Bipolar disorder is a major psychiatric illness characterised by its early onset, severity and alternating mood episodes.Reference Ferrari, Stockings, Khoo, Erskine, Degenhardt and Vos1 A considerable proportion of bipolar disorder cases have been significantly associated with cognitive deficits, although to varying degrees, even when the bipolar disorder is in remittance.Reference Bora, Hidiroglu, Ozerdem, Kacar, Sarisoy and Civil Arslan2 Studies have also suggested that patients with bipolar disorder can experience accelerated cognitive ageing, which presents as a steeper decline in function.Reference Seelye, Thuras, Doane, Clason, VanVoorst and Urosevic3,Reference Chen, Huang, Lee, Chang, Lin and Chiu4 Cognitive impairment in patients with bipolar disorder may worsen as the disease progresses, which may negatively affect patient recovery.Reference Duarte, Becerra and Cruise5 In addition, age can dynamically, neurodevelopmentally and neuroprogressively interact with the neuropathology of bipolar disorder.Reference Kohler and Marlinge6 Although cognitive dysfunction has increasingly been recognised as a key symptom in patients with bipolar disorder, the biomarkers related to cognitive dysfunction have rarely been investigated, especially in different age groups of patients with bipolar disorder.
Evidence suggests that vitamin D deficiency may be a key marker of cognitive decline.Reference Pavlovic, Abel, Barlow, Farrell, Weiner and DeFina7 Moreover, higher vitamin D levels and higher vitamin D supplement intake have been associated with a slower cognitive decline.Reference Goodwill, Campbell, Simpson, Bisignano, Chiang and Dennerstein8,Reference Beydoun, Hossain, Fanelli-Kuczmarski, Beydoun, Canas and Evans9 This indicates that vitamin D may play an essential role in brain health and function, and may exert various neuroprotective effects on numerous brain areas.Reference Mayne and Burne10 A survey revealed that more than a third of patients with severe mental disorders, including bipolar disorder, were deficient in vitamin D.Reference Boerman, Cohen, Schulte and Nugter11 In addition, clinical studies suggested that vitamin D may play a role in the pathophysiology or cognitive profile of bipolar disorder. For example, a negative and moderate correlation was identified between vitamin D levels and mania severity or disease severity in bipolar disorder.Reference Altunsoy, Yuksel, Cingi Yirun, Kilicarslan and Aydemir12 Polymorphism in the vitamin D receptor gene was associated with lower expression of the dopamine D1 receptor gene and may contribute to bipolar disorder development.Reference Ahmadi, Mirzaei, Hossein-Nezhad and Shariati13 Furthermore, vitamin D deficiency has been associated with poor cognitive functioning in individuals experiencing major depressive episodes and in psychotic disorders, including psychotic bipolar disorder.Reference Belzeaux, Annweiler, Bertrand, Beauchet, Pichet and Jollant14,Reference Nerhus, Berg, Simonsen, Haram, Haatveit and Dahl15 An image study suggested a significant and positive association between vitamin D and intracranial volume in psychotic bipolar disorder.Reference Gurholt, Nerhus, Osnes, Berg, Andreassen and Melle16 In summary, although bipolar disorder has been reported to be associated with altered cognitive functioning and hypovitaminosis D, the associations between vitamin D levels, bipolar disorder pathophysiology and cognitive features are not well understood.
The mechanism underlying vitamin D's influence on brain function is complex and likely to involve direct induction of neurotrophic factors and indirect anti-inflammatory and metabolic effects.Reference Cui, Gooch, Petty, McGrath and Eyles17 Research suggests a connection between inflammation and the pathogenesis of bipolar disorder.Reference Jones, Vecera, Pinjari and Machado-Vieira18 In addition, inflammatory markers were identified, suggesting that inflammation could contribute to the neurocognitive deficits observed in bipolar disorder.Reference Poletti, Mazza, Calesella, Vai, Lorenzi and Manfredi19,Reference Castaño-Ramírez, Sepúlveda-Arias, Duica, Díaz Zuluaga, Vargas and López-Jaramillo20 Therefore, the anti-inflammatory neuroprotective role of vitamin D may preserve axonal integrity in the face of neuro-damage, which can be identified as neurofilament light chain (NfL) levels.Reference Mayne and Burne10,Reference Naifar, Maalej Bouali, Guidara, Ellouze, Jmal and Omri21 The NfL is a unique biomarker related to axonal damage and neural cell death. It demonstrates promise as a potential marker of neuropsychiatric disease activity and progression associated with cognitive deficit.Reference Bavato, Cathomas, Klaus, Gütter, Barro and Maceski22,Reference Jakobsson, Bjerke, Ekman, Sellgren, Johansson and Zetterberg23 Patients with bipolar disorder have been reported to have higher NfL levels, both in their central nervous systems and peripheral blood, compared with healthy controls.Reference Jakobsson, Bjerke, Ekman, Sellgren, Johansson and Zetterberg23,Reference Aggio, Fabbella, Finardi, Mazza, Colombo and Falini24 However, studies analysing the association between vitamin D, NfLs and the cognitive profile of bipolar disorder have not been conducted.
Aims of the study
We hypothesised that both vitamin D levels and NfLs have individual effects on cognition in bipolar disorder, with vitamin D indicating an anti-inflammatory modifiable nutrition status and NfLs indicating neuroaxonal damage over the disease course. Cognitive impairment in bipolar disorder may be neurodevelopmental, neuroprogressive or a combination of the two. Therefore, the aim of the study was to investigate the interaction of these two biomarkers in patients with bipolar disorder of different ages, to enable future studies to clarify the underlying mechanism of bipolar disorder cognition.
Method
Participants
In this cross-sectional study, we recruited 100 individuals who had been given a diagnosis of bipolar disorder type 1 in accordance with the DSM-5, from the out-patient clinic of a tertiary psychiatric hospital. The individuals were aged from 20 to 65 years and had not taken vitamin D supplements within 3 months of the study. We excluded individuals with (a) a known substance use disorder (with the exception of a nicotine use disorder); (b) any disorder with neurological symptoms or complications, such as brain injury or stroke; (c) an active physical illness such as renal impairment or hepatic failure, or pregnancy; (d) a previous diagnosis of intellectual disability, schizophrenia or schizoaffective disorders or (e) an inability to complete the standard clinical assessment or provide informed consent. The information regarding psychiatric comorbidities and the exclusion criteria was obtained through patient medical records and the Chinese version of the modified Schedule of Affective Disorder and Schizophrenia-Lifetime. Individuals were also required to be euthymic and under stable medication (no change in psychotropic medications in the previous month).
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by the Research Ethics Committee of Taipei City Hospital (approval number TCHIRB-10912011). Written informed consent was obtained from all patients.
Measurements
Demographic data, clinical course and mood symptoms
Patients’ demographic and clinical course data were collected from medical records and interviews with psychiatrists, if required. Participant clinical characteristics included number of affective episodes (total, manic and major depressive), number of episodes with psychotic features, number of hospital admissions and age at illness onset. Because the duration of illness varied across individuals, we calculated episode density by dividing the number of episodes by the duration of illness, to obtain the total episode density, manic episode density, major depressive episode density and episodes with psychotic features density. The psychopharmacological medications the patients used at the time of assessment were recorded and converted to a defined daily dose (DDD). A DDD is a unit of measurement representing the assumed average maintenance dose per day for a drug used for its main indication in adults, which can be used for comparisons of drug consumption. Mood symptoms were obtained through clinician-administered measures, that is, through the 17-item Hamilton Rating Scale for Depression (HRSD) and the Young Mania Rating Scale (YMRS). We defined euthymia as both HRSD and YMRS ≤8, screening within 1 week before the cognitive assessment.
Cognitive measurements
The enrolled patients were assessed with the Brief Assessment of Cognition in Affective Disorders (BAC-A), which has extensively been used as a quick and reliable cognitive assessment of patients with a wide range of clinical affective disorders.Reference Bauer, Keefe, Sanches, Suchting, Green and Soares25 The BAC-A can be administered in approximately 35 min. The assessment measures affective memory and emotional inhibition through the Affective Auditory Verbal Learning Test and Emotional Stroop Task, and measures six standard neurocognitive domains, namely working memory (Digit Sequencing Task), motor speed (Token Motor Task), verbal fluency (Category Instances and Controlled Oral Word Association Test), processing speed (Symbol Coding), verbal memory (List Learning) and executive function (Tower of London), through a comparison with norm references.Reference Wang, Huang, Hung, Chen, Chen and Lee26 The criterion and construct validity for each test of cognitive impairment, as well as the sensitivity of these tests to changes in cognition, have been demonstrated in the literature. Each test has also been demonstrated to be valid for use in different cultures and language groups.Reference Wang, Huang, Hung, Chen, Chen and Lee26 Premorbid IQ was estimated through the Adult Reading Test from the Wechsler Adult Intelligence Scale, by a licensed psychologist.
Physical activity and dietary patterns
Physical activity and dietary patterns are key variables affecting both vitamin D levels and cognition. We used the Chinese version of the International Physical Activity Questionnaire, which was reported to be a valid and reliable self-reported assessment of physical activity,Reference Macfarlane, Lee, Ho, Chan and Chan27 to assess participants’ regular physical activity levels. We used the food frequency questionnaire to assess participants’ basic dietary patterns because vitamin D levels may be influenced by dietary habits. The food frequency questionnaire can measure long-term dietary intake, which is essential information for understanding dietary composition.
Vitamin D and NfL level analysis
The patients underwent fasting blood sampling in the early morning on the same day of cognitive assessment. A venous blood sample of 10 mL was taken from each participant, and the serum was separated and stored at −80°C until analyses were conducted. Serum vitamin D levels, measured as 25-hydroxyvitamin D (25(OH)D), were determined by chemiluminescent immunoassay technology with a commercially available kit (LIAISON®, DiaSorin Inc., Stillwater, MN, USA). Serum vitamin D levels were considered deficient if the serum 25(OH)D values were <20 ng/mL.Reference Kennel, Drake and Hurley28 NfL levels were measured by Quanterix Simoa® assay (Quanterix Corp., Lexington, MA, USA), a digital immunoassay with a lower detection limit of 0.104 pg/mL, according to the manufacturer's instructions.
Statistical analyses
We first tested the influence of age on cognitive outcomes and assessed the associations between age and vitamin D and NfL levels. We compared the demographic and clinical characteristics of the patients with bipolar disorder with and without a vitamin D deficiency, stratified by age, with a cut-off value of 45 years, to balance the sample size and age distribution of the two age strata. χ²- and Student t-tests were used for assessing categorical and continuous variables, respectively. The normality of the data was assessed with the Kolmogorov–Smirnov test. For nonnormally distributed data, we used the nonparametric Wilcoxon rank-sum test. General linear models adjusted for age and gender were used to determine the associations between vitamin D and NfLs, and their interaction effects on cognitive domains. Furthermore, general linear models with additional adjustment for years of education, psychotropic medication DDD and the variables that significantly differed between the groups with and without vitamin D deficiencies were used in a second model. We determined an interaction plot representing the interaction effect of vitamin D and NfL levels on cognition in each age group. All analyses were conducted with SAS software (version 9.4 for Windows; SAS Institute, Cary, NC, USA), and significance was set at P < 0.05.
Results
Patient characteristics and age effect
We recruited 100 patients (48 men and 52 women) with bipolar disorder who were in a euthymic state. The mean age was 47.49 years (s.d. 11.71), and the mean age at onset was 24.78 years (s.d. 9.79). The median and mean NfL levels were 9.26 and 11.10 pg/mL, respectively. The mean vitamin D level was 16.46 ng/nL, with 72% of the patients exhibiting vitamin D deficiency. A significant positive association was identified between age and NfL levels (r = 0.377, P < 0.001), and a nonsignificant negative association was identified between age and vitamin D levels (r = −0.63, P = 0.266; scatterplot in Supplementary Fig. 1 available at https://doi.org/10.1192/bjo.2022.608). Age also had a significant negative correlation with composite neurocognitive score (r = −0.031, P < 0.001) and the neurocognitive domains of verbal memory (r = −0.16, P = 0.019), working memory (r = −0.039, P < 0.001), processing speed (r = −0.031, P < 0.001) and executive function (r = −0.028, P = 0.001) as determined through the BAC-A. With a cut-off value of 45 years, significant differences were identified in the composite neurocognitive score, working memory and executive function scores between age subgroups (Supplementary Table 1).
Comparison of vitamin D status
Table 1 presents the sociodemographic and clinical characteristics of the patients with bipolar disorder with and without vitamin D deficiencies, stratified by age. In the younger age group, 62.79% exhibited vitamin D deficiency; in the older age group, 78.95% exhibited vitamin D deficiency. No significant differences were identified in the disease course, amount of physical activity, NfL levels or DDD of the younger patients with and without vitamin D deficiency. In the older age group, we noted significantly higher numbers of hospital admissions in the patients with versus without vitamin D deficiency (8.49 ± 8.55 v. 2.92 ± 2.88; P = 0.031). Furthermore, the patients without vitamin D deficiency reported more physical activity than those with (1552.67 ± 429.54 v. 666.56 ± 603.16; P = 0.016). No differences were noted in the ages, premorbid estimated IQs, gender, marital or job statuses, smoking or alcohol use habits, nutrient supplement intakes or proportions of physical comorbidities of the patients with and without vitamin D deficiency in both age groups. Moreover, the differences in cognitive function in the patients with and without vitamin D deficiency and in those with higher and lower median NfL levels were nonsignificant for both age groups (Supplementary Tables 2 and 3).
Table 1 Sociodemographic and clinical characteristics of patients with bipolar disorder with and without vitamin D deficiency, stratified by age

HRSD, Hamilton Rating Scale for Depression; YMRS, Young Mania Rating Scale; IPAQ, International Physical Activity Questionnaire; NfL, neurofilament light chain; DDD, defined daily dose.
* P < 0.05.
Interaction effect of vitamin D and NfL level on cognition
General linear models adjusted for age and gender, and models further adjusted for years of education, psychotropic DDD, number of hospital admissions and physical activity levels, were used to determine the effects of vitamin D, NfLs and their interaction on various cognitive domains in younger (Table 2) and older patients (Table 3) with bipolar disorder. In the younger age group, we identified a significant association between serum vitamin D, NfLs and their interaction to composite neurocognitive score. The association was particularly noted on verbal fluency and processing speed domains (model 2 in Table 2). In the younger age group, NfL levels had a considerable negative influence and vitamin D had a minor negative influence. Furthermore, the interaction of NfL and vitamin D levels positively affected cognitive function. In the older age group, the interaction between vitamin D and NfLs significantly and negatively affected composite neurocognitive score. The association was also particularly noted on verbal fluency (model 2 in Table 3). Moreover, vitamin D had a positive association with composite neurocognitive score; in addition, both vitamin D and NfLs had a significant positive association with verbal fluency in the older age group. In summary, we identified interaction effects of vitamin D and NfLs on composite neurocognitive function and verbal fluency domain for both age groups. Furthermore, age influenced the direction of the interaction effects. The crossed lines in the interaction plot (Fig. 1) indicate that the association between vitamin D status and cognition was influenced by NfL levels for both younger and older patients with bipolar disorder. In the younger patients with bipolar disorder with vitamin D levels <10 ng/mL, those with lower NfL levels exhibited more favourable cognitive function. However, when vitamin D levels were higher, the negative effect of NfLs was not present (Fig. 1(a)). In the older patients with bipolar disorder, the patients with lower NfL levels generally demonstrated increased cognitive function as their vitamin D status improved. However, the plot for the older patients with bipolar disorder with higher NfL levels revealed no notable cognitive changes in vitamin D levels (Fig. 1(b)).
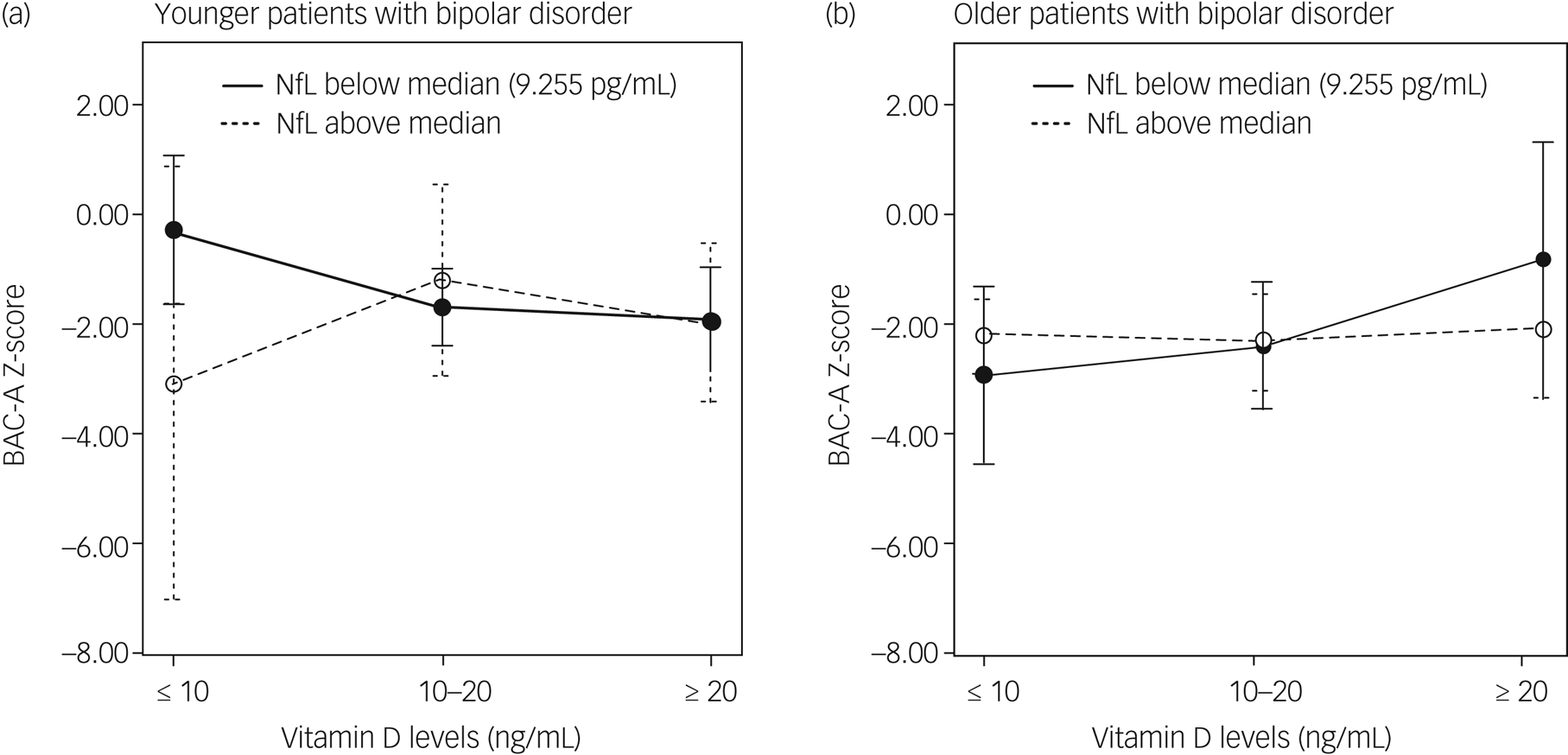
Fig. 1. Interaction plots of effect of vitamin D and neurofilament light chain levels on cognition. (a) Younger patients with bipolar disorder and (b) older patients with bipolar disorder. BAC-A, Brief Assessment of Cognition in Affective Disorders; NfL, neurofilament light chain.
Table 2 Associations between total serum vitamin D and neurofilament light chain and their interaction effect on cognitive domains in younger patients (aged 20–45 years) with bipolar disorder

Model 1: age, gender, vitamin D level, NfL level and interaction of vitamin D and NfLs. Model 2: age, gender, defined daily dose of total psychotropic medications, years of education, number hospital admissions, level of physical activity determined with the International Physical Activity Questionnaire, vitamin D level, NfL level and interaction of vitamin D and NfLs. NfL, NfL, neurofilament light chain.
* P < 0.05.
** P < 0.01.
*** P < 0.001.
Table 3 Association between total serum vitamin D and neurofilament light chain and their interaction effect on cognitive domains in older patients (aged 46–65 years) with bipolar disorder
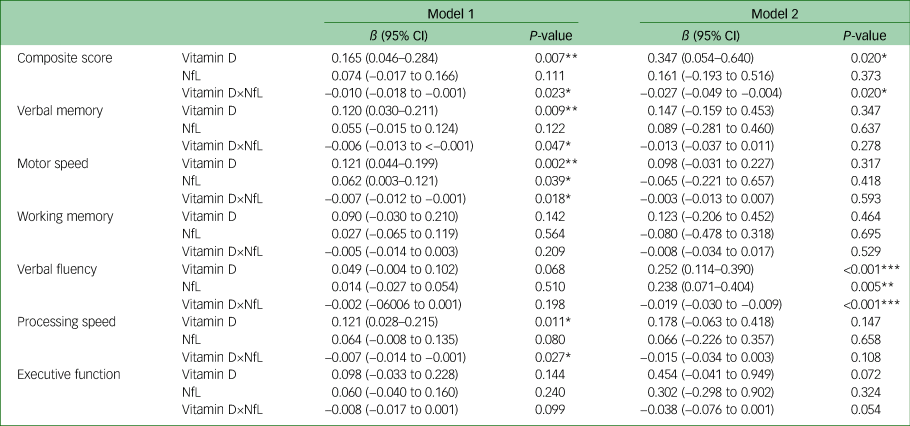
Model 1: age, gender, vitamin D level, NfL level and interaction of vitamin D and NfLs. Model 2: age, gender, defined daily dose of total psychotropic medications, years of education, number of hospital admissions, level of physical activity determined with the International Physical Activity Questionnaire, vitamin D level, NfL level and interaction of vitamin D and NfLs. NfL, neurofilament light chain.
* P < 0.05.
** P < 0.01.
*** P < 0.001.
Discussion
Main findings
In the younger patients with bipolar disorder, we noted a negative association between NfLs and vitamin D, and composite neurocognitive score and two neurocognitive domains (verbal fluency and processing speed). In the older patients, we noted a positive association between NfLs and vitamin D, and the cognitive domain of verbal fluency. Our results revealed a consistently significant antagonistic interaction effect on composite score and verbal fluency for both age groups. In addition, age was likely an effect-modified factor in the association. If NfLs can be regarded as a marker of neuroaxonal change over the course of bipolar disorder, clarifying the association of an intervening factor, such as vitamin D status, at different ages could considerably influence future intervention designs.
Complex interaction of NfLs and vitamin D in bipolar disorder
In interpreting the positive correlation between NfLs and verbal fluency in older patients with bipolar, researchers must consider that older patients have a longer disease course; therefore, early axonal damage could later have been affected by remyelination and axonal regeneration. This would be consistent with neuroimaging findings indicating that NfL level is positively associated with axial diffusivity in bipolar disorder.Reference Aggio, Fabbella, Finardi, Mazza, Colombo and Falini24 Our finding of a negative correlation between vitamin D and cognition in young patients with bipolar disorder contrasts with that of most studies on neuropsychiatric diseases.Reference Byrn and Sheean29 However, the parallel association (both negative or positive) identified between NfLs and vitamin D and cognition was consistent with the results of trials involving patients with multiple sclerosis, which has a similar neuropathology to bipolar disorder.Reference Magioncalda, Martino, Conio, Piaggio, Teodorescu and Escelsior30,Reference Magioncalda, Martino, Tardito, Sterlini, Conio and Marozzi31 In the aforementioned trials, disease status modified the association between vitamin D and NfL levels. In patients with active multiple sclerosis, the theoretical negative correlation of vitamin D and NfLs (non-parallel) have been reported to affect their brain function. However, in patients with multiple sclerosis in remittance or who received disease-modifying therapy, a parallel correlation or no correlation has been reported between vitamin D and NfLs and brain function.Reference Hänninen, Jääskeläinen, Herukka and Soilu-Hänninen32,Reference Røsjø, Lindstrøm, Holmøy, Myhr, Varhaug and Torkildsen33 In this study, the patients with bipolar disorder were in a euthymic state and were receiving medication. This may explain the parallel association between vitamin D, NfLs and cognitive function in the two age groups, similar to the aforementioned multiple sclerosis findings. Our findings may further be explained by the following. First, in the early stages of the disease in younger patients with bipolar disorder, NfLs play a significant role in determining disease severity, which negatively influences cognition. Meanwhile, the influence of an individual's vitamin D status on cognition may be modified by other factors, such as medication. For example, although mood stabilisers are generally provided when treating bipolar disorder, they are frequently associated with reduced vitamin D levels.Reference LoPinto-Khoury, Brennan and Mintzer34 Second, in the older patients with bipolar disorder in our study, a positive association between vitamin D and cognitive function was identified, and vitamin D may enhance the remyelination process. This, together with the elevated NfL levels, led to a positive association between vitamin D and NfL levels and the verbal fluency domain. In addition, the age-modified interaction effect could contribute to evidence supporting the neuroimmune development of bipolar disorder, in which, at the onset of the illness, individuals experience an increased proinflammatory state in adolescence, an anti-inflammatory state during young adulthood and a nearly normalised immune state in later stages of the disease course.Reference Guglielmo, Miskowiak and Hasler35 The interaction plot revealed an antagonistic interaction effect of vitamin D and NfLs, indicating that in the early stages of bipolar disorder in younger patients, NfL level may serve as a marker for neuro-damage resulting from neuroinflammation, and cognition differed in patients with higher and lower NfL levels, particularly those with extremely low vitamin D levels. In older patients with bipolar disorder who have a longer disease course, neuroinflammatory processes become stable, and the positive effects of vitamin D become more prominent in patients with lower rather than higher NfL levels. Therefore, our findings are consistent with the most current overview of the immune-inflammatory model in bipolar disorder,Reference Magioncalda and Martino36 and further add the role of neuroaxonal damage and vitamin D status (Fig. 2).
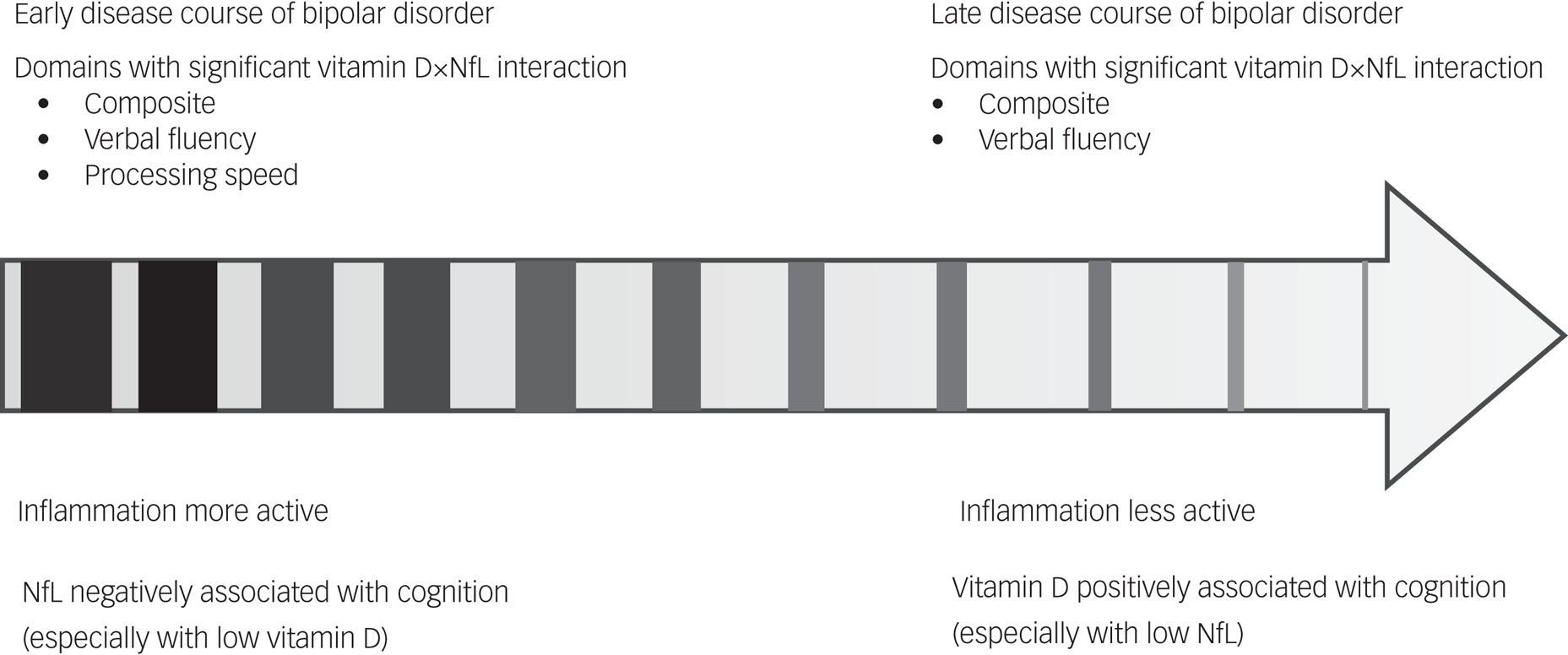
Fig. 2. Inflammatory model and age-modified interaction effects in treated euthymic bipolar disorder. NfL, neurofilament light chain.
Possible causal inferences of the interaction
Studies involving animal models have reported effects of vitamin D on reversing neuro-cytoskeleton imbalances, but not memory deficits, and have reported the protective effects of vitamin D to be more favourable before and during, rather than after, the demyelination process.Reference Nystad, Torkildsen and Wergeland37 The cross-sectional design of this study prevented us from identifying any temporal association between vitamin D and NfLs. Therefore, we could not determine whether neuroinflammation with high NfL levels caused vitamin D depletion or vitamin D deficiency aggravated axonal abnormality over the disease course. In addition, some studies have suggested that the effects of vitamin D should be identified through vitamin D receptor polymorphisms rather than serum levels.Reference Oliveira, Magalhães, Loures, Fraga, de Souza and Guimarães38 Because vitamin D receptor polymorphisms also contribute to bipolar disorder, gene polymorphisms may influence both neuroaxonal integrity and the effects of vitamin D on brain function in bipolar disorder.Reference Eghtedarian, Ghafouri-Fard, Bouraghi, Hussen, Arsang-Jang and Taheri39 Future longitudinal studies are required to investigate potential temporal associations between these factors. We also analysed the association between vitamin D, NfL levels and the interaction and clinical variables, such as clinical course and DDD, for each pharmacological category. We found only one positive association, between first-generation antipsychotic dose and NfL levels (data not shown). We need further studies to capture the whole picture of antipsychotics used during the disease course to understand the impact of antipsychotics on neuroaxonal integrity. Furthermore, few trials have investigated the influence of vitamin D levels on cognitive outcomes in psychiatric patients, with only one study involving patients with schizophreniaReference Krivoy, Onn, Vilner, Hochman, Weizman and Paz40 and one study on multiple sclerosis.Reference Darwish, Haddad, Osman, Ghassan, Yamout and Tamim41 Although both of the trials suggested a potential influence of vitamin D supplements on cognitive performance, the scarcity of studies on the subject prevents us from determining whether vitamin D can improve cognition in bipolar disorder. Our review of the literature revealed only two studies investigating the influence of vitamin D on bipolar disorder. One trial investigated bipolar depression with vitamin D deficiency, and revealed no impact of vitamin D intervention on depressive symptoms.Reference Marsh, Penny and Rothschild42 Another open-label trial involving patients aged <18 years with bipolar disorder with mania revealed that vitamin D3 supplementation improved mood symptoms and brain neurochemistry.Reference Sikoglu, Navarro, Starr, Dvir, Nwosu and Czerniak43 Neither trial included cognitive outcome measurements or changes in NfL levels. Further clinical trials focused on the influence of vitamin D intervention on cognitive outcomes in bipolar disorder and the influence of NfLs are required to verify our findings.
Strengths and weaknesses
To our knowledge, this study is the first to provide evidence of the interaction effect of vitamin D and NfLs on neurocognitive domains in patients with bipolar disorder of different ages. Our study has several limitations. First, our study sample was recruited from a tertiary psychiatric hospital. Therefore, the patients likely had a more severe degree of illness and cognitive dysfunction. In addition, only the euthymic patients with bipolar disorder were recruited in this study, who were screened for their mood status and then referred for cognitive assessment within 1 week; therefore, some of the patients may be in early remission. This potential bias in our sample ascertainment may limit the generalisability of the current findings to the entire bipolar disorder population. Second, we do not have the longitudinal cognitive profile to ensure cognitive stability across episodes. Third, we did not have a healthy control to determine whether vitamin D deficiency or the age-modified interaction effect of NfL and vitamin D levels on cognitive function were unique to bipolar disorder. Fourth, the age restrictions we used in our sample limited our findings to older and teenaged patients with bipolar disorder. Additional bipolar disorder subgroups with new onset and longer disease duration should be included in future samples. Fifth, vitamin D status was assessed by measuring total serum 25(OH)D concentrations. However, only free (unbound) and albumin-bound vitamin D reflect biological activity;Reference Speeckaert and Delanghe44 in addition, the effects of vitamin D should be identified through vitamin D receptor polymorphisms. Nevertheless, a study involving an Asian sample reported that total 25(OH)D concentrations were closely correlated with bioavailable 25(OH)D.Reference Kim, Chung, Lê, Na and Cho45 Therefore, 25(OH)D can be regarded as a primary marker of vitamin D status. Sixth, peripheral NfL levels do not represent total NfL levels in the central nervous system. However, serum measurements of NfL levels have been considered reliable in many studies.Reference Travica, Berk and Marx46 Finally, as the cross-sectional study design, we precluded the causal effect of our findings, and further studies to clarify the linking mechanism are needed.
Implications
We identified the interaction effect of vitamin D and NfLs on cognitive function in bipolar disorder. In addition, we discovered that age might play the role of an effect modifier. The effects of vitamin D on serum NfLs and cognitive outcomes in bipolar disorder warrant further study, with inclusion of patients with bipolar disorder with a range of mood statuses and disease courses. Longitudinal or randomised controlled trial designs are required to validate our findings and achieve more robust causal inferences and clinical implications.
Supplementary material
Supplementary material is available online at http://dx.doi.org/10.1192/bjo.2022.608
Data availability
The data that support the findings of this study are available from the corresponding author, P.-H.K., upon reasonable request.
Acknowledgements
This manuscript was edited by Wallace Academic Editing. We thank the Center for Public Health of the Department of Education and Research at Taipei City Hospital, Taiwan, for their valuable contributions with respect to data management and our statistical analyses.
Author contributions
W.-Y.C. completed study conceptualisation, funding acquisition, project administration, analyses and writing of the original draft of the manuscript. M.-C.H., P.-Y.C. and C.-J.K. performed the investigation and obtained resources. C.C.C. and Y.-C.C. suggested the investigation and completed resource and data curation. P.-H.K. participated in supervision, methodology and reviewing of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Ministry of Science and Technology (grants MOST 107-2314-B-532-001, MOST 108-2314-B-002-136-MY3 and MOST 110-2314-B-532-007). The funders played no role in the study design; collection, analysis and interpretation of data; manuscript writing or the decision to submit the paper for publication.
Declaration of interest
None.









eLetters
No eLetters have been published for this article.