Article contents
Organic Synthesis and the Unification of Chemistry—A Reappraisal
Published online by Cambridge University Press: 05 January 2009
Extract
Proclaiming Louis Pasteur as the “Founder of Stereochemistry”, the distinguished Scottish chemist, Crum Brown, addressing a late nineteenth-century audience of Edinburgh savants, drew attention—as Pasteur had incessantly done—to the intimate relationship between living organisms and the optical activity of compounds sustaining them. It seemed to Crum Brown “that we must go very much further down in the scale of animate existence than Buridan's ass, before we come to a being incapable of giving practical expression to a distinct preference for one of two objects differing only in being one to the right and the other to the left”. Crum Brown's lecture must have been entertaining, but it was also motivated by a serious desire to do justice to a particular assertion of Pasteur—an assertion which had, moreover, been misunderstood and dismissed by no less a chemical genius than Wilhelm Ostwald. Writing at a time when the majority of his colleagues were stressing the resemblances between inorganic and organic compounds, Pasteur had insisted that he “could not point out the existence of any more profound distinction between the products formed under the influence of life and all others” than that “artificial products have … no molecular asymmetry”. Pasteur was obliged to concede that the chemist might produce enantiomorphic pairs of isomers, but without resorting to a manual separation of crystals he was powerless to imitate Nature's performance, powerless to fabricate by chemical means a separate optical isomer, divorced from its partner. Now it was not only Crum Brown who felt that chemists had brushed aside this proposition of Pasteur. In his 1898 Presidential Address to the chemical section of the British Association, Professor F. R. Japp also complained that the possible vitalistic implications of Pasteur's distinction between natural and artificial products had been misapprehended or tacitly ignored.
- Type
- Research Article
- Information
- Copyright
- Copyright © British Society for the History of Science 1971
References
1 Brown, A. Crum, “Pasteur as the Founder of Stereochemistry”, Revue française d'Edimbourg, 07 1897, 211–226 (217).Google Scholar
2 Ibid., 225.
3 Japp, F. R., “Stereochemistry and Vitalism”, British Association for the Advancement of Science Report, 1898, pp. 813–828.Google Scholar
4 Pasteur, L., Researches on Molecular Asymmetry (1860). Alembic Club Reprint No. 14, pp. 26–46Google Scholar [italics mine].
5 Pasteur, L., C.r. hebd. Séanc. Acad. Sci., Paris, lxxviii (1874). 1516.Google Scholar
Earlier in his career Pasteur had thought that the artificial preparation of racemic mixtures—optically inactive because they contain equal quantities of the active d- and l-forms—would probably elude the chemist. Accordingly, to the suggestion that the malic acid obtained from Dessaignes' artificial aspartic acid might be in the racemoid form, Pasteur had replied that this was “improbable, for then not only should we have made an active body from an inactive one, but we should have made two—a right and a left” (C.r. hebd. Séanc. Acad. Sci., Paris, xxxiii (1851), 217Google Scholar). Not for long though could Pasteur doubt the possibility of artificially prepared racemates. By 1860 Perkin and Duppa had obtained from dibromosuccinic acid a form of tartaric acid which Pasteur acknowledged to be the racemate (Ann. chim. phys. [3], lxi (1860), 484–488 ff.Google Scholar). They had produced active species, albeit in neutralized pairs, from an acid thought to be inactive. Even at this point, however, Pasteur had not been immediately prepared to abandon his principle that optically active compounds could only be procured from prior naturally occurring and optically active substances. Perhaps the succinic acid, the starting point for the racemate, was itself active? If the acid employed by Perkin and Duppa appeared inactive this might be due either to internal compensation (was it perhaps the meso-form?) or to its exhibiting a finite, but immeasurably small, activity (Ann. chim. phys. [3], lxi (1860), 486Google Scholar). In the paper in which Pasteur reviewed the work of Perkin and Duppa, he had nonetheless been sufficiently open-minded to predict that Maxwell Simpson's artificial production of succinic acid from ethylene would help to settle the issue. And this indeed was the case: when, in 1873, Jungfleisch, a student of Berthelot, prepared racemic acid from Simpson's synthetic succinate, Pasteur had been obliged to retreat (Japp, , op. cit. (3), 820).Google Scholar
But he only retreated so far. It had now been demonstrated that the chemist could, from inactive material, prepare inactive pairs of active species. It had not yet been demonstrated by chemical means—as distinct from a manual separation of the enantiomorphs—that individual isomers could be obtained in isolation from their partners. The following proposition, Pasteur maintained with perfect justification, therefore remained intact: “Jusqu'a présent, on n'a jamais formé un corps actif simple à l'aide de corps inactifs” (C.r. hebd. Séanc. Acad. Sci., Paris, lxxviii (1874), 1516).Google Scholar
6 Japp, , op. cit. (3), 823.Google Scholar
7 Wurtz, A., “On oxide of Ethylene, considered as a link between Organic and Mineral Chemistry”, J. Chem. Soc., xv (1862), 387–406.CrossRefGoogle Scholar
8 A Scott-Couper, “On a New Chemical Theory”—translation of a paper presented to the French Academy of Sciences by Dumas, J. B. [C.r. hebd. Séanc. Acad. Sci., Paris, xlvi (1858), 1157–1160]Google Scholar, and reprinted in Benfey, O. T. (ed.), Classics in the Theory of Chemical Combination (Dover, New York, 1963), 132–135Google Scholar. For Couper's presupposing a unified chemistry see Benfey, , 141Google Scholar. Kekulé, A., Annalen der Chemie und Pharmacie, cvi (1858), 129–159CrossRefGoogle Scholar, translated by Benfey, ibid., 109–131.
9 Berzelius, J. J., “Experiments to Determine the Definite Proportions in which the Elements of Organic Nature are Combined”, Annals of Philosophy, iv (1814), 323–331; 401–409.Google Scholar
10 Ibid., 323.
11 Ibid., 328 and 329.
12 Naquet, A., Principles of Chemistry Founded on Modern Theories (translated from 2nd edn. by Cortis, W.), London, 1868, 341.Google Scholar
13 For just one example, see Miller, W. A., Elements of Chemistry, 3 vols., London, 1855–1857, iii, 1.Google Scholar
14 Dumas, J. B., The Faraday Lecture, 06 1869. Chem. News, xx (1869), 6.Google Scholar
15 Ibid., 4.
16 A worthy exception here is Benfey, O. T., From Vital Force to Structural Formulas, Boston, 1964Google Scholar. Benfey recognizes, for example, the unifying rôle of Gerhardt's types, but the very fact that he can assert that with these “types” “the break with Berzelius was now complete” (pp. 48 and 49) reveals a certain insensitivity towards the organic-inorganic interface. To suppose that Berzelius was responsible for an absolute barrier which the type theories finally overthrew is a little crude when one remembers that the Swedish chemist frequently acknowledged the existence of compounds like ammonia and calcium phosphate which bridged the two territories. [Cf. Berzelius, J. J., Théorie des Proportions, Paris, 1835, 21–23Google Scholar.] One must also add, as will become clear from the text, that it was Berzelius' regulative principle which, by its ordering the assimilation of organic to inorganic compounds during the 1830's, had already removed the supports of any such barrier.
17 McKie, Even, who questioned the unifying rôle of Wöhler's urea (Nature, cliii (1944), 608–610)CrossRefGoogle Scholar, nevertheless attached considerable importance to the syntheses of Berthelot.
18 Hofmann, A. W., Berichte d. deutsch, chem. Gesell, xv (1882), 3152–3153Google Scholar. Reproduced in Thorpe, , Essays in Historical Chemistry, 2nd ed.London, 1902, 302–303.Google Scholar
19 Jungfleisch, E., Notice sur la vie et les travaux de M. Berthelot, Extrait du Bulletin de la Société Chimique de France, Paris, 1913, 77.Google Scholar
20 Brooke, John H., “Wöhler's urea, and its Vital Force?—A verdict from the Chemists”, Ambix, xv (1968), 84–114.CrossRefGoogle Scholar
21 Gay-Lussac, , Cours de Chimie, Paris, 1828, vol. 11, Lesson 32.Google Scholar
22 For the electrochemical background here see Williams, L. Pearce, Michael Faraday, London, 1965Google Scholar. Russell, C. A., “The Electrochemical Theory of Berzelius”, Annals of Science, xix (1963), 117–126; 127–145.CrossRefGoogle Scholar
23 The Positive Philosophy of Auguste Comte, translated and condensed by H. Martineau, London, 1875, i, 262.Google Scholar
24 Ibid., i, 293. In other respects Comte's review of the urea synthesis was unusually perceptive. As I have suggested before (op. cit. (20), 89–98Google Scholar), a crucial distinction that must be remembered when appraising the significance of early syntheses is that between a direct and an indirect synthesis. Wöhler undoubtedly achieved a complete synthesis of urea, but not by combining its elements directly. The distinction is important because it helps to explain the prevalence of pessimistic assertions about organic synthesis long after 1828: what remained in question was the possibility of direct synthesis from carbon, hydrogen, nitrogen and oxygen alone. Few, if any, historians have drawn attention to this point, and yet to Comte it was critical and crystal clear. Having observed the tendency of his contemporaries to “exhibit the impossibility of reproducing by synthesis vegetable and animal substances”, he asked whether this impossibility might not be “owing to our persisting in an elementary [direct] synthesis when we ought to proceed by an immediate synthesis…” (ibid., i, 261). “The most striking achievement,” he continues, “is that of M. Wöhler, in producing the animal substance urea,” but “he could not have done this if he had tried, according to the common prejudice, to combine directly oxygen, hydrogen, carbon, and azote … Is there any reason to suppose that it is otherwise in any other case?” (ibid.).
25 Cf. Jungfleisch, E., op. cit. (19), 56–80Google Scholar. This account, while giving the facts about Berthelot's syntheses, is not to be trusted when it comes to their interpretation.
26 Jacques, J., Le vitalisme et la chimie organique pendant la première moitié du XIXe siècle. Revue d'Histoire des Sciences, iii (1950), 35–48Google Scholar. According to Berthelot, a mere two or three substances analogous to organic compounds had been prepared prior to his own efforts, and “the majority” of chemists allegedly still regarded all hope of organic synthesis as chimerical. Although Jacques' discussion is itself suspect in parts [cf. Brooke, , op. cit. (20), 86–87Google Scholar] he is surely right to insist that Berthelot was underestimating his predecessors. By 1850, urea (Wöhler), acetic acid (Kolbe), alcohol (Hennell), methane (Melsens), ethane (Kolbe), chloroform, acetone, oxalic acid and many other organic compounds most certainly had been synthesized.
27 Cf. note 24, for the significance of this distinction.
28 Kapoor, S. C., “Dumas and Organic Classification”, Ambix, xvi (1969), 20.Google Scholar
29 Berthelot, M., Leçons sur les méthodes générales de synthèse en chimie organique, Paris, 1864, 17. (Italics mine.)Google Scholar
30 Ibid.
31 C. R. hebd. Séanc. Acad. Sci., Paris, lxxviii (1874), 1513.Google Scholar
32 Ibid., 1515.
33 Ibid., 1515–1516.
34 Berthelot, M., Leçons (1864), op. cit., 180 f.Google Scholar
35 Berthelot had prepared formic acid from carbon monoxide, so he took the widespread occurrence of this acid in vegetables as evidence for the participation of carbon monoxide in vegetable syntheses. His only other admissible evidence was a report that experiments of Boussingault had revealed the presence of free carbon monoxide during plant respiration “au moins lorsque cette respiration s'opère d'une manière imparfaite” [Ibid., 181].
36 I owe this point to Dr. Kapoor.
37 Cf. my discussion of this same point in op. cit. (20), 101–103Google Scholar, where I suggest that in exorcizing vital agents from the physiologists' vocabulary tranquil transformations were more influential than drastic syntheses.
38 Berthelot, (1864), op. cit., 182.Google Scholar
39 Ibid., 183. [Italics mine.]
40 Ibid., 181.
41 Thus DrTeich, M., in his invaluable account of “The Historical Foundations of Modern Biochemistry” (Clio Medica, i (1965), 41–57)Google Scholar, though he recognizes the errors in the late Professor McKie's discussion of Wöhler's urea, none the less condones, perhaps a little too readily, a presupposition of McKie's account—namely that vitalist beliefs could eventually collapse under a pile of facts. “The preparation of [urea did reveal],” Dr. Teich insists, “one of those contradictory facts which McKie thinks—and rightly so—helped to banish vitalism not only from organic chemistry but from science as a whole.”
42 It is for this reason that I cannot fully endorse Dr. Lipman's definition of “vitalism”, proferred in his very helpful study of “Vitalism and Reductionism in Liebig's Physiological Thought” (Isis, lviii (1967), 167–185Google Scholar). “We can … define vitalism,” Lipman suggests, “as the belief in the existence of some operating principle which is not found in inorganic nature and which distinguishes a living organism from the physico-chemical world” (p. 168). This is an unassailable definition as far as it goes, but it does seem to do less than justice to the way in which current physiological and physico-chemical concepts may be seen to change, even by a vitalist. For a classification of different vitalist positions with specific reference to the time factor see Kemeny, J. G., A Philosopher Looks at Science, Princeton, 1959, Ch. 12, p. 209.Google Scholar
43 Cf. Magendie, F., Leçons sur les phénomènes physiques de la vie, Paris, 1839, i, 93Google Scholar. Graham, T., “Bakerian Lecture on Osmotic Force”, Medical Times and Gazette, ix (1854), 3–4.Google Scholar
44 The reaction of the physiologists to the urea synthesis is to be examined in a pending historiographical study by Everett Mendelsohn.
45 Du Bois Reymond, E., Reden, ii, 219Google Scholar, as cited by Merz, J. T., A History of European Thought in the Nineteenth Century, Dover edn., New York, 1965, i, 217–218.Google Scholar
46 Odling, W., Lectures on Animal Chemistry, London, 1866, 57.Google Scholar
47 Ibid., 78.
48 Ibid., 55.
49 From their joint paper on uric acid, translated by Hofmann, A. W., The Life Work of Liebig, London, 1876, 89.Google Scholar
50 For this latter example I am indebted to Dr. C. A. Russell who, in an unpublished manuscript entitled “The Beginnings of Synthetic Organic Chemistry”, has abo emphasized the accidential nature of many of the early successes.
51 Berzelius reiterated this principle throughout the 1830's and defended it to the last. Traité de Chimie, 5th ed. (2nd French ed.), trans, by Hoefer, and Esslinger, , Paris, 1845–1850, v, 28.Google Scholar
By adopting the term “regulative principle” I hope to emphasize its presuppositional, heuristic, methodological and selective properties rather than its hypothetical or dogmatic character. The inorganic-organic analogy was not so much a theory about the composition of organic compounds, in general, as a means of discriminating between rival hypotheses for the constitution of particular compounds.
52 Metzger, H., Les Concepts Scientifiques (Paris, 1926), 15–52.Google Scholar
53 Hesse, M. B., Models and Analogas in Science (London, 1963). 75.Google Scholar
54 In 1833, for example, Gay-Lussac, and Dumas, felt no compunction in writing: “Les recherches si variées, les analyses si délicates des chimistes modernes … ont amené la chimie minérale à un degré de perfection qui laisse peu de chose à découvrir aux chimistes qui essaient d'exploiter encore l'étude des diverses combinaisons inorganiques…”, J. de Pharmacie, xix (1833), 93–99.Google Scholar
55 Cf. Berzelius, , “Si nous cherchons à nous créer une idée sur les combinaisons organiques, nous n'avons jusqu' à présent qu'une seule voie dont la sûreté soit incontestable, et qui soit éclairée par les faits sans nombre. Je veux dire que nous devons prendre pour point de départ la comparison des combinaisons inorganiques”. J. de Pharmacie, xix (1833), 618.Google Scholar
56 The róle of analogical argument in the context of chemical discovery has been briefly treated by Farber [Isis, xli (1950), 20Google Scholar] who, although drawing attention to particular nineteenth-century examples of inorganic-organic analogies, has not sufficiently emphasized the systematic way in which such analogies were employed and explored.
57 Cf. Robiquet, (J. de Pharmacie, xx (1834), 493)Google Scholar who made an immediate appeal to the authority of the Swedish chemist in order to crush Dumas' contention that organic compounds were just as dualistic as inorganic species.
58 Cf. Bussy, A., J. de Pharmacie, xxii (1836), 684Google Scholar. Prout, W., Bridgewater Treatise: Chemistry, Meteorology and the Function of Digestion considered with reference to Natural Theology (2nd edn., London, 1834), 417Google Scholar. Of the laboratory simile it has recently been said that the case for a chemical physiology “could hardly have been put more strongly”. Knight, David M., “Reduction in Physiology”, History of Science, v, 139.Google Scholar
59 For a more elaborate defence of this assertion than I have space here to give, the reader is referred to Ch. II of my Cambridge Ph.D. thesis (1969), The Rôle of Analogical Argument in the Development of Organic Chemistry.
60 Comte, A., Martineau, , op. cit. (23), i, 274.Google Scholar
61 Ibid., 258. Thus Comte differed from Berzelius considerably in emphasis, though trivially in principle. Whereas for Berzelius the possibly unity of chemistry was a question to be decided empirically with the aid of his regulative principle, Comte intrepidly proclaimed the irrationality of a divided science (ibid., 253). Whereas Comte advocated a universal dualism that could stand independently of electrochemical theory, Berzelius was undoubtedly committed to a more intimate relationship between the two (cf. ibid., 286).
62 Cf. note 11.
63 Dr. Wightman, among many, has been content to say that “Berzelius remained an uncompromising vitalist to the day of his death”. Wightman, W. P. D., The Growth of Scientific Ideas (London, 1966), 435.Google Scholar
64 Thenard, , Traité de Chimie (4th edn., Paris, 1824), 560 fGoogle Scholar. He and his colleague, Gay-Lussac, had suggested, for example, that a non-nitrogenous compound having an O: H ratio which exceeded that of water was sure to be an acid. If the O: H ratio were identical to that in water then the organic compound would be neutral, and analogous to sugars and gums …
65 This example appeared time and time again throughout Berzelius's works, and it is readily accessible in his Traité de Chimie, v (Paris, 1831), 9 f.Google Scholar
66 Berzelius, J. J., “Essay on the Allotropy of Simple Bodies”, read before the Academy of Sciences, Stockholm, 13 09 1843Google Scholar. A French translation appeared in Revue Scientifique et Industrielle (ed. Quesneville), xv (1843), 137 fGoogle Scholar. There was also an English translation in Taylor, 's Scientific Memoirs, iv (1846), 240–252.Google Scholar
This highly speculative paper has not received the attention it deserves from historians of isomerism. In rather whiggish fashion they have pounced on Berzelius's “spatiar”/structural explanation of this phenomenon, without appreciating the fact that he constantly entertained alternative kinds of explanation. Allotropy became for him not simply a phenomenon analogous to isomerism, but a means of explaining it: Was the carbon, present in two isomeric organic compounds, perhaps in different allotropie states?
67 Liebig, J., Traité de Chimie Organique, trans. Gerhardt, C. F. (Paris, 1840–1844).Google Scholar
68 Liebig, J. and Dumas, J. B., “Note sur l'état actuel de la chimie organique. Comptes Rendus Acad. Sci., v (1837), 567–572.Google Scholar
69 For Bunsen's own view of the significance of what he had isolated, see Ann. chim. phys. [3], viii (1843), 356–362.Google Scholar
As Laurent and Gerhardt were later to show, Bunsen had not in fact isolated the conjectured radical within cacodyl chloride, but rather its dimer. Had Liebig had the privilege of hindsight he would have been obliged to concede that the objection to the radical concept was stronger still!
70 Liebig, , op. cit. (67), i (1840), p. vGoogle Scholar. “Aujourd'hui”, Liebig maintained, “dans l'intérêt de la science, l'admission des radicaux composés est indispensable, et peu nous importe qu'on parvienne à les isoler ou non, pourvu que par eux on arrive au but qu'on s'est proposé en les introduisant. Or, ce qu'on a voulu, atteindre par leur intermédiaire, e'est le moyen d'étendre à certains groupes de corps, les principes qui nous ont guidés dans l'étude de la chimie minérale”. (Italics mine.)
71 Brooke, J. H., “Analogical Argument in the Laurent-Berzelius Correspondence”. Read before the Summer Conference of the British Society for the History of Science, 07 1967Google Scholar. An expanded version of this paper is currently being prepared for publication.
72 (Ed.) Söderbaum, H. G., Jacob Berzelius Bref (Stockholm & Uppsala, 1912–1932), vol. 3, section 7, 180–209Google Scholar. For a succinct account of the Laurent-Berzelius debate in the context of early controversies as to which of the nature or the position of its elements was foremost in the determination of a compound's properties, see Levere, T. H., “Affinity or Structure: An Early Problem in Organic Chemistry”, Ambix, xvii (1970), 111–126.CrossRefGoogle Scholar
73 The earliest animosities had been associated with Laurent's claim that chlorine could play a rôle identical to hydrogen in organic compounds. And this was in defiance of a Berzelius who insisted that chlorine could not even take the place of hydrogen, let alone simulate its function. By 1843, however, the framework of the debate had shifted. Berzelius had now conceded a parallel between acetic and trichloracetic acids, such that chlorine was allowed to occupy the niche vacated by hydrogen: “All one can say for certain”, Berzelius wrote to Laurent, “is that the chlorine occupies the same place as the hydrogen”. (Ber zelius Bref., op. cit. (72), part VII, 188Google Scholar.) Secondly, Laurent was no longer suggesting that chlorine could completely imitate the function of hydrogen: “certainly”, he admitted, “the chlorine introduces modifications into these compounds” (ibid., 184).
74 Laurent, to Berzelius, , 01 1844Google Scholar, ibid., 184 f.
75 Berzelius, to Laurent, , 06 1844Google Scholar, ibid., 208.
76 Cf. Holton, G., “Presupposition in the Construction of Theories”, in (ed.) H. Woolf, Science as a Cultural Force (1964), 97 and 104.Google Scholar
77 Liebig, to Berzelius, , 26 04 1840Google Scholar. Translated by Teich, M., op. cit. (41), 46.Google Scholar
78 Ann. chim. phys. [2] lxxii (1839), 409.Google Scholar
79 Comptes Rendus Acad. Sci., viii (1839), 609–622.Google Scholar
80 It seems probable that the predicaments of Laurent and Gerhardt career-wise were exaggerated both by themselves and by their biographers, Messrs. Gerhardt Jnr., Grimaux, and Tiffeneau. It cannot be denied, however, that their theoretical innovations did meet with resistance, and in France it was not until well into the 1860's that Wurtz was able to teach their new doctrines to students at the Faculty of Medicine in Paris. In the preface to the first edition of his Principles of Chemistry (Paris, 1864)Google Scholar Alfred Naquet complained that the innovations of Laurent and Gerhardt were still not generally taught in the country where they arose. Some three years later he could at last allude to a “revolution in teaching which has taken place at the Faculty of Medicine, where M. Wurtz now inculcates the new views”. [Principles of Chemistry…, translated from 2nd edn., by Cortis, W. (London, 1868), viii.]Google Scholar
81 Leicester, H. M., The Historical Background of Chemistry (New York, 1965), 179.Google Scholar
82 Revue Scientifique et Industrielle (ed. Quesneville), x (1842), 145–218.Google Scholar
83 For a delineation of this range see Ch. VI of my Ph.D. thesis, op. cit. (59) (1969).Google Scholar
84 See the preface which Odling wrote to his translation of Laurent's Chemical Method (London, 1855).Google Scholar
85 Cf. the Williamson-Kolbe debate concerning the formulation of ethers. J. Chem. Soc., vii (1855), 111 f; 122 f.Google Scholar
86 Griffin, J. J., The Radical Theory in Chemistry (London, 1858), 38 and 39.Google Scholar
87 Ibid.
88 Wurtz, A., “Sur la Formation Artificielle de la Glycérine”, Ann. chim. phys. [3], li, 904–101.Google Scholar
89 Ibid., 100.
90 Ibid. The respective modes of preparation were as follows; just as Wurtz compared them: 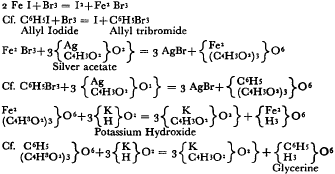
91 Kapoor, S. C., op. cit. (28), 18.Google Scholar
92 Bussy, A., Compte rendu des travaux de la Société de Pharmacie, J. de Pharmacie, xxii (1836), 680–684.Google Scholar
93 Dumas, J. B., Ann. chim. phys. [2], xxxvii (1828), 15–53.Google Scholar
94 Dumas, J. B. and Liebig, J., Note sur l'état actuel de la Chimie Organique. Comptes Rendus Acad. Sci., v (1837), 567–572.Google Scholar
95 One obvious question which still remained open was whether the properties of a complex organic radical could be reduced to those of its constituents. Precisely because this question was not yet resolved, it would be a mistake to suggest that the application of Berzelius's electrochemical theory to organic compounds sufficed to seal a unified chemistry.
96 Revue Scientifique et Industrielle (ed. Quesneville), xiv (1843), 103–104.Google Scholar
97 Comptes Rendus des travaux de Chimie (ed. Laurent & Gerhardt), Sixieme année (1850), 440.Google Scholar
Of his “Introduction …” Gerhardt wrote, “Cet ouvrage est le premier et le seul où la chimie minérale soit exposée d'après un système différent du système dualistique, et où l'on ait fait la fusion complète de la chimie minérale et de la chimie organique.”
98 Traité de Chimie Organique, 4 vols. (Paris, 1853–1856).Google Scholar
99 A. Williamson, Papers on Etherification, Alembic Club Reprint no. 16. Both Laurent and Gerhardt had earlier employed the same water type.
100 Wurtz, A., J. Chem. Soc., xv (1862), 406.Google Scholar
101 Ibid., 387 f.

103 Chemical News, xx (1869), 1–7.Google Scholar
104 Ibid., 4.
105 Ibid., 5.
106 Ibid., 6.
107 Ibid., 5.
What Dumas has in mind here are analogies of regular progression along atomic and molecular weight series, e.g.: 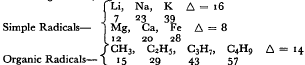
108 Ibid.
109 Medical Times and Gazette, vi (1853), 131 f.Google Scholar
110 Ibid., 132.
111 Ibid., 134.
112 Papillon, J. F., Introduction à l'étude de la philosophie chimique (Paris, 1865), 18.Google Scholar
- 21
- Cited by


