Introduction
Stroke survivors risk developing psychiatric disorders such as anxiety and depression (Burton et al., Reference Burton, Murray, Holmes, Astin, Greenwood and Knapp2013; Hackett & Pickles, Reference Hackett and Pickles2014). Depression occurs in 31% of stroke survivors and is associated with poorer rehabilitation outcomes and increased mortality (Hackett & Pickles, Reference Hackett and Pickles2014; Lincoln, Kneebone, Macniven & Morris, Reference Lincoln, Kneebone, Macniven and Morris2012). Anxiety is also common, with a point prevalence of approximately 25%, and is associated with poorer quality of life and rehabilitation outcomes (Burton et al., Reference Burton, Murray, Holmes, Astin, Greenwood and Knapp2013; Lincoln et al., Reference Lincoln, Kneebone, Macniven and Morris2012). Those with cognitive and communication deficits may be more vulnerable to developing anxiety and depression and experience worse outcomes (Kutlubaev & Hackett, Reference Kutlubaev and Hackett2014; Menlove et al., Reference Menlove, Crayton, Kneebone, Allen-Crooks, Otto and Harder2015; Morris, Eccles, Ryan & Kneebone, Reference Morris, Eccles, Ryan and Kneebone2017).
Stroke Clinical Practice Guidelines internationally have consistently highlighted the need for routine mood screening to ensure early identification and management of anxiety and depressive symptoms (Bowen, James & Young, Reference Bowen, James and Young2016; Eskes et al., Reference Eskes, Lanctôt, Herrmann, Lindsay, Bayley, Bouvier and Green2015; Jolliffe, Lannin, Cadilhac & Hoffmann, Reference Jolliffe, Lannin, Cadilhac and Hoffmann2018; Stroke Foundation of New Zealand, 2010). However, the implementation of these training programmes has been variable. In the United Kingdom, staff training resources have been developed to support the implementation of mood screening as routine practice (Kneebone, Baker & O'Malley, Reference Kneebone, Baker and O'Malley2010; Morris, Jones, Wilcox & Cole, Reference Morris, Jones, Wilcox and Cole2012). Consequently, data from the most recent national stroke audit reveals that 90% of stroke patients receive mood screening before discharge from hospital (Sentinel Stroke National Audit Programme, 2017). In contrast, in Australian stroke services, where training pathways are not commonplace, screening compliance is low (Stroke Foundation, 2017), ranging from only 28% of acute inpatients (Stroke Foundation, 2019) to 53% of subacute rehabilitation patients (Stroke Foundation, 2018).
The literature highlights a number of potential barriers to post-stroke mood screening, including, a lack of clarity around responsibility for screening, workload pressures, inadequate screening knowledge, low self-efficacy and poor awareness of screening guidelines (Baker, Worrall, Rose & Ryan, Reference Baker, Worrall, Rose and Ryan2021). There is also a significant gap between the care needs of patients and clinical resources, most notably psychologists available to support psychological health in people with stroke (Baker et al., Reference Baker, Worrall, Rose, Hudson, Ryan and O’Byrne2018; Baker et al., Reference Baker, Worrall, Rose and Ryan2021; Ryan, Bohan & Kneebone, Reference Ryan, Bohan and Kneebone2019). These findings highlight the demand for all professions from the multidisciplinary team to receive adequate support and training in mood screening.
Currently there is no gold standard protocol for mood screening in stroke patients. However, the screening training pathways evaluated in the UK provide a logical template, as they are well regarded by health professionals and improve screening rates (Kneebone et al., Reference Kneebone, Baker and O'Malley2010; Morris et al., Reference Morris, Jones, Wilcox and Cole2012). The most recent Australian training evaluation provides encouraging, albeit mixed, results. While social workers significantly increased screening rates in a sub-acute rehabilitation setting, training occupational therapists in an acute setting encountered a number of barriers to screening including low self-efficacy, lack of time and privacy on the wards (McLean, Torkington & Ratsch, Reference McLean, Torkington and Ratsch2019). These findings support the need for further studies on the feasibility and acceptability of mood screening training programmes and pathways, that consider a range of structural, organisational and provider factors, before training can be administered on a wider scale (Baker et al., Reference Baker, Worrall, Rose and Ryan2021; McLean et al., Reference McLean, Torkington and Ratsch2019; Ryan et al., Reference Ryan, Bohan and Kneebone2019).
Feasibility studies are a necessary and cost-saving step when evaluating interventions as their results help determine which elements of the study’s intervention, methodology and design are achievable and worth investigating (Arain, Campbell, Cooper & Lancaster, Reference Arain, Campbell, Cooper and Lancaster2010; Lancaster, Dodd & Williamson, Reference Lancaster, Dodd and Williamson2004; Thabane et al., Reference Thabane, Ma, Chu, Cheng, Ismaila, Rios and Goldsmith2010). Most importantly, feasibility trials help researchers evade faults in the methodological design of their studies and ultimately reduce research waste (Arain et al., Reference Arain, Campbell, Cooper and Lancaster2010; Lancaster et al., Reference Lancaster, Dodd and Williamson2004; Thabane et al., Reference Thabane, Ma, Chu, Cheng, Ismaila, Rios and Goldsmith2010). Further, the literature highlights the importance of acknowledging stakeholder’s preferences and recommends evaluating acceptability of the intervention as best practice in feasibility studies (Lancaster et al., Reference Lancaster, Dodd and Williamson2004). In this study, the Mood Assessment Post-Stroke (MAPS) training was piloted with the MDT of a sub-acute inpatient rehabilitation MDT. The participating service was approached by the researchers because of their historically low mood screening rates (Stroke Foundation, 2016) and their interest in establishing mood screening as part of routine clinical care.
Aims and objectives
The overarching objective of this study was to assess the feasibility and acceptability of administering and evaluating the “MAPS” training within an inpatient rehabilitation setting.
Specifically, this study investigated the feasibility of:
-
a. Recruitment.
-
b. The modality and strategy of the training.
-
c. Data collection – i.e., assessing trainee knowledge and skills.
Methods
Study design
This study utilised a concurrent mixed methods design (Creswell, Plano Clark, Gutmann & Hanson, Reference Creswell, Plano Clark, Gutmann and Hanson2003) involving quantitative data collection immediately followed by qualitative data collection.
Participants
All 28 health professionals who routinely worked on a sub-acute inpatient rehabilitation service at a major metropolitan hospital in Sydney, Australia, were invited to participate in the training using convenience sampling.
Ethical considerations
This project was approved by the relevant Human Research Ethics Committees of the participating hospital (HREC REF NO. 2018/ETH00669) and universities (HREC REF NO. 2019002456). All participants provided written consent to participate in this study.
The ‘Mood Assessment Post-Stroke’(MAPS) training
Through a combination of didactic lecture and interaction (e.g., role play, group discussion), the MAPS training pathways delivered evidence-based information about depressive and anxiety disorders following stroke, including the rationale for and how to undertake routine mood screening. Subsequently, participants were shown how to administer screening instruments for patients with or without cognitive/communication impairment and utilise the screening protocol decision making aides (see Figs. 1–4). The training ended with suggested short-term care strategies to support patients experiencing anxiety and depressive symptoms. Details on the development of the training, the screening instruments used, and other training materials are reported on, following the Template for Intervention Description and Replication (TIDieR) checklist and guide (Hoffmann et al., Reference Hoffmann, Glasziou, Boutron, Milne, Perera, Moher and Johnston2014) (See Appendix 1).
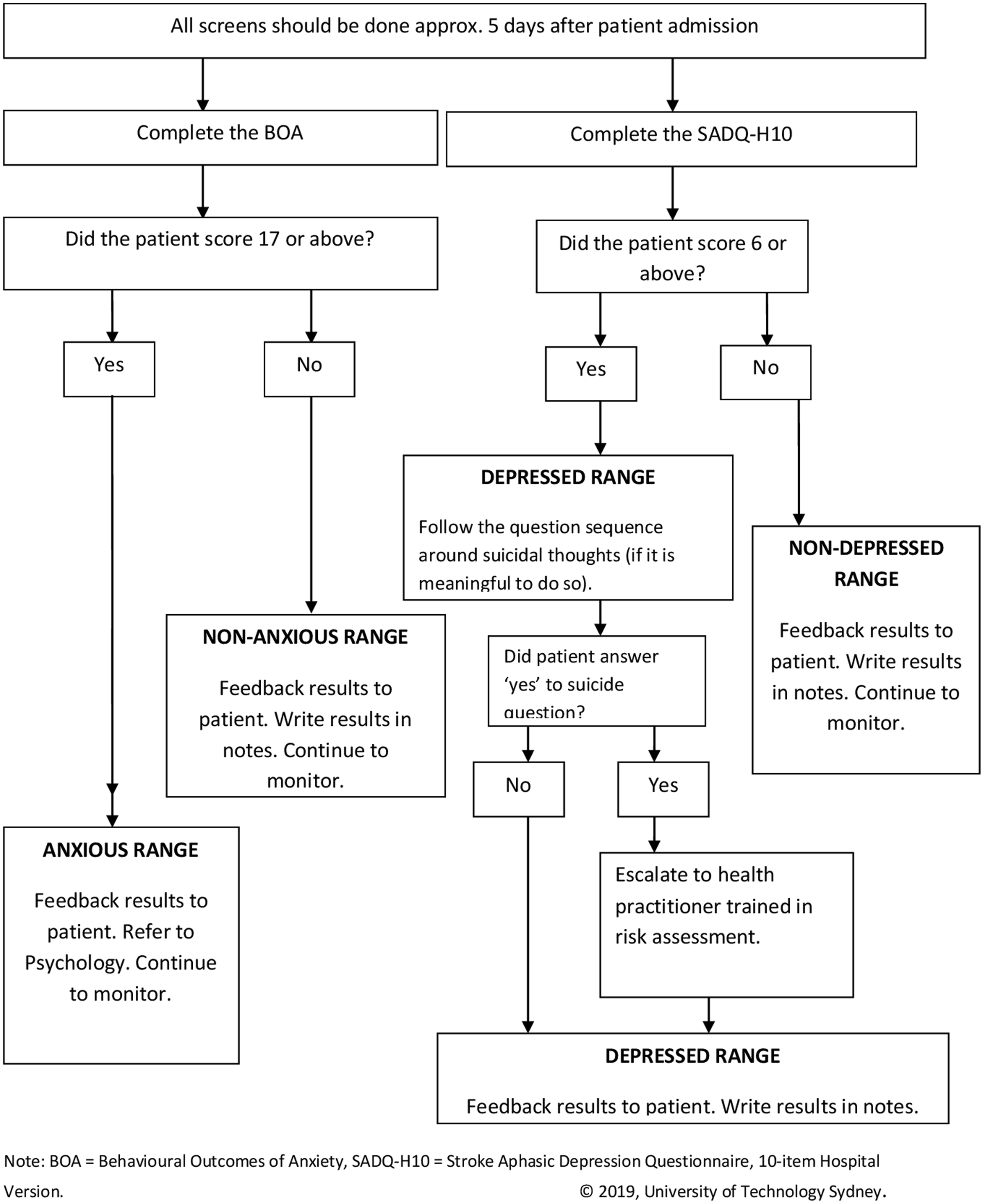
Figure 1. Screening protocol for those with a significant cognitive and/or communication disability.

Figure 2. Screening protocol for those without a significant cognitive or communication disability.

Figure 3. Specific phobia screening questions.
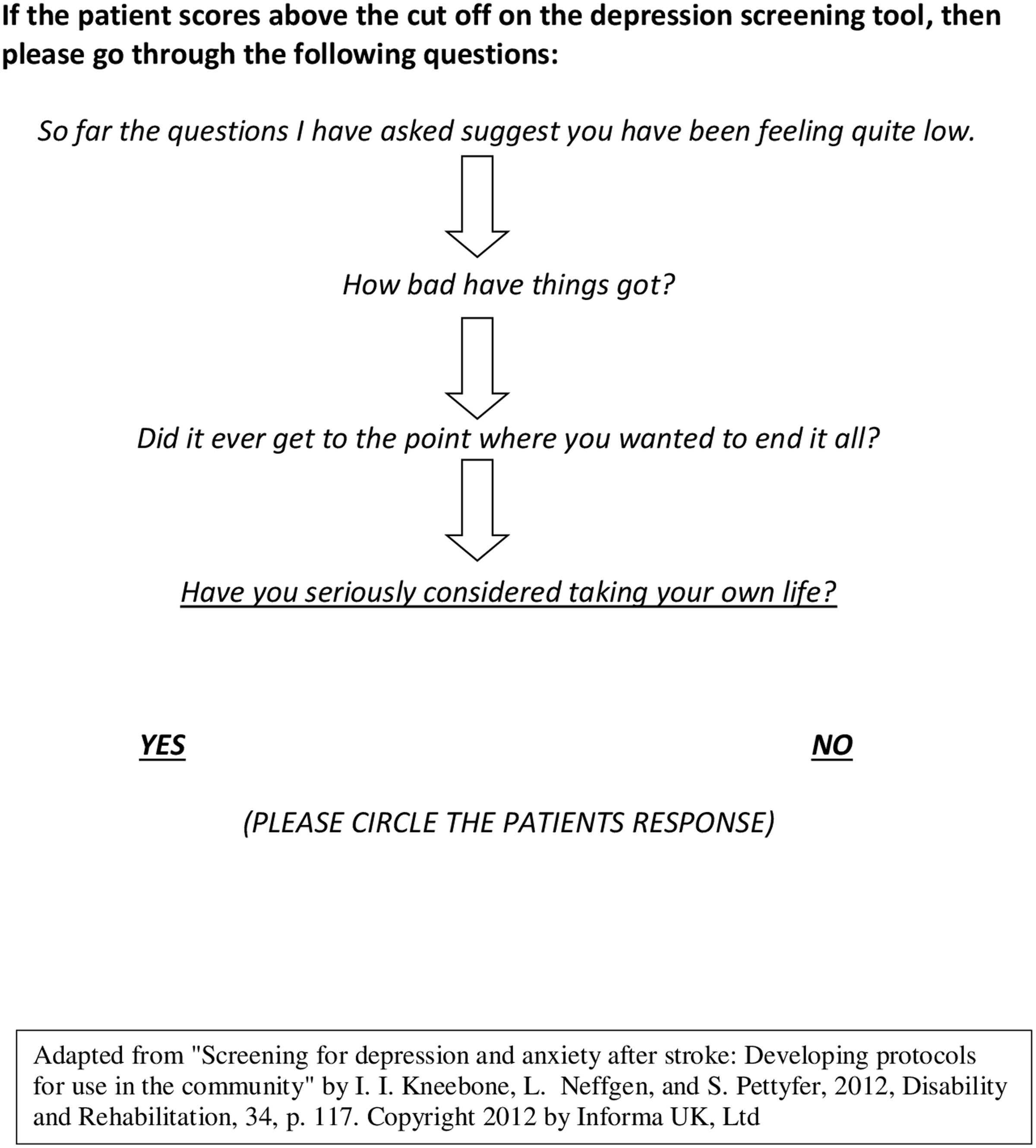
Figure 4. Screening for suicidal ideas.
Development of the “MAPS” training
The MAPS training was based on one developed in the UK (Kneebone et al., Reference Kneebone, Baker and O'Malley2010) and demonstrated as effective via audit (Kneebone, Stone, Robertson & Walker-Samuel, Reference Kneebone, Stone, Robertson and Walker-Samuel2013). This pre-existing training developed by Kneebone et al. (Reference Kneebone, Baker and O'Malley2010) was modified by the authors specifically for the fast-paced inpatient ward environment. During the initial stages of development, the authors attended a meeting with the medical lead of the rehabilitation service at the hospital to discuss the development and implementation of the training. One of the researchers was previously a clinician on the rehabilitation ward and was involved in modifying the training to ensure it was relevant to the local context. Specifically, the screening instruments used were updated based on relevant literature reviews to ensure all screening instruments were validated in a stroke population and were suitable (Bennett & Lincoln, Reference Bennett and Lincoln2006; Lincoln et al., Reference Lincoln, Kneebone, Macniven and Morris2012; Chun, Carson, Dennis, Mead & Whiteley, Reference Chun, Carson, Dennis, Mead and Whiteley2017). Notably screening for specific phobias was introduced to the screening pathway following emerging research on the high prevalence of specific phobias in stroke survivors (Chun, Whiteley, Dennis, Mead & Carson, Reference Chun, Whiteley, Dennis, Mead and Carson2018).
The training also included a new module on short term care strategies for the MDT to employ when supporting stroke survivors experiencing anxiety and depressive symptoms. Other modifications included giving the MDT a template for recording screening results in patient notes and an information sheet on anxiety and depression for patients and their family members which also provided a rationale for mood screening. Case scenario questions were also changed, and a new post-training quiz was introduced to help participants consolidate their learning.
Screening instruments for anxiety and depression
Identification of appropriate screening instruments for the current training program were based on reviews of the literature (e.g., Lincoln et al., Reference Lincoln, Kneebone, Macniven and Morris2012), and other relevant work (Chun et al., Reference Chun, Carson, Dennis, Mead and Whiteley2017). All had been validated in stroke and were free, brief, and easy to administer based on the extensive clinical experience of the authors.
To screen for depression, the Patient Health Questionnaire – 9 item (PHQ-9) (Williams et al., Reference Williams, Brizendine, Plue, Bakas, Tu, Hendrie and Kroenke2005) was chosen. The Generalised Anxiety Disorder – 7 item questionnaire (Chun et al., Reference Chun, Carson, Dennis, Mead and Whiteley2017) was chosen to screen for anxiety. Given that at least 35% of people who have had a stroke have significant cognitive impairment (Tatemichi et al., Reference Tatemichi, Desmond, Stern, Paik, Sano and Bagiella1994) and at least 12% have persisting significant communication disorders (Wade, Hewer, David & Enderby, Reference Wade, Hewer, David and Enderby1986), the training also included two observational mood screening instruments that had been validated in the stroke population with cognitive and communication impairments. Specifically, the Behavioural Outcomes of Anxiety Scale (Eccles, Morris & Kneebone, Reference Eccles, Morris and Kneebone2017; Linley-Adams, Morris & Kneebone, Reference Linley-Adams, Morris and Kneebone2014) and the Stroke Aphasic Depression Questionnaire – hospital version (10 items) (Lincoln et al., Reference Lincoln, Kneebone, Macniven and Morris2012) were selected as the observational screening instruments for anxiety and depression, respectively.
Screening for specific phobias and suicidal ideas
The training also included brief screening questions for specific phobias (see Fig. 3), and suicidal ideation (see Fig. 4), given the prevalence of specific phobias (Chun et al., Reference Chun, Whiteley, Dennis, Mead and Carson2018) and increased risk of suicide in stroke survivors (Pompili et al., Reference Pompili, Venturini, Campi, Seretti, Montebovi, Lamis and Girardi2012). Importantly, participants were reassured that they were not assessing suicide risk and could refer patients on to specialist mental health professions (i.e. psychology and psychiatry) to manage any identified risk concerns.
It was suggested by the researchers that initial screening should occur approximately 5 days after admission to the rehabilitation ward, to ensure patients had adequate time to adjust to their new environment (Kneebone et al., Reference Kneebone, Baker and O'Malley2010). Given that anxiety and depressive symptoms can arise at any point in time after a stroke, the researchers also recommended that staff continue to screen patients during their recovery whenever concerns arose (Kneebone et al., Reference Kneebone, Baker and O'Malley2010). Note that this was a pragmatic recommendation and guideline based on previous protocols developed in the UK (Kneebone et al., Reference Kneebone, Baker and O'Malley2010) and there is no consensus on when is best to screen (Towfighi et al., Reference Towfighi, Ovbiagele, El Husseini, Hackett, Jorge, Kissela and Williams2017). Also note that this training was developed for an inpatient rehabilitation setting and thus suitable screening times may differ in other contexts. It is noted providing a specific timeframe for screening can be a useful behavioural change strategy to improve compliance (Michie, Van Stralen & West, Reference Michie, Van Stralen and West2011). That is, if there is no set time, screening is less likely to occur.
Training strategy
Participants were informed about the training days by senior staff members and volunteered to participate based on their availability. Training was then delivered in person in small groups (<10 staff) by two members of the research team, both registered psychologists, with clinical and research expertise in stroke. The training was delivered twice on separate days at the hospital, to facilitate maximum staff attendance. Both training days included the same content and the same procedures for data collection were followed.
Other aspects of the training
Participants were also trained in responding to and documenting screening results. Trainers also provided a number of evidence-based short-term care strategies staff could use to support patients experiencing anxiety and depressive symptoms.
Feasibility of knowledge and skills assessment: quantitative data
Measures
During the training, participants completed a “consolidation exercise” i.e., a series of questions based on case scenarios to practice applying their mood screening knowledge and skills (see Appendix 2). The “consolidation exercise” was purposefully designed for the training by the researchers, with higher scores (max = 20) indicative of greater mastery of the content.
Participants were also given a quiz before and after the training with the same questions (Appendix 3). The quiz was designed purposefully for the training by the researchers and included questions asking about the symptoms of anxiety and depression, the screening instruments and actions to take following screening. A higher score (max = 11) again indicated greater content mastery.
Data analysis
Descriptive statistics, including mean scores and measures of variance (range and standard deviation), were calculated for performance on the consolidation exercise and the quiz taken after the training only. No descriptive statistics were reported on for the quiz taken before training, as most participants arrived late and did not complete the quiz. Given the preliminary nature of this study, inferential statistics were not applied to the quantitative data.
Acceptability of the “MAPS” training pathways: qualitative data
Measures
Immediately following the post-training quiz, a focus group was conducted on both of the separate training days. Both focus groups were led by the same member of the research team (RE), a female, who at the time was a provisionally registered psychologist and doctoral candidate. RE received ongoing supervision from a member of the research team (BR), a female, with extensive experience in conducting focus groups and qualitative research. The interviewer had not been involved in delivery of the training and had no relationship with the focus group members prior to study commencement. The participants also had no knowledge of RE’s personal goals or reasons for doing the research.
During both focus groups, the interviewer asked the same questions about the acceptability of the training using a topic guide (Appendix 4). Both focus groups were approximately 40 min in duration. No field notes were made during the interviews. The interviewer utilised audio-visual recording for both sessions which were then transcribed verbatim by the author on Microsoft Word.
Data analysis
A thematic analysis that followed the steps outlined by Braun and Clarke (Braun & Clarke, Reference Braun and Clarke2006) was completed by the interviewer using Microsoft Word (Appendix 5). An inductive approach was utilised whereby themes were derived from the raw data. Results of the qualitative analysis were presented according to the Consolidated Criteria for Reporting Qualitative Research (COREQ) (Tong, Sainsbury & Craig, Reference Tong, Sainsbury and Craig2007) (Supplementary File 2). However, in order to avoid potentially identifying information (e.g., demographic and professional details) and outcomes stratified by discipline, were not reported. The analysts, RE and BR, acknowledge there may be potential biases in the data given they were both aware of the low screening rates at the hospital, and that there was a dearth of training programmes and pathways available to healthcare professionals in Australia at the time. Thus, the researchers may have had preconceived assumptions about the need and value of the training.
Feedback from the hospital
Feedback on the success of implementation was also collected as an additional measure, 1 month after the training days. This was obtained in a follow up email from the research team to the medical lead on the rehabilitation ward, broadly enquiring about their progress and future intentions.
Results: quantitative data
From a pool of 28 ward staff, 12 multidisciplinary rehabilitation clinicians (mean age = 35.7, SD = 11.39) participated in MAPS training, the consolidation exercise and post-training quiz. Those who did not complete the training were either unable to attend due to workplace demands, did not have a rostered shift during the training, or declined to participate. Six professions from the MDT were represented (Medicine = 2; Physiotherapy = 3; Occupational therapy = 4; Social work = 1; Dietetics = 1; Nursing = 1). On the consolidation exercise 10 out of 12 (83%) participants scored ≥19 out of 20. The mean performance score was 19 out of 20 (SD = 1.5, range 15–20, i.e. 95% accuracy),with skewness of −2.00. Participants demonstrated the ability to select the correct screening instruments to administer based on the level of the patient’s disability, accurately identify cut-off points for different screening instruments, and identify appropriate steps to take depending on different outcomes of the screening assessment.
On the post-training quiz, all participants scored ≥10 out of 11. The mean performance score was 10.72 (SD = 0.48, range 10–11), i.e. 97% accuracy, with skewness of −1.89. Participants demonstrated accurate understanding of mood screening procedures and general concepts regarding anxiety and depression. The pre-training quiz results are not reported on because many participants arrived late to the training and therefore did not take the quiz.
Results: qualitative data
Focus group
Two optional focus groups with four and five participants (respectively) were conducted. Participants volunteered to participate in the focus groups. Those who did not attend were noted to be those who had to return to their workplace duties. From the thematic analysis two overarching or core themes were identified along with two subthemes (Table 1). Other miscellaneous themes are reported in Appendix 6.
Table 1. Outline of Core Themes and Associated Sub-themes with Quotations
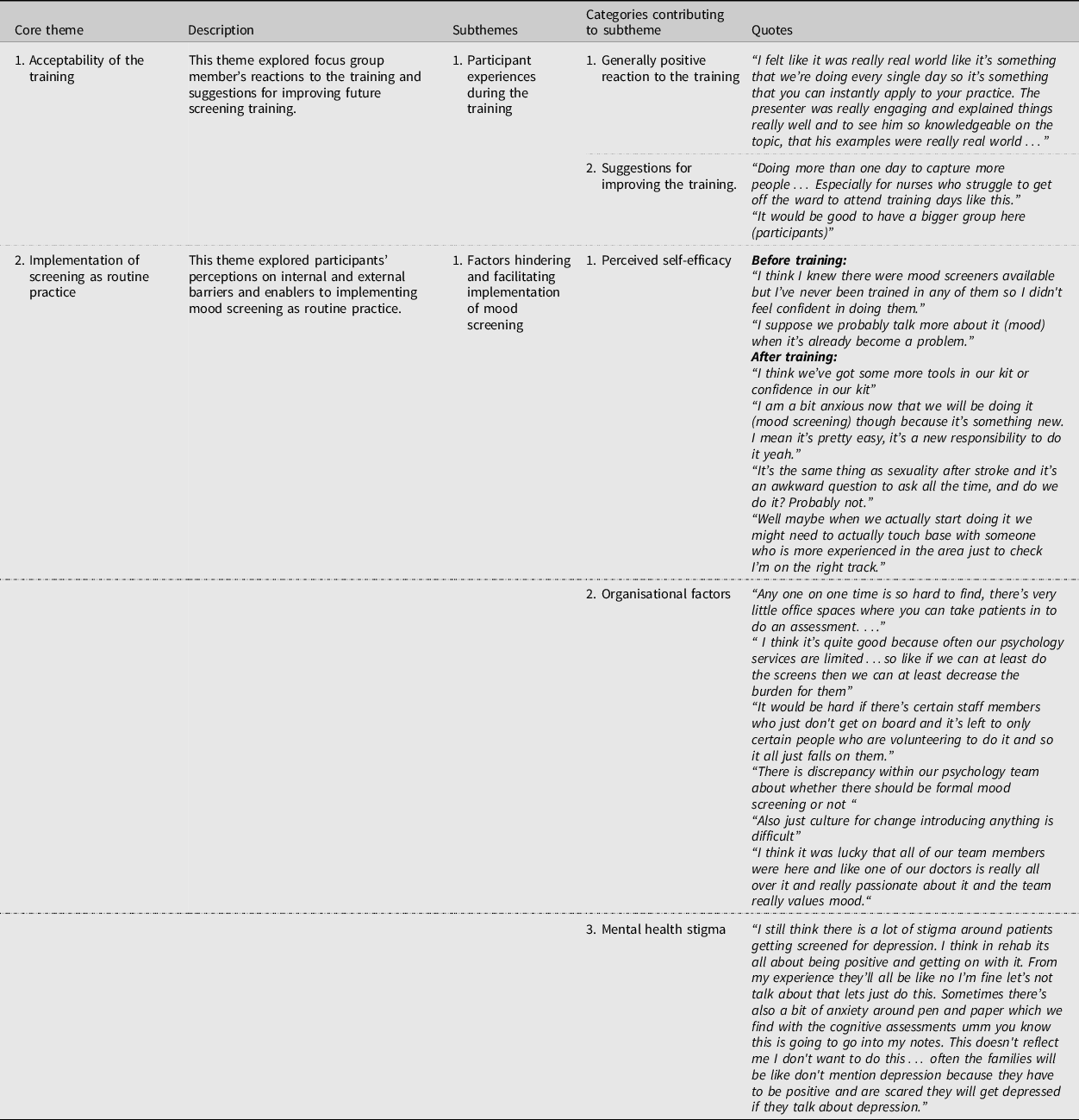
Core theme 1: Acceptability of the training – participant experiences during the training
All participants reacted positively to the training. It was agreed that the training was concise, relevant and worth recommending to colleagues. Most focus group members also commented that the presenters were very knowledgeable and engaging.
Core theme 1: Acceptability of the training – suggestions for future training
While many participants reported that they wouldn't change anything about the training, other participants commented that the training could have provided video role plays and training packs for the entire MDT. When asked about the limitations of the training, participants agreed that there weren't enough members of the MDT present for the training and requested more training days so that their colleagues could attend.
Core theme 2: Implementation of screening into practice – perceived self-efficacy
Focus group findings revealed common sub-themes regarding participants’ self-beliefs about their screening skills and abilities both prior to and after training.
Prior to training, focus group members shared the common perception that their low self-efficacy around formal mood screening contributed to their poor screening rates. Specifically, most focus group members reported that they had limited knowledge of screening instruments and no training in procedural screening skills. Others commented on their confusion around the continuum of mood change possible following stroke and the threshold for clinically significant symptoms that warranted intervention. Consequently, the MDT had been informally triaging patients (i.e. having brief conversations around mood and motivation), and only referring patients onto psychology once mood was clearly and significantly impacting on physical rehabilitation. The focus group members also reported that “a very small portion” of their work with patients was in relation to their mental health, with the exception of one participant, who believed their work did involve frequent discussion around mental health. With respect to how staff supported patients experiencing anxiety and depressive symptoms, focus group members reflected that they were likely ‘indirectly’ managing and improving patients’ mood through assisting with physical recovery, offering general support and encouragement, and goal setting.
Following training, most participants’ self-efficacy appeared to have increased. Those focus group members reported that the training had made formal mood screening seem quick, easy, and feasible and found the training “empowering”. These participants reported feeling more confident in their ability to perform screening and make decisions based on screening results. A smaller portion of participants, however, reported low self-efficacy around implementing routine mood screening following the training. Specifically, these focus group members reported that screening would provoke some anxiety given it was a new and unfamiliar practice.
Further, there was a consensus amongst participants that screening for suicidal ideas would remain an “uncomfortable” and “awkward” conversation after the training. Some participants also discussed their lack of confidence in their ability to respond to suicidal ideation but acknowledged that this confidence could be built over repeated practice with patients. Focus group members also reported that improving their self-efficacy would facilitate implementation of routine screening. For example, a participant suggested that the MDT could improve their self-efficacy with further training and ongoing support during the early stages of implementation.
Core theme 2: Implementation of screening into practice – organisational factors
Almost all participants voiced concerns about organisational factors that could potentially hinder the implementation of screening. In particular, lack of time, resources and privacy on the rehabilitation ward seemed to be the most concerning organisational barriers for focus group members. However, a focus group member reported that routine screening could improve time management in the rehabilitation ward. Specifically, sharing the responsibility of screening could help relieve the burden on the very limited psychology services in the hospital.
Another organisational barrier discussed was the ambivalence within the team around which health professionals should be screening, and at what time points should screening occur. This theme also raised discussion around the need for screening to be the responsibility of the whole MDT.
Other participants believed staff attitudes might also be a barrier to successful implementation. For example, a focus group member reported that screening was not part of their job role. It was also commonly acknowledged that there was a divide within the treating team as to whether routine screening should be implemented. However, there was no discussion around why there was this divide during the focus group. A focus group member also spoke of the difficulty of making any changes within any organisation and the anxiety and uncertainty associated with organisational change.
Despite the perception some staff attitudes would hinder the implementation of routine screening, most focus group members believed there was widespread support for routine screening, particularly from a leading member of the physician team. Further, all focus group members reported valuing patient mental health as part of the team culture of the rehabilitation service.
Core theme 2: Implementation of screening into practice – mental health stigma
Focus group members also raised concerns that mental health stigma may prevent patients and their families from wanting to engage with the screening. Others reported that the screening may make the patients anxious as the outcome would be documented in their medical record.
Feedback from the hospital
Following email contact with the medical lead of the rehabilitation service at the hospital, researchers were advised that the Medical Officers (physicians) had embarked upon routine formal screening. Within the email, the medical lead also disclosed that other disciplines within the MDT were eager to implement mood screening, however lacked the “confidence” to administer the screening instruments. The medical lead also expressed the department’s aim for the MDT to share the responsibility of screening once their confidence in screening improves.
Discussion
Despite the prevalence of anxiety and depression and their adverse consequences in the stroke population (Burton et al., Reference Burton, Murray, Holmes, Astin, Greenwood and Knapp2013; Hackett & Pickles, Reference Hackett and Pickles2014; Kutlubaev & Hackett, Reference Kutlubaev and Hackett2014; Lincoln et al., Reference Lincoln, Kneebone, Macniven and Morris2012; Pompili et al., Reference Pompili, Venturini, Campi, Seretti, Montebovi, Lamis and Girardi2012), screening rates remain low in some Australian hospitals (Stroke Foundation, 2017, 2018, 2019). The literature suggests that mood screening training can improve screening knowledge, skills and compliance in the UK (Hart & Morris, Reference Hart and Morris2008; Kneebone et al., Reference Kneebone, Baker and O'Malley2010; Kneebone, Neffgen & Pettyfer, Reference Kneebone, Neffgen and Pettyfer2012; McLean et al., Reference McLean, Torkington and Ratsch2019; Morris et al., Reference Morris, Jones, Wilcox and Cole2012), but there are no studies on the feasibility of training evaluations for inpatient rehabilitation settings within the Australian healthcare system.
Regarding the feasibility of evaluating screening training, the results of this study illustrate the many limitations of delivering and evaluating in person training. Firstly, this modality of training hindered recruitment with only 40% of the MDT attending the training sessions. It was noted that many staff, in particular nursing and medical staff, could not attend due to lack of time/availability during their shifts or because they had not been rostered on at work when the two training days were held. This limitation was also identified by focus groups members who expressed their desire to have more members of their team attending the training. While a broad range of health professionals from the MDT were represented in this study, only a few members of each discipline participated in the study. A limitation may be that those participants may have been those particularly interested in mood screening. Furthermore, given participants were colleagues this may have affected their focus group responses and different or additional information may have been provided if offered the opportunity to be individually interviewed. Additionally, data collection was also negatively affected by the modality of the training. Notably, no baseline measures of screening knowledge could be taken as several participants arrived late to the training again due to workplace demands. Thus, it was not possible to determine the effect of the training on screening knowledge.
A viable solution to address participation and knowledge assessment rates could be delivering the training online via pre-recorded training sessions. (Pei & Wu, Reference Pei and Wu2019; Richmond, Copsey, Hall, Davies & Lamb, Reference Richmond, Copsey, Hall, Davies and Lamb2017). Future projects are encouraged to explore making training materials available in a format that participants can access at any time of their convenience, complete at their own pace, and refer back to for “booster sessions” as needed. Possibly more accessible training will allow Australian stroke services to match the success of their UK counterparts. Developing an online training would also allow for more participants from each discipline to attend the training thereby enhancing the internal validity of the data and allowing examination of intra-discipline and inter-discipline specific differences in attitudes towards the training and its implementation. Furthermore, this training was only evaluated a single rehabilitation ward, at a single point in time. Further research is needed to evaluate the effectiveness of MAPS training on screening uptake and its impact on patient treatment and mood outcomes in a variety of Australian healthcare contexts.
Regarding the acceptability of the training, the results of this study revealed that whilst the “MAPS” training was viewed very positively by all participants of the focus groups, opinions on the prospect of implementing the training as routine practice varied across participants. These results are consistent with findings from other studies examining the perceptions of multidisciplinary stroke clinicians’ addressing psychological problems in the workplace (Baker et al., Reference Baker, Worrall, Rose and Ryan2021; Hart & Morris, Reference Hart and Morris2008; McCluskey, Vratsistas-Curto & Schurr, Reference McCluskey, Vratsistas-Curto and Schurr2013; Ryan et al., Reference Ryan, Bohan and Kneebone2019; Sekhon, Douglas & Rose, Reference Sekhon, Douglas and Rose2015; Stroyde, Reference Stroyde2019). Based on the informal feedback from the medical lead of the rehabilitation service, self-efficacy in screening remained the most problematic barrier within MDT 1 month following training (excluding the team of physicians). This low self-efficacy persisted despite participants demonstrating excellent screening knowledge/skills in the quiz and consolidation exercise and many reporting increased self-efficacy at the focus groups. This finding falls in line with other studies that have found stroke clinicians’ resistance to addressing emotional problems in stroke is primarily related to their perception of being under-skilled (Baker et al., Reference Baker, Worrall, Rose and Ryan2021; Hart & Morris, Reference Hart and Morris2008; McLean et al., Reference McLean, Torkington and Ratsch2019; Sekhon et al., Reference Sekhon, Douglas and Rose2015; Stroyde, Reference Stroyde2019). This highlights the need for future training to provide health professionals with online access to training materials, continuing professional development in this area, and ongoing support from their psychology team particularly during the initial stages of implementation, as recommended by both the literature (Hart & Morris, Reference Hart and Morris2008) and study participants. Including role plays of screening in training (as suggested by focus group participants) may further enhance screening self-efficacy. Future training evaluations may also wish to consider using self-report measures of self-efficacy before and after training as well as during implementation.
Notably, despite the impediments identified, the MAPS training pathways appeared successful in supporting implementation of the screening. Following communication with the medical lead of the rehabilitation service, it was identified the medical officers (physicians) within the MDT were providing this routinely. Self-efficacy did not appear to be a barrier to screening for this discipline. This outcome is likely attributed to the fact that MAPS training addressed significant barriers to screening previously identified, such as a lack of clarity around responsibility for screening, workload pressures, poor screening knowledge, and poor awareness of screening guidelines (Baker et al., Reference Baker, Worrall, Rose, Hudson, Ryan and O’Byrne2018; Baker et al., Reference Baker, Worrall, Rose and Ryan2021; Hart & Morris, Reference Hart and Morris2008; Ryan et al., Reference Ryan, Bohan and Kneebone2019). Specifically, the MAPS training addressed these barriers by outlining responsibility for screening within the MDT, encouraged clinicians to share screening responsibility to reduce workload pressures, advised when screening should occur and provided simple protocols that clearly outlined the tools, the procedure for screening and provided templates for documenting results. These findings are promising and support the need for future training evaluation studies to address the above-mentioned barriers to screening.
The focus groups provide additional insights into why the medical team chose to implement routine screening. During one of these, a participant disclosed that the leading member of the medical team displayed behaviours that were consistent with the behaviours of a clinical champion (Flanagan et al., Reference Flanagan, Plue, Miller, Schmid, Myers, Graham and Damush2018). The literature suggests that clinical champions in stroke care are dedicated to the process of implementation, influence and inspire their team members to make changes, and persist when faced with barriers (Flanagan et al., Reference Flanagan, Plue, Miller, Schmid, Myers, Graham and Damush2018; Wood et al., Reference Wood, Giannopoulos, Louie, Baillie, Uribe, Lee and Morley2020). Future research might also explore whether involving/nominating a clinical champion from each health discipline, improves the success of implementation for a MDT.
The authors acknowledge that, quantitative audit data on screening compliance before and after training should be collected and reported on in future training evaluation studies to further support the efficacy of the training. Other suggestions for future training evaluations include follow-up assessments of knowledge and implementation after some weeks/months to allow researchers to monitor the stability of findings. Further, future research may also consider including measures of impact to determine how training influences patient mood and day-to-day functioning.
Conclusion
Delivering training for post-stroke mood screening fosters staff acceptance and facilitates knowledge. Encouragingly, the training also inspired the initiation of a routine screening procedure on the ward. Given the challenges of delivering training for a MDT face to face (and taking into account delivery during and beyond COVID-19), future studies should seek to provide flexible online options to improve training participation rates and data collection. Future training should address organisational and socio-cultural barriers to screening and potentially explore inter-disciplinary and intra-disciplinary attitudes towards screening. Most importantly, training programmes and pathways might incorporate ongoing support to stroke clinicians to boost screening self-efficacy, and encourage the use of clinical champions, to formally promote successful and sustained implementation.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/BrImp.2022.34
Acknowledgements
Early development work of the training was supported by the Stroke Foundation.
Financial support
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







