use within 4 weeks before the start of the study; pregnancy; blood donation within 4 months before the start of the study; BMI < 18·5 or >35 kg/m2; clinical results at screening judged by the medical advisor to affect study outcome or be indicative of a health problem. Subject characteristics were: weight 73·9 (sd 14·1) kg (range 50·3–101 kg), BMI 24·8 (sd 3·0) kg/m2 (range 20·7–32·2 kg/m2); age 49 (sd 11) years (range 26–64 years). The study was explained to participants and written informed consent was obtained before participation. The study protocol was approved by the Human Research Governance Committee of the Institute of Food Research and the Norwich Research Ethics Committee.
The study was a randomised two-phase cross-over design investigating the bioavailability of flavanones from fresh and processed oranges. Each test phase comprised a 5 d period of intervention separated by a washout period of at least 1 week. During each period of intervention, subjects followed a low-polyphenol diet and to aid compliance a list of authorised and prohibited foods were given. On day 3 of the intervention, fasted subjects had an intravenous catheter inserted and a baseline blood sample (10 ml) was obtained. Subjects were given a standard breakfast consisting of two slices of white toast (72 g) with spread (10 g) followed by either 150 g fresh orange segments or 300 g orange juice. To limit variation in food and drink intakes, subjects refrained from drinking and eating for 1·5 and 4 h respectively. Blood samples (10 ml) were collected into lithium heparin tubes at 15, 30 and 45 min and 1, 1·5, 2, 3, 4, 6, 8, 24 and 48 h and immediately centrifuged at 1500 g for 10 min. Plasma samples were subsequently mixed with ascorbate (final concentration 1 mm) to prevent flavanone degradation and frozen at − 20°C until analysis. Urine was collected in 24 h fractions the day before consumption of orange flavanones and for 48 h after consumption into collection bottles containing 1 g ascorbic acid. The amount of urine in each fraction was measured, and samples were stored at − 20°C until analysis.
Quantification of flavanone glycosides in orange fruits and juices
Orange fruits and juices were analysed using HPLC. All solvents used were HPLC grade, all water was ultra-pure and the purity of commercial standards was confirmed by HPLC. All analyses were performed at least in triplicate. Fresh oranges were weighed and samples of fruit purée (30 g) were mixed with 70 ml of 70 % aqueous methanol and blended to homogeneity. A sample (1 ml) was removed and mixed with 50 μl rhamnetin in methanol (0·1 mg/ml; internal standard). Juices were prepared by adding 700 μl methanol and 50 μl rhamnetin (0·1 mg/ml) to 300 μl of juice. Both fruit and juice samples were heated to 70°C for 20 min with occasional vortex mixing, centrifuged (13 000 rpm × 10 min; 4°C) and filtered before HPLC analysis.
Samples were analysed by HPLC with online UV-diode array and LC-MS detectors (see later). Absorbance at 270 nm was used for quantification, and tandem MS (positive-ion mode) to confirm the identity of the analytes. Recoveries of internal standards for the food and beverage analyses were 93·1 (sd 4·8) and 95·4 (sd 4·4) % for fruits and juices, respectively. Intra-day variance values for narirutin, hesperidin and didymin in fruits and juices were < 10 %.
Extraction and quantification of flavanones and metabolites in plasma and urine
Acidified plasma samples (2·0 ml) were hydrolysed enzymically by incubating with 2·0 ml phosphate buffer (pH 5·0), 0·2 ml β-glucuronidase (2000 units) and 0·2 ml sulfatase (200 units) at 37°C for 2 h. Acidified urine samples (10·0 ml) were incubated with 5·0 ml phosphate buffer (pH 5·0), 1·0 ml β-glucuronidase (10 000 units) and 1·0 ml sulfatase (1000 units) at 37°C for 2 h. Then 50 μl galangin (0·1 mg/ml) was added as an internal standard before incubation. Flavanones in hydrolysed plasma and urine samples were extracted using a solid-phase extraction (SPE) cartridge (Varian Bond Elute C18) conditioned with methanol (5 ml) followed by water (10 ml). Following application of urine or plasma, the cartridge was washed with water (10 ml) and flavanones eluted directly into vials with 1 % HCl in methanol (1·0 ml for urine) or 1 % HCl in acetonitrile (0·5 ml for plasma). Pelagonidin-3-glucoside (50 μl of 0·1 mg/ml) was added to the SPE eluate as a volume marker immediately before HPLC analysis.
Samples (1 μl) of hydrolysed plasma and urine extracts were analysed by HPLC (Agilent HP1100; Agilent Technologies, Waldbronn, Germany) using a Gemini C18 column (150 × 2·00 mm, 5 μm particle size; Phenomonex, Macclesfield, Cheshire, UK) eluted at 0·3 ml/min with a gradient of increasing solvent B (0·1 % trifluoroacetic acid in acetonitrile) from solvent A (0·1 % aqueous trifluoroacetic acid) at 30°C over 36 min (plasma) or 65 min (urine). The eluent was scanned over 200–600 nm by a diode array detector and subsequently an ESI-MS (Agilent Technologies, Waldbronn, Germany). The mass spectrometer was operated in negative ionisation mode (cone voltage 22 V, source block temperature 120°C, desolvation temperature 300°C) with multiple reaction monitoring. Quantification was based on peak areas at 270 nm. Quantification of orange flavanones in plasma and urine samples was based on standard curves (range 0·1–100 μg/ml) for naringenin and hesperetin. Standard curves were linear with regression coefficients >0·99 and recoveries of the internal standard were 91·4 (sd 9·38) %. The precision of the urine and plasma assays was assessed using replicate (n 4) analyses of spiked blank samples: precision was excellent with mean values of 97·8 (sd 2·0) and 99·8 (sd 2·5) % of expected analyte recovered for hesperetin and naringenin, respectively. Assay repeatability was assessed by repeated analyses (four different days) of replicate (n 5) spiked samples; the inter-day variance was < 5 % (sem as percentage of the mean). Recoveries of internal standards for the plasma analyses were 91 (sd 10·5) %.
Identification of flavanone metabolites in urine after orange consumption
A sample of urine from a typical volunteer was analysed as above, except directly without any enzyme hydrolysis. An authentic sample of synthetic naringenin 7-glucuronide(Reference Davis, Needs, Kroon and Brodbelt29) was run in comparison to allow its identification in the urine sample. Samples treated separately with glucuronidase or sulfatase were compared with untreated equivalents to assist identification of glucuronidated and sulfated metabolites. A second analysis of the urine samples was performed independently and blindly at another facility (see below). Liquid chromatography-tandem MS (LC-MSn) was the only identification technique employed for this parallel analysis. Negative-ion-mode LC-MSn provided the mass of each metabolite, the number of sulfate and/or glucuronic acid moieties attached, and the identity of the flavonoid aglycone. The locations of the glucuronic acid moieties were determined by metal complexation with the Co2+ ion and an auxiliary ligand, 4,7-dpphen. The fragmentation patterns of the [Co(II) (flavonoid glucuronide–H) (4,7-dpphen)2]+ complexes are highly indicative of the position of the glucuronic acid moieties, as demonstrated in a recent study(Reference Davis, Needs, Kroon and Brodbelt29). Similar types of metal complexes have been used recently to identify flavonoid glycosides in various plant extracts(Reference Davis and Brodbelt30, Reference Wojcińska, Williams, Mabry, Ahmed, Davis, Tóth, El-Sayed, Matławska and Clevinger31). Methanol (7·5 ml) was added to 2·5 ml samples of urine for protein precipitation. The mixture was vortexed and centrifuged for 15 min at 1380 g. The supernatant fraction was collected and evaporated with N2 down to approximately 500 μl. Injection volumes were 20–40 μl. Chromatography was performed on an Alliance 2695 HPLC system (Waters, Milford, MA, USA). The column was a Waters Symmetry C18 (2·1 × 50 mm, 3·5 μm particle size). The mobile phases were water containing 0·05 % formic acid (A) and methanol containing 0·05 % formic acid (B). The gradient began at 25 % B, increased to 30 % B over 10 min, then increased to 50 % B over 10 min, then to 100 % B over 5 min, and was held constant at 100 % B for an additional 2 min. The flow rate was 0·3 ml/min throughout. The column effluent was directed without splitting into an LCQ Duo quadrupole ion trap mass spectrometer (ThermoFinnigan, San Jose, CA, USA) equipped with an ESI source. For negative-ion-mode experiments, the spray voltage was − 4·5 kV. For metal complexation experiments, a methanolic solution of 5 μm-CoBr2 and 4,7-dpphen was added post-column via a T joint and syringe pump flowing at 20 μl/min. The positive-ion mode was employed for these experiments, using a spray voltage of +4·5 kV. Collision-induced dissociation (CID) with the He bath gas was used to fragment ions of interest for structural characterisation.
Data analysis
Statistical analyses were performed using the R data analysis software (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). In the comparison of data from the fruit and juice cross-over study, repeated-measures models were used to analyse the data and ‘volunteer’ was included as a ‘random effect’. Regression diagnostics were checked to determine if data transformations, outlier omissions, or alternative non-parametric models were required. All results from the models were considered significant if P < 0·05. When the metabolite data were split into their two separate components (naringenin and hesperetin) and analysed for differences in fruit v. juice, a Wilcoxon rank sum test was used. Again, results were considered significant if P < 0·05. For the single portion juice intervention, the urinary excretion data were analysed using standard ANOVA models. For all models, regression diagnostics were checked to determine if data transformations, outlier omissions, or alternative non-parametric models were required. All results from the models were considered significant if P < 0·05.
Results
Comparison of flavanone absorption and excretion from orange fruit and juice
In a randomised cross-over study, twenty volunteers ingested orange juice and orange fruit segments and the appearance of flavanones in plasma and urine was measured. The oranges used in the interventions contained 11·8 (sd 5·5) mg naringenin (as narirutin) and 79·7 (sd 17·7) mg hesperetin (as hesperidin)/150 g portion. The orange juice used in the interventions contained 9·4 (sd 0·7) mg naringenin (as narirutin) and 71·8 (sd 8·1) mg hesperetin (as hesperidin)/300 g portion. The fruits and juices also contained small amounts of didymin (data not shown). The stereochemical nature of the flavanones was not determined directly, but it is usual for citrus flavanones to be present as the 2S conformer.
Plasma and urine samples were analysed for total flavanone aglycones (naringenin and hesperetin) following hydrolysis with aryl sulfatase and β-glucuronidase. Flavanones were absent from all baseline samples, indicating good compliance with the flavonoid avoidance diet. Low concentrations of flavanones were detected in plasma within 15 min of ingestion of the fruit or juice. The pharmacokinetic profile for total flavanones in plasma was similar for both naringenin and hesperetin; a small peak at 1–1·5 h followed by the major peak at about 6 h with a subsequent decline to near baseline over the next 48 h (Fig. 2). The absolute mean peak plasma concentration (Cmax) and area under the plasma concentration time curve (AUC) for hesperetin were significantly higher than that for naringenin for both the fruit and juice matrices (Table 1). However, since the hesperetin oral dose was substantially lower (about 7-fold) than the naringenin dose, naringenin was more efficiently absorbed to the plasma than hesperetin on a dose-adjusted basis. The mean time to reach Cmax was greater for hesperetin compared with naringenin but the effect was not significant. Urinary excretion (% of oral dose) of naringenin was greater than for hesperetin for both the juice and fruit matrices, which reflects the more efficient absorption of naringenin to plasma. There were no significant differences between fruit and juice in the urinary yields (% of dose excreted) for hesperetin or naringenin. For all the parameters described (Cmax, time to peak concentration, AUC, urinary yield), the individual variation was substantial and the variance was high (see Table 1). The stereochemical nature of the flavanones in plasma and urine was not determined.

Fig. 2 Plasma pharmacokinetic profiles for orange flavanones following consumption of oranges. In a randomised cross-over design, twenty subjects consumed 300 g orange juice (a) and 200 g orange fruit (b) and total naringenin (○) and hesperetin (●) were measured following hydrolysis of samples with glucuronidase and aryl-sulfatase. Data are means (n 20), with standard deviations represented by vertical bars.
Table 1 Plasma pharmacokinetic data, urinary excretion data and statistical parameters
(Mean values and standard deviations)

Cmax, peak plasma concentration; Tmax, time to peak plasma concentration; AUC, area under the plasma concentration time curve; NT, not tested.
* P values obtained using the Wilcoxon rank sum test.
† Calculated only for urinary excretion in the n 129 subject group.
‡ Naringenin and hesperetin doses were calculated from the narirutin and hesperidin content of the fruit or juice.
§ Data represent the total flavanone in plasma determined following enzyme hydrolysis (Mr naringenin and hesperetin are 272·3 and 302·3 g/mol, respectively).
∥ These data include the twenty volunteers consuming both fruit and juice and an additional 109 volunteers who consumed only juice.
The observed individual variation in absorption and excretion parameters detailed in Table 1 was investigated further. First, the association between parameters was compared as individual paired dose-adjusted data (n 20). The correlation (Spearman's) between AUC and Cmax was strong for naringenin from juice (R 2 0·88) and fruit (R 2 0·74), and hesperetin from juice (R 2 0·73) and fruit (R 2 0·92) (all P values < 0·01). The correlations between percentage urinary excretion and AUC or Cmax were weaker for both hesperetin and naringenin for both matrices (R 2 values 0·00–0·45 (P>0·05) in all cases except for naringenin from fruit).
Urinary excretion of orange flavanones in an extended study investigating the relationship with various subject characteristics
The urinary excretion of flavanones from orange juice was extended to include a further 109 volunteers aged between 18 and 80 years to produce a large dataset suitable for evaluating the effects of volunteer anthropometric and other variables. For the extended study population, the excretion of naringenin and hesperetin was 14·5 (sd 11·9) and 3·9 (sd 3·6) % (n 129), respectively. The range for naringenin was 1·6–59·0 % (37-fold), with a median of 9·9 %. The range for hesperetin was between not detected (three volunteers) to 25·4 %, with a median of 3·1 %. The naringenin:hesperetin ratio was 4·4 (sd 2·8) (range 0·7–13, median 3·7).
The urinary excretion data were fitted to a standard linear model (ANOVA) with log (percentage excretion) as the response and several subject variables (age, BMI, sex, contraceptive pill use amongst the female volunteers) as the explanatory variables. After systematically removing non-significant terms and re-fitting the data, a possible effect of age on excretion of hesperetin (P = 0·033) and naringenin (P = 0·058) was detected. For naringenin, the apparent effect of increasing age was to decrease excretion in males but increase excretion in females. However, separation of the data into males and females did not indicate a significant effect of age. For hesperetin, the effect of increasing age was to decrease excretion, although the magnitude of the effect was very small – equivalent to a 4 % decrease in urinary excretion between the ages of 20 and 80 years (Fig. 3). There were no significant effects of sex, BMI or contraceptive pill use on the excretion of either flavanone (Table 1).
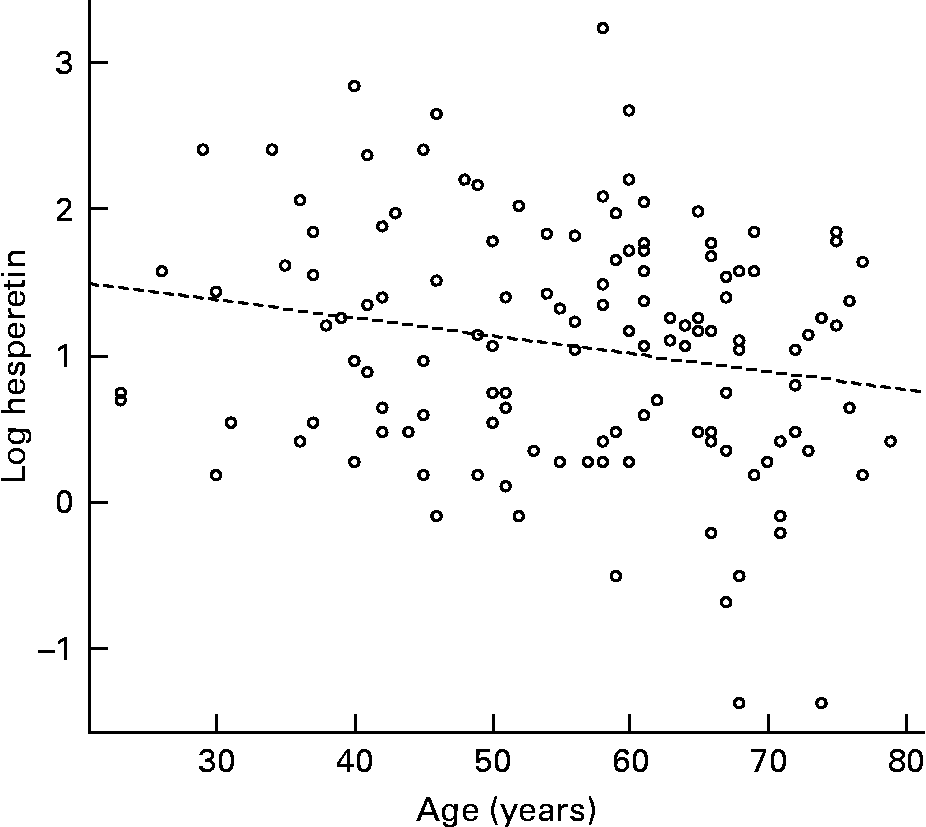
Fig. 3 Relationship between urinary excretion (% oral dose) of orange flavanones from juice and subject age.
Structural identification of flavanone metabolites in plasma and urine
The urine of a typical volunteer, analysed by reverse-phase chromatography, and monitored at 270 nm, is shown in Fig. 4. The sample was simultaneously analysed by full scan positive- and negative-ion ESI-MS. Data for labelled peaks, which were absent from a baseline chromatogram, are summarised in Table 2. Each displayed [M+H]+ and [M − H]− ions consistent with a glucuronidated or sulfated flavanone, and each was removed by a combined glucuronidase and sulfatase treatment. Hence the labelled components were tentatively identified as follows: A and B, naringenin monoglucuronides; C and D, hesperetin monoglucuronides; E, a hesperetin monosulfate. Component A was confirmed as naringenin 7-glucuronide by comparison with an authentic synthetic standard(Reference Davis, Needs, Kroon and Brodbelt29). The UV spectra of components B to E were then examined; all were consistent with a flavanone structure. Naringenin-7-glucuronide (A) and C exhibited very similar spectra (Table 1 and Fig. 2), strongly suggesting that C was also a 7-substituted flavanone; from this, C was provisionally identified as hesperetin 7-glucuronide. D and E exhibited very similar spectra, suggesting that they were both substituted in the same position. (We have observed that the UV spectra of, for example, quercetin glucuronides and sulfates with the same glucuronidation pattern display almost identical UV profiles (PW Needs and PA Kroon, unpublished results).) B displayed its own distinctive spectrum. 5-Substituted flavonoid metabolites have not been reported; and if 5-substution is discounted, B must be naringenin 4′-glucuronide; and hence D and E are hesperetin 3′-glucuronide and hesperetin 3′-sulfate respectively.
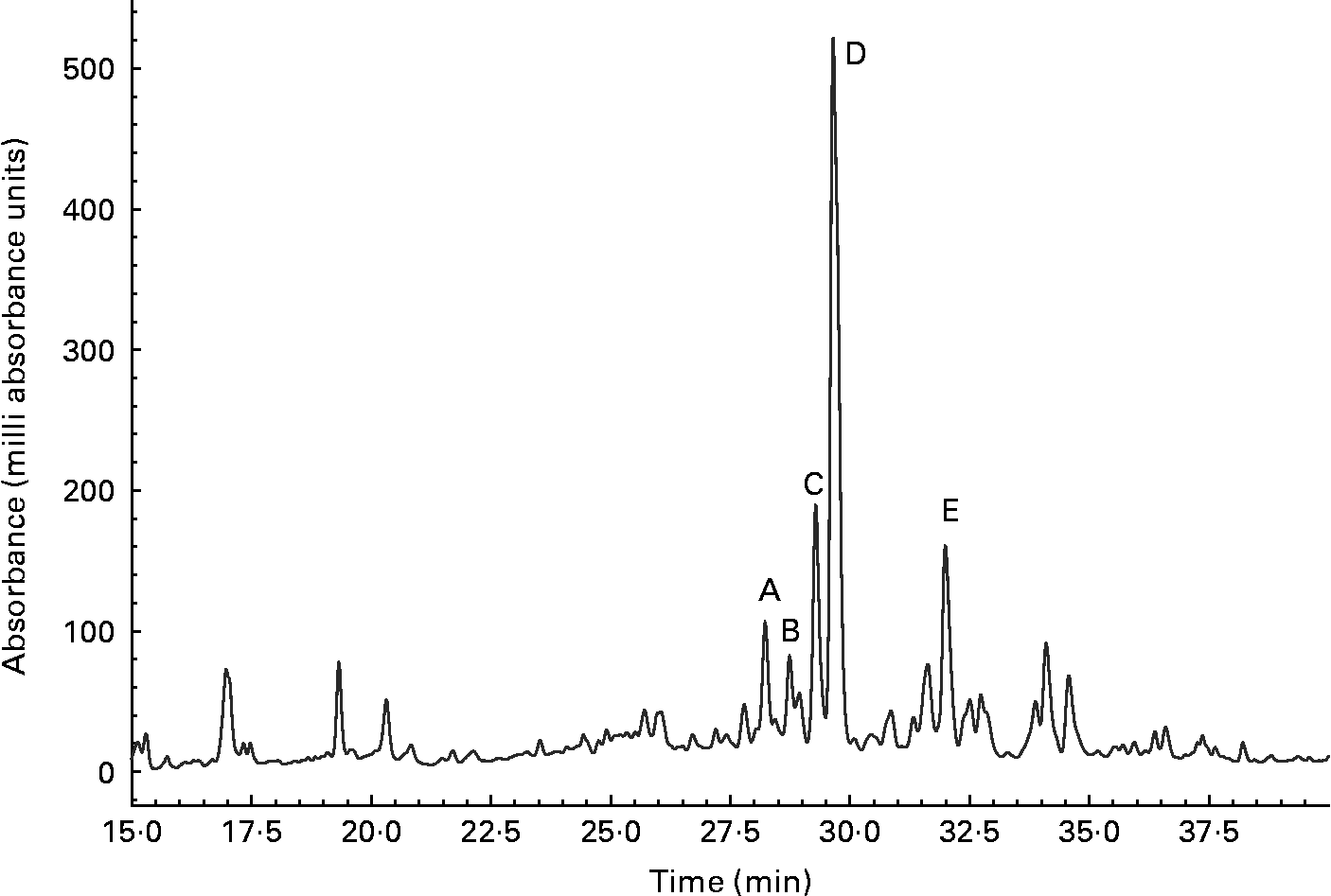
Fig. 4 Flavanone metabolites in urine detected at 270 nm. A, Naringenin 7-glucuronide; B, naringenin 4′-glucuronide; C, hesperetin 7-glucuronide; D, hesperetin 3′-glucuronide; E, hesperetin 3′-sulfate.
Table 2 Characterisation of flavanone plasma and urinary metabolites

These assignments were independently confirmed using LC-MSn techniques as well as recently discovered metal complexation techniques(Reference Davis, Needs, Kroon and Brodbelt29). Negative-ion-mode analysis of components A (retention time (RT) = 11·7 min) and B (RT = 12·9 min) revealed the masses of both of these compounds to be 448 Da. In each case, CID led to the loss of 176 Da, corresponding to a glucuronic acid moiety and giving the product of m/z 271. The second-generation fragment ions stemming from m/z 271 are shown in Figs. 5 (a) and (b), and match the fragmentation pattern of deprotonated naringenin (Fig. 5 (c)). Post-column metal complexation was then performed as described in the Materials and methods section in order to provide metal complexes of the target compounds that are useful for pinpointing the specific sites of glucuronidation(Reference Davis, Needs, Kroon and Brodbelt29). The fragmentation of the metal complexes of A and B are shown in Figs. 5 (d) and (e). The former shows a fragmentation pattern highly indicative of glucuronidation at the 7 position based on characteristic losses such as those of the aglycone group and of the glucuronic acid moiety. However, the latter pattern is consistent with B-ring glucuronidation, as the only fragment ions observed stem from the loss of an auxiliary ligand, with or without the glucuronic acid moiety. The only possible site for B-ring glucuronidation on naringenin is the 4′ hydroxyl group. Hence A was identified as naringenin 7-glucuronide and B as naringenin 4′-glucuronide. Similar evidence was used to identify unknowns C (RT = 14·9 min) and D (RT = 17·8 min). Negative-mode ESI-LC-MS revealed two compounds of mass 478 Da (based on the formation of deprotonated molecular ions at m/z 477) that fragmented to yield a single neutral loss of 176 Da, corresponding to the glucuronic acid moiety. The second-generation fragment ions from subsequent MSn experiments are indicative of hesperetin (Figs. 6 (a)–(b)), as revealed based on comparison with the fragmentation pattern of deprotonated hesperetin (Fig. 6 (c)). Fragmentation of the metal complex of C indicates a glucuronic acid moiety at the 7 position (Fig. 6 (d)), whereas the metal complex of D indicates B-ring glucuronidation (Fig. 6 (e)), i.e. at the 3′ position of hesperetin. Thus C was positively identified as hesperetin 7-glucuronide and D as hesperetin 3′-glucuronide. E was partially identified as a hesperetin sulfate based on its mass (382 Da), the loss of 80 Da upon fragmentation (corresponding to SO3), and second-generation fragment ions that mirror those of hesperetin (data not shown). To our knowledge, there is no purely MS-based method for determining the location of sulfate groups on flavonoids.

Fig. 5 Mass spectra used in identifying naringenin glucuronides. (a) Component A, MS3 447 → 271 → ; (b) component B, MS3 447 → 271 → ; (c) naringenin standard, MS/MS 271 → ; (d) [Co(II) (A–H) (4,7-diphenyl-1,10-phenanthroline)2]+, MS/MS 1170 → ; (e) [Co(II) (B–H) (4,7-diphenyl-1, 10-phenanthroline)2]+, MS/MS 1170 → . Aux, auxiliary ligand 4,7-diphenyl-1,10-phenanthroline; Agl, aglycone portion of flavonoid; GlcA, glucuronic acid moiety.

Fig. 6 Mass spectra used in identifying hesperetin glucuronides. (a) Component C, MS3 477 → 301 → ; (b) component D, MS3 477 → 301 → ; (c) hesperetin standard, MS/MS 301 → ; (d) [Co(II) (C–H) (4,7-diphenyl-1,10-phenanthroline)2]+, MS/MS 1200 → ; (e) [Co(II) (D–H) (4,7-diphenyl-1, 10-phenanthroline)2]+, MS/MS 1200 → . Aux, auxiliary ligand 4,7-diphenyl-1,10-phenanthroline; Agl, aglycone portion of flavonoid; GlcA, glucuronic acid moiety.
In addition to the above compounds, which were also discovered in the previously described LC-UV-MS analysis, the LC-MSn analysis additionally revealed several di-substituted metabolites in the urine samples. A hesperetin glucuronide sulfate (RT = 16·6 min) was identified based its mass (558 Da), neutral losses of 80 Da and 176 Da upon CID, and an aglycone fragmentation signature matching that of hesperetin. Surprisingly, metal complexation was able to reveal the location of the glucuronic acid moiety of this compound. The metal complexation technique was developed originally for the characterisation of flavonoid monoglucuronides, but the hesperetin glucuronide sulfate also formed a metal complex of the correct stoichiometry, observed at m/z 1280 in the positive-mode ESI mass spectrum. The first stage of CID led to the loss of the sulfate group ( − 80 Da), effectively resulting in a metal complex of a flavonoid monoglucuronide, which can be characterised based on its fragmentation pattern as described for the other compounds above. The second stage of fragmentation, using m/z 1200 as the precursor ion, yielded a CID mass spectrum similar to that shown in Fig. 6 (d). This strongly suggests that the glucuronic acid moiety of this hesperetin glucuronide sulfate is located at the 7 position. A similar strategy has been used previously to partially identify diglycosyl flavonoids while employing metal complexes designed to characterise monoglycosyl flavonoids(Reference Davis and Brodbelt30). Finally, a few diglucuronidated flavonoids were found in the urine samples. Two early-eluting components (RT = 4·8 and 9·9 min) were identified as hesperetin diglucuronides. These analytes both had a mass of 654 Da, exhibited two sequential losses of glucuronic acid ( − 176 Da), and yielded fragmentation patterns of the aglycone group that corresponded to hesperetin. In addition, there was at least one other low-intensity compound resembling a naringenin diglucuronide that appeared in only a few urine samples. This analyte had a mass of 624 Da, had an RT of 1·9 min, and lost two glucuronic acid moieties upon sequential stages of CID. However, the low abundance of this analyte combined with signal losses due to scattering during each stage of fragmentation led to an MS4 spectrum with a poor signal:noise ratio. While there is evidence of naringenin aglycone, the spectrum is too noisy to make a conclusive identification. Metal complexes could not be formed for any of the purported naringenin or hesperetin diglucuronides, possibly due to low abundance of these compounds.
Discussion
A number of questions have been addressed in the present report. First, what is the ‘exposure’ following ingestion of typically consumed portions of orange fruit and orange juice? Second, are there differences between orange fruit and orange juice in the absorption and excretion of flavanones? Third, what is the inter-individual variation in absorption and excretion and how much of the observed variation is due to differences in subject variables such as age, sex, BMI and use of oral contraceptives? Finally, what is the nature of the flavanones in plasma and urine? Methods appropriate for the quantification of flavanones in human plasma and urine were used to measure the absorption and urinary excretion of orange flavanones in volunteers following a single (physiological) dose of orange juice or orange fruit. The resulting data show that both orange fruit segments and commercial orange juice provide reasonable quantities of flavanones (as 7-rutinosides of hesperetin and naringenin), and that the flavanone aglycones can be recovered in plasma and urine following hydrolysis with a mixture of glucuronidase and sulfatase. For the first time, we provide evidence to show there is no significant difference between fresh fruit and a commercial processed juice in the bioavailability of the component flavanones. Following consumption of either a portion of fruit or a glass of juice (equivalent to one medium orange or a large glass of juice), the mean peak plasma concentrations of hesperetin and naringenin were all in the range 0·05–0·10 μmol/l (Table 1). The time to reach Cmax was 6–7 h for both the fruit and the juice. This indicates that orange flavanones are absorbed predominantly from the large intestine, and not from the small intestine, in agreement with previously reported studies(Reference Erlund, Meririnne, Alfthan and Aro26, Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27).
In a previous report, Manach et al. (Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27) reported the oral bioavailability of flavanones for two doses of orange juice, both large compared with that used in the study reported here. Although only two doses were tested, the data suggest there may be a threshold effect since the plasma concentrations achieved were not linear with dose: Instead, there was a 3-fold increase in the peak plasma concentration from the 2-fold higher dose for both naringenin and hesperetin. The data presented in this report add support to the notion of a threshold effect for hesperetin from oranges. The concentration of hesperetin in the fruits and juices used in the study described here (Table 1) were similar to those in the juice used in a study reported previously(Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27). But, the mean plasma concentrations observed in the present study with doses of hesperetin 3·3- and 1·6-fold lower than reported by Manach et al. (Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27) were 13- and 5-fold lower, respectively. For naringenin, the peak plasma concentration in the present study (dose = 300 ml orange juice) was similar to that reported by Manach et al. for a 500 ml dose. Regardless of the nature of the oral dose–Cmax relationship, the data presented here indicate that the maximum exposure for a typically consumed portion of orange juice or fruit, described by the Cmax or AUC, is rather low compared with the concentrations of flavanones that have been required to elicit responses in cell and animal models, which is generally 1–100 μm(Reference So, Guthrie, Chambers, Moussa and Carroll15, Reference Borradaile, Carroll and Kurowska32–Reference Jin, Han, Zhang, Lee, Lim, Chung and Yun38). Previously published reports describing the feeding of orange flavanones to animals have shown that high oral doses can be used to achieve the concentrations reported to elicit responses in vitro (Reference Felgines, Texier, Morand, Manach, Scalbert, Regerat and Remesy39, Reference Silberberg, Morand, Mathevon, Besson, Manach, Scalbert and Remesy40). Further, it is possible to increase the bioavailability of citrus flavanones by treating the juices with rhamnosidase which leads to cleavage of the rhamnose moiety from naringin and hesperedin to yield flavanone monoglucosides with improved bioavailabilities(Reference Nielsen, Chee, Poulsen, Offord-Cavin, Rasmussen, Frederiksen, Enslen, Barron, Horcajada and Williamson41).
One of the main objectives of these studies was to determine the inter-individual variation with respect to absorption and excretion of flavanones from a physiologically relevant oral dose, and to try and identify factors that contribute to the observed variation. The between-subject variation we observed was strikingly large for all the parameters that estimate the amount absorbed to plasma or excreted (plasma Cmax, AUC, urinary excretion). In an extended study in which we quantified the urinary excretion of flavanones from a single 300 g oral dose of orange juice in 129 subjects, urinary excretion ranged from not detected ( < 0·1 %) to 25·4 % of the hesperetin dose, and from 1·6 to 59 % of the naringenin dose. Previously reported studies concerned with flavanone bioavailability have presented data as mean parameters along with the standard error of the mean or the standard deviation(Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27, Reference Nielsen, Chee, Poulsen, Offord-Cavin, Rasmussen, Frederiksen, Enslen, Barron, Horcajada and Williamson41), and have not reported ranges or discussed the individual variation. A report by Erlund et al. (Reference Erlund, Meririnne, Alfthan and Aro26) is an exception; the authors note the large inter-individual differences in Cmax and AUC and indicate the observed ranges for these parameters (for example, Cmax varied 12-fold between the eight volunteers). In fact, the previously reported studies with orange flavanones have involved very limited numbers of subjects (less than ten) and often from a very limited age range (for example, Manach et al. (Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27); five men of very similar age, weight and BMI). In contrast, our data have been derived from a very large pool of male and female subjects (n 129) that cover a broad range of ages (23–79 years), heights (1·50–1·89 m), weights (50·3–112·5 kg) and BMI (22·5–34·7 kg/m2). However, when we examined the data, we found no effect of age, sex or BMI on the absorption (Cmax, AUC) or urinary excretion parameters (Table 1).
The absorption of flavonoid rutinosides such as hesperidin, naringin and rutin (quercetin rutinoside) takes place from the large intestine (time to peak plasma concentration about 6 h), in contrast to flavonoid glucosides such as quercetin glucosides from onions and pelargondin-3-glucoside from strawberries that are absorbed predominantly from the small intestine (time to peak plasma concentration about 1 h)(Reference Hollman, Bijsman, van Gameren, Cnossen, de Vries and Katan42–Reference Hollands, Brett, Dainty, Teucher and Kroon44). This is because the human small intestine contains β-glucosidases capable of hydrolysing flavonoid glucosides but not rutinosides (rhamnoglucosides)(Reference Nemeth, Plumb, Berrin, Juge, Jacob, Naim, Williamson, Swallow and Kroon45, Reference Berrin, Czjzek, Kroon, McLauchlan, Puigserver, Williamson and Juge46). Therefore, the rutinosides cannot be hydrolysed until these compounds reach the colon and are exposed to α-rhamnosidases originating from certain of the resident bacteria. Therefore, a likely source of variation in the absorption of citrus flavanones is the composition of the gut microbiota which can give rise to different levels of α-rhamnosidase and β-glucosidase hydrolysing activities, and also flavonoid-degrading capabilities. The reported inter-subject variation in plasma pharmacokinetic data for the aglycones hesperetin and naringenin are small(Reference Kanaze, Bounartzi, Georgarakis and Niopas47) compared with those reported here and elsewhere(Reference Erlund, Meririnne, Alfthan and Aro26, Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27) for the glycosides, which is indicative of an important role for the flora. Another probable source of variation in the absorption will be differences in the composition of enzymes and transporters in the intestinal epithelial cells. Extensive research using cell and animal models has identified a number of enzymes and transporters in the human gut epithelial cells that interact with flavonoids and flavonoid glycosides and may play a role in their uptake, metabolism and transport across the epithelial cells. These include β-glucosidases (lactase phloridzin hydrolase, cytosolic β-glucosidase(Reference Nemeth, Plumb, Berrin, Juge, Jacob, Naim, Williamson, Swallow and Kroon45, Reference Berrin, McLauchlan, Needs, Williamson, Puigserver, Kroon and Juge48)), the apical Na-dependent GLUT-1(Reference Day, Gee, DuPont, Johnson and Williamson49), apical and basolateral multi-drug resistance protein (MRP) transporters such as MRP-2 and breast cancer resistance protein (BCRP)-1(Reference O'Leary, Day, Needs, Mellon, O'Brien and Williamson50, Reference Sesink, Arts, de Boer, Breedveld, Schellens, Hollman and Russel51) and UDP-glucuronosyl transferase, sulfotransferase and catechol-O-methyltransferase conjugating enzymes(Reference O'Leary, Day, Needs, Mellon, O'Brien and Williamson50, Reference Boersma, van der Woude, Bogaards, Boeren, Vervoort, Cnubben, van Iersel, van Bladeren and Rietjens52). Since dietary flavonoid glycosides appear in plasma as glucuronidated and sulfated conjugates of the flavonoid aglycone or its methylated derivatives, and not as the original glycoside, it is clear that certain enzyme activities (β-glucosidase, UDP-glucuronosyl transferase, sulfotransferase) are essential in the absorption process. However, the identity of the specific proteins involved is currently not clear, although the evidence for a key role for lactase is substantial(Reference Nemeth, Plumb, Berrin, Juge, Jacob, Naim, Williamson, Swallow and Kroon45). Individual differences in the ability of the enterocytes to hydrolyse β-glucosides, transport β-glycosides, glucuronidate, sulfate or methylate flavonoids, and efflux flavonoid phase-2 conjugates would probably have profound effects on the absorption of flavonoids from the gut.
Finally, we have reported, for the first time, the structure of the naringenin and hesperetin conjugates in human plasma. The type and degree of conjugation and the conjugation position(s) are known to have dramatic effects on the biological activity of flavonoids, yet there is a paucity of information as to the absolute structure of these metabolites in humans following their absorption from the gut(Reference Kroon, Clifford, Crozier, Day, Donovan, Manach and Williamson28). Although previous reports have provided evidence that, following ingestion of citrus products containing flavanone glycosides(Reference Erlund, Meririnne, Alfthan and Aro26, Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Demange and Remesy27, Reference Nielsen, Chee, Poulsen, Offord-Cavin, Rasmussen, Frederiksen, Enslen, Barron, Horcajada and Williamson41) or isolated flavanones(Reference Kanaze, Bounartzi, Georgarakis and Niopas47, Reference Ameer, Weintraub, Johnson, Yost and Rouseff53) by human subjects, the flavanones in plasma and urine are present as a mixture of glucuronides and sulfates, the conjugation positions have not been determined. Using a combination of methods, including synthesis of an authentic standard (naringenin-7-glucuronide) and application of a novel post-column metal complexation tandem MS fingerprinting technique(Reference Davis, Needs, Kroon and Brodbelt29), we have elucidated the specific structure of all four major naringenin and hesperetin monoglucuronide conjugates present in human urine following consumption of orange juice or fruit (Table 2; Figs. 4–6). In addition, we show the presence of a hesperetin monosulfate and several di-substituted compounds. This knowledge should be used to inform the design of future studies concerned with quantifying the biological activities of flavanones using in vitro (for example, cultured cell) models.
In conclusion, we have shown that a proportion of the flavanones from a typically consumed portion of orange fruit or juice are absorbed from the human gut and appear in plasma with mean peak plasma concentrations in the 0·05–0·10 μm range. There was no significant difference in the bioavailability of flavanones from processed long-life orange juice or fresh fruit. The flavanones in plasma and urine were present exclusively as phase-2 conjugates, and the four major metabolites were identified as the 4′- and 7-glucuronides of naringenin and the 3′- and 7-glucuronides of hesperetin. We also report an enormous inter-individual variation in the excretion of orally dosed orange flavanones, and show that this variation is not due to the age, sex or BMI of the subjects, or use of the contraceptive pill.
Acknowledgements
The authors thank all the staff (Aliceon Blair, Yvonne Clements, Trudy Harrison and Ruth Whitlam) at the Human Nutrition Unit for their consistent professionalism and attention to detail in looking after our volunteers. We are very grateful to all our dedicated volunteers for making these studies possible. The present study was funded by the Food Standards Agency (UK). B. D. D. and J. S. B. gratefully acknowledge support from the National Institutes of Health (NIH RO1 GM63512) and the Welch Foundation (F1155). The authors have no conflicts of interest to declare. P. A. K. and B. T. designed the study; G. M. B., W. H., P. W. N., J. S. B. and B. D. D. undertook the interventions and analytical work; J. R. D. performed the statistical analysis; P. A. K. drafted the manuscript and all authors provided input to the final manuscript.










