Compared with research about the associations between socioeconomic status and risk for bipolar affective disorder (BPD), Reference Tsuchiya, Agerbo, Byrne and Mortensen1,Reference Schoeyen, Birkenaes, Vaaler, Auestad, Malt and Andreassen2 fewer studies have examined the relationships between baseline socioeconomic status and long-term outcomes for individuals living with BPD. Although several studies suggested higher socioeconomic status may be related to better symptomatic or functional consequnces, Reference Keck, McElroy, Strakowski, West, Sax and Hawkins3–Reference Tse, Chan, Ng and Yatham5 other studies failed to detect significant influences of socioeconomic status on readmissions, global assessment of functioning and time with depressive/manic symptoms in longitudinal follow-ups. Reference Perlick, Rosenheck, Clarkin, Sirey and Raue6,Reference Carlson, Kotov, Chang, Ruggero and Bromet7 In comparison with the general population, the mortality rate is substantially higher for individuals with BPD, considered as one of the serious mental illnesses, Reference Hoang, Stewart and Goldacre8,Reference Wahlbeck, Westman, Nordentoft, Gissler and Laursen9 and this mortality gap may be getting wider in recent years. Reference Lawrence, Hancock and Kisely10 Given the well-recognised differences in mortality across socioeconomic groups, Reference Stringhini, Dugravot, Shipley, Goldberg, Zins and Kivimaki11 data exploring the impacts of socioeconomic deprivation on excess mortality in people with BPD remain wanting.
Regarding the existing evidence, there are some flaws. First, earlier studies on socioeconomic status and treatment outcomes only recruited hospitalised individuals from a single centre. Reference Keck, McElroy, Strakowski, West, Sax and Hawkins3,Reference Perlick, Rosenheck, Clarkin, Sirey and Raue6 The generalisability is questionable because socioeconomic status may vary considerably across catchment areas. Second, nearly all of the previous studies were from the USA, Reference Keck, McElroy, Strakowski, West, Sax and Hawkins3,Reference Craig, Grossman, Mojtabai, Gibson, Lavelle and Carlson4,Reference Perlick, Rosenheck, Clarkin, Sirey and Raue6,Reference Carlson, Kotov, Chang, Ruggero and Bromet7 where financial barriers to healthcare services were extremely high to the most disadvantaged people. Reference Woods12 Third, the applied socioeconomic status measurements mainly focused on the affected person's education level and recent occupations which could be the consequences of illness resulting from early social decline. Reference Bebbington and Ramana13 Lastly, very few studies explored the socioeconomic status effects on outcome of excess mortality in the populations with serious mental illnesses. Reference Martin, McLean, Park, Martin, Connolly and Mercer14 None of them specifically examined the relationships between socioeconomic deprivation and all-cause mortality in people with BPD.
Therefore, the current exploratory study, using the claims data from the National Health Insurance Research Database (NHIRD) in Taiwan, sought to explore the impacts of baseline socioeconomic status at both personal and household levels on longitudinal outcomes of hospital treatment and mortality in a nationally representative sample of people with newly diagnosed BPD. Considering the need to understand factors affecting healthcare costs and to alleviate healthcare cost burden, the associations between socioeconomic status measurements and healthcare costs were also explored in a follow-up period of consecutive 3 years, from the perspectives of healthcare providers.
Method
Setting
Taiwan is a country with a population of around 23 million. On a purchasing power parity basis, its gross domestic product per capita in 2008/2009 was 31 100/32 000 international dollars. National Health Insurance (NHI) in Taiwan is a single-payer compulsory social insurance system which centralises the disbursement of healthcare funds and guarantees equal access to healthcare for all citizens. In 2008, a total of 22.92 million individuals were involved in Taiwan's NHI programme with a coverage rate of 99.48%. 15 The NHIRD consists of data characterising healthcare utilisation of insured residents, including expenditures, medical procedures/treatments and basic demographic characteristics. Diagnosis in NHIRD is given with the International Classification of Diseases, 9th revision, clinical modification diagnoses (ICD-9-CM). In this study, all participants were first identified from the NHIRD, and the index date was defined as the date on which the participant was first diagnosed with BPD (ICD-9-CM codes: 296.0, 296.1, 296.4–296.7) in 2008. Data on all NHI information for each participant were extracted for the 1 year preceding, and 3 years following the index date. The study protocol was approved by the Research Ethics Review Committee of Far Eastern Memorial Hospital, Taiwan (No. 102006-F).
Participants
All participants meeting the following criteria in NHIRD were included in the study.
-
They were diagnosed with BPD in 2008.
-
They were aged 18 years or above on the index date.
-
Data were available for a minimum of 12 months before and 36 months after the index date.
-
They did not have a diagnosis of BPD in the year preceding the index date.
To ensure the validity of the clinical diagnoses, participants needed to have at least four out-patient visits because of treatment of BPD or one hospital treatment with BPD as the primary diagnosis in the year following the index date.
Service use and costs
The extracted service use components included out-patient services, emergency attendances and in-patient stays. Service use during the preceding year and over the consecutive 3-year study period following the index date was extracted. All costs were calculated from the actual claims data, and expressed in 2008–2009 New Taiwan Dollar (NTD; the implied purchasing power parity conversion rate between 2008–2009 NTD and International Dollar is 16.99:1). 16
Demographic/clinical information and baseline socioeconomic status measurements
Demographic and clinical data were extracted, including age, gender, physician specialty clinical setting and type of mood episode on the index date. The baseline socioeconomic status variables included low-income household as recognised by the government (those whose total income divided by the number of household members is lower than the minimum cost of living, which is set at 60% of the average monthly per capita non-productive expenditure during the past year), insurance premium level (the monthly salary-based income of the insured, which is categorised into four levels: above NTD 72 801, NTD 36 301–72 800, NTD 17 281–36 300, below NTD 17 280 (International Dollar 1=NTD 16.99)), 16 and urbanisation level of residence (urbanisation stratifications of townships in Taiwan according to population density, population ratio of people with college or above educational levels, population ratio of people of agricultural employment population ratio of elderly people and physician density) Reference Liu, Hung, Chuang, Chen, Weng and Liu17 on the index date. For all participants, comorbidities of mental and physical illnesses, as well as healthcare utilisation/expenditure, were traced back for the 12 months prior to the index date.
Healthcare utilisation pattern within the first year of index diagnosis
Healthcare utilisation pattern was operationally defined by whether the individual had been admitted for treatment of BPD as well as the frequency of out-patient visits for BPD treatment within the first year of diagnosis. Because the individual who had been admitted for treatment of BPD may differ considerably from those receiving treatments exclusively at out-patient settings, we categorised study participants into three mutually exclusive groups based on the healthcare utilisation pattern for BPD treatment within the first year: (1) those who were admitted for treatment of BPD; (2) those having ≥7 out-patient clinic visits; and (3) those having four to six out-patient clinic visits.
Data analysis
Basic demographic/clinical data, socioeconomic status measurements and healthcare expenditure for the preceding year were described for the overall sample and compared among groups according to their first-year healthcare utilisation patterns. Survival analyses with Kaplan–Meier curves and Cox regressions were employed to estimate the effects of baseline socioeconomic status factors on the risks of being hospitalised within the consecutive 3 years. The considered confounding factors included demographic/clinical information and comorbid physical/mental illnesses within the preceding year.
To further identify characteristics predictive of healthcare costs over the second and third years respectively generalised linear regression models with a log link and gamma variance function and baseline socioeconomic status variables as major predictors of interest were employed. Reference McCullagh and Nelder18 The generalised linear model allows a generalisation of the response distribution to members of the exponential family, including gamma, inverse Gaussian and binomial distributions. Given the assumption for a constant coefficient of variation, the gamma distribution has been found to be appropriate for cost analyses. In a generalised linear model, the regression equation is called the ‘linear predictor’ but this linear predictor is not equated with the expected cost, but via ‘link function’. The log link is commonly used when analysing cost data because it guarantees non-negative outcomes and has a close connection to the logarithmic transformation of data. This generalised linear model approach has been commonly used in analyses of economic cost data. Reference Desai, Lawson, Barner and Rascati19
Standardised mortality ratios (SMRs) were calculated for the 3-year observation period. Death was defined as withdrawal of the person from the NHI programme. Reference Wu, Chen, Ho, Hsu, Kuo and Wu20 SMRs were calculated using age (namely 18–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, 85–89 and 90+) and gender strata. Reference Chang, Hayes, Broadbent, Fernandes, Lee and Hotopf21 The number of deaths observed in these 3 years represented the numerator. The denominator was the expected number of deaths in a year estimated by age- and gender-specific mortality statistics for the Taiwanese population in 2009/2010 multiplied by three. To assure for the validity of this proxy definition of death status, a set of sensitivity analysis for SMRs was calculated with another target population consisting of people who had no diagnoses of mental illnesses during medical encounters in 2008, retrieved from a random sample of 200 000 beneficiaries from the Longitudinal Health Insurance Database 2005, which provided a population representative cohort including nearly one million of the year 2005 Registry of Beneficiaries (22.17 million) under Taiwan's NHI programme. All statistical analyses were performed via SPSS version 17.0 (Chicago, Illinois, USA). Alpha level was set at 0.05 (P-value) for statistical significance.
Results
Initially, there were 15 254 adult participants with newly diagnosed BPD. Among them, 7267 were subsequently excluded because they did not have at least four out-patient visits because of treatment of BPD or one hospital treatment with BPD as the primary diagnosis in the year following the index date. There are totally 7987 participants with newly diagnosed BPD included in the current analyses.
On the index date, the mean age was 44.3 (s.d.=16.2), around 40.8% male and 3.3% classified as from low-income households. The income premium distributions from the lowest to the highest level were 58.5%, 33.6%, 7.1% and 0.8% respectively. At first diagnosis, the affective presentations were manic (47.1%), mixed (13.6%), depressed (26.5%) and unspecified (12.8%). Within the first year, 29.2% of the participants were admitted at least once for BPD treatments (Table 1).
Table 1 Sociodemographic and clinical characteristics, stratified by healthcare utilisation pattern a within the first year of index diagnosis

| Characteristics | Hospital treatment (n=2329) | OPD
≥7 (n=3823) |
OPD
4–6 (n=1835) |
Total (n=7987) |
Significance |
|---|---|---|---|---|---|
| Age [mean (s.d.)] | 43.84 (16.23) | 44.05 (15.78) | 45.48 (16.97) | 44.32 (16.20) | F=6.252, P=0.002* |
| Gender [n (%)] | χ2=59.600, P<0.001** | ||||
| Male | 1104 (47.4) | 1442 (37.7) | 715 (39.0) | 3261 (40.8) | |
| Female | 1225 (52.6) | 2381 (62.3) | 1120 (61.0) | 4726 (59.2) | |
| Low-income household [n (%)] | 98 (4.2) | 117 (3.1) | 49 (2.7) | 264 (3.3) | χ2=8.968, P=0.011* |
| Insurance premium b [n (%)] | χ2=59.702, P<0.001** | ||||
| Level (1) | 1487 (63.8) | 2155 (56.4) | 1033 (56.3) | 4675 (58.5) | |
| Level (2) | 725 (31.1) | 1325 (34.7) | 631 (34.4) | 2681 (33.6) | |
| Level (3) | 110 (4.7) | 310 (8.1) | 149 (8.1) | 569 (7.1) | |
| Level (4) | 7 (0.3) | 33 (0.9) | 22 (1.2) | 62 (0.8) | |
| Urbanisation level c [n (%)] | χ2=42.778, P<0.001** | ||||
| Level (1) | 669 (28.7) | 1183 (30.9) | 631 (34.4) | 2483 (31.1) | |
| Level (2) | 648 (27.8) | 1191 (31.2) | 527 (28.7) | 2366 (29.6) | |
| Level (3) | 332 (14.3) | 541 (14.2) | 264 (14.4) | 1137 (14.2) | |
| Level (4) | 358 (15.4) | 489 (12.8) | 212 (11.6) | 1059 (13.3) | |
| Level (5) | 37 (1.6) | 44 (1.2) | 28 (1.5) | 109 (1.4) | |
| Level (6) | 84 (3.6) | 105 (2.7) | 54 (2.9) | 243 (3.0) | |
| Level (7) | 201 (8.6) | 270 (7.1) | 119 (6.5) | 590 (7.4) | |
| Clinical setting at index visit [n (%)] | χ2=4487.668, P<0.001** | ||||
| OPD | 721 (31.0) | 3743 (97.9) | 1777 (96.8) | 6241 (78.1) | |
| ER | 227 (9.7) | 80 (2.1) | 58 (3.2) | 365 (4.6) | |
| In-patient | 1381 (59.3) | 0 (0.0) | 0 (0.0) | 1381 (17.3) | |
| Physician type at index visit [n (%)] | χ2=113.512, P<0.001** | ||||
| Non-psychiatrist | 599 (25.7) | 683 (17.9) | 545 (29.7) | 1827 (22.9) | |
| Psychiatrist | 1730 (74.3) | 3140 (82.1) | 1290 (70.3) | 6160 (77.1) | |
| Catastrophic illness card d [n (%)] | 863 (37.1) | 883 (23.1) | 323 (17.6) | 2069 (25.9) | χ2=232.449, P<0.001** |
| Initial diagnosis codes e [n (%)] | χ2=716.779, P<0.001** | ||||
| 296.0 | 308 (13.2) | 635 (16.6) | 435 (23.7) | 1378 (17.3) | |
| 296.1 | 228 (9.8) | 220 (5.8) | 132 (7.2) | 580 (7.3) | |
| 296.4 | 813 (34.9) | 693 (18.1) | 292 (15.9) | 1798 (22.5) | |
| 296.5 | 316 (13.6) | 1245 (32.6) | 559 (30.5) | 2120 (26.5) | |
| 296.6 | 208 (8.9) | 627 (16.4) | 253 (13.8) | 1088 (13.6) | |
| 296.7 | 456 (19.6) | 403 (10.5) | 164 (8.9) | 1023 (12.8) | |
| Total healthcare costs in
the preceding year (in 1000 NTD) [mean (s.d.)] |
77 (133) | 56 (100) | 51 (99) | 61 (111) | F=34.863, P<0.001** |
| Comorbid physical illness f [n (%)] | |||||
| Hypertension | 454 (19.5) | 736 (19.3) | 387 (21.1) | 1577 (19.7) | χ2=2.774, P=0.250 |
| Diabetes mellitus | 248 (10.6) | 408 (10.7) | 181 (9.9) | 837 (10.5) | χ2=0.964, P=0.618 |
| Renal disease | 100 (4.3) | 138 (3.6) | 81 (4.4) | 319 (4.0) | χ2=2.863, P=0.239 |
| Cancer | 84 (3.6) | 118 (3.1) | 67 (3.7) | 269 (3.4) | χ2=1.790, P=0.409 |
| Cardiovascular disease | 406 (17.4) | 692 (18.1) | 411 (22.4) | 1509 (18.9) | χ2=19.517, P<0.001** |
| Chronic obstructive pulmonary disease | 273 (11.7) | 424 (11.1) | 209 (11.4) | 906 (11.3) | χ2=0.578, P=0.749 |
| Stroke | 76 (3.3) | 123 (3.2) | 74 (4.0) | 273 (3.4) | χ2=2.736, P=0.255 |
| Parkinson's disease | 51 (2.2) | 75 (2.0) | 48 (2.6) | 174 (2.2) | χ2=2.490, P=0.288 |
| Comorbid painful physical symptom f [n (%)] | |||||
| Headache/migraine/dizziness | 699 (30.0) | 1382 (36.1) | 692 (37.7) | 2773 (34.7) | χ2=33.459, P<0.001** |
| Back pain | 525 (22.5) | 1060 (27.7) | 522 (28.4) | 2107 (26.4) | χ2=25.273, P<0.001** |
| Comorbid mental illness f [n (%)] | |||||
| Major depression | 556 (23.9) | 1360 (35.6) | 474 (25.8) | 2390 (29.9) | χ2=113.530, P<0.001** |
| Other depression | 598 (25.7) | 1238 (32.4) | 502 (27.4) | 2338 (29.3) | χ2=35.666, P<0.001** |
| Schizophrenia | 421 (18.1) | 353 (9.2) | 103 (5.6) | 877 (11.0) | χ2=185.993, P<0.001** |
| Other psychotic disorder | 228 (9.8) | 199 (5.2) | 76 (4.1) | 503 (6.3) | χ2=70.309, P<0.001** |
| Substance use disorder | 187 (8.0) | 215 (5.6) | 90 (4.9) | 492 (6.2) | χ2=20.981, P<0.001** |
| Alcohol use disorders | 77 (3.3) | 37 (1.0) | 19 (1.0) | 133 (1.7) | χ2=54.097, P<0.001** |
| Hyperkinetic syndrome | 3 (0.1) | 14 (0.4) | 10 (0.5) | 27 (0.3) | χ2=5.448, P=0.066 |
| Panic disorder | 49 (2.1) | 207 (5.4) | 69 (3.8) | 325 (4.1) | χ2=41.221, P<0.001** |
| Deaths in first year of follow-up [n (%)] | 92 (4.0) | 47 (1.2) | 47 (2.6) | 186 (2.3) | χ2=47.670, P<0.001** |
| Deaths in second year of follow-up [n (%)] | 151 (6.5) | 107 (2.8) | 78 (4.3) | 336 (4.2) | χ2=48.770, P<0.001** |
| Deaths in third year of follow-up [n (%)] | 215 (9.2) | 164 (4.3) | 118 (6.4) | 497 (6.2) | χ2=60.742, P<0.001** |
| Hospital treatment in first year of follow-up g [n (%)] | 1883 (80.9) | 205 (5.4) | 106 (5.8) | 2194 (27.5) | χ2=4775.633, P<0.001** |
| Hospital treatment in second year of follow-up g [n (%)] | 1907 (81.9) | 382 (10.0) | 157 (8.6) | 2446 (30.6) | χ2=4161.064, P<0.001** |
| Hospital treatment in third year of follow-up g [n (%)] | 1917 (82.3) | 477 (12.5) | 188 (10.2) | 2582 (32.3) | χ2=3841.458, P<0.001** |
s.d., Standard deviation; OPD, out-patient department; ER, emergency room.
a Study participants were grouped by first-year healthcare utilisation pattern for treatment of BPD: hospital treatment, participants who had been admitted at least once during the first year; OPD ≥7, participants who had not been admitted and had ≥7 out-patient visits during the first year; OPD 4–6, participants who had not been admitted and had 4–6 out-patient visits during the first year.
b Insurance premium was classified into four different levels: Level (1): Under 17 280 NTD; Level (2): Between 17 281 NTD and 36 300 NTD; Level (3): Between 36 301 NTD and 72 800 NTD; Level (4): Above 72 801 NTD.
c Urbanisation was classified into seven different levels: Level (1): Metropolitan city; Level (2): City; Level (3): Developing city; Level (4): Town; Level (5): Ageing population town; Level (6): Agricultural town; Level (7): Rural area.
d If a patient is diagnosed with a catastrophic illness by a physician under Ministry of Health and Welfare guidelines, the patient can apply for a catastrophic illness card with which the patient does not need to pay a copayment for out-patient or in-patient care for related conditions.
e 296.0, 296.1, 296.4 represents manic states; 296.5 represents depressive states; 296.6 represents mixed states; and 296.7 represents unspecified mood states.
f Comorbid physical and mental illnesses were measured over the 12-month pre-index period. Costs were expressed in New Taiwan Dollar (NTD). Chi-squared test was used for comparing categorical variables between groups by first-year healthcare utilisation pattern and ANOVA was used for comparisons of continuous variables.
g Hospital treatment during which the patients were first diagnosed on the index dates were not included.
*P<0.05, **P<0.001.
Baseline socioeconomic status in relation to hospital treatment outcome
The Kaplan–Meier curves showed that individuals from low-income households had remarkably worse survival curves in terms of hospital treatment compared with the others with a log-rank test of P<0.01 in each of 1-, 2- and 3-year follow-ups. Individuals with lower premium levels also significantly differed in survival curves regarding hospital treatment compared with those with higher premium levels (Fig. 1). The results of Cox regression revealed that the group of low-income household had an over 40% increase in the risk of hospital treatment in each of the 1-year, 2-year and 3-year follow-ups. Compared with the group with the highest insurance premium, the lower premium levels predicted hospital treatment in the 2-year and 3-year follow-ups. Urbanisation level was not significantly associated with hospital treatment. Apart from the socioeconomic status factors, younger age and male gender were shown to be associated with higher rates of being hospitalised (Table 2).
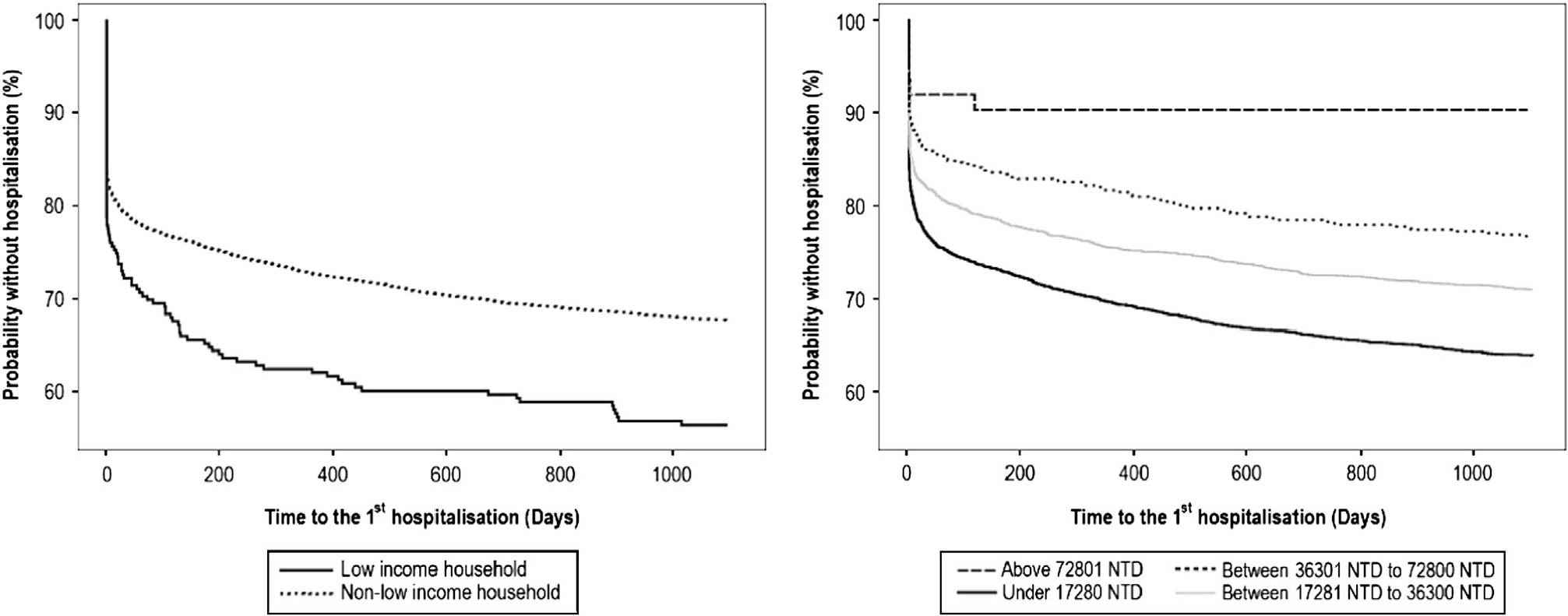
Fig. 1 Socioeconomic status groups and time to the first hospital treatment.
Table 2 Factors predicting hospital treatment in 1-year, 2-year and 3-year follow-up

| 1-year follow-up | 2-year follow-up | 3-year follow-up | ||||
|---|---|---|---|---|---|---|
| Exp(β) | 95% CI | Exp(β) | 95% CI | Exp(β) | 95% CI | |
| Age | 0.993 | (0.990, 0.997)** | 0.993 | (0.990, 0.996)** | 0.993 | (0.989, 0.996)** |
| Gender | ||||||
| Male v. female | 1.115 | (1.021, 1.218)* | 1.129 | (1.038, 1.227)* | 1.141 | (1.052, 1.238)* |
| Low-income household | ||||||
| Yes v. no | 1.429 | (1.152, 1.771)* | 1.430 | (1.163, 1.758)* | 1.461 | (1.196, 1.785)** |
| Insurance premium a | ||||||
| Level (1) v. Level (4) | 2.100 | (0.870, 5.069) | 2.572 | (1.066, 6.205)* | 2.837 | (1.176, 6.842)* |
| Level (2) v. Level (4) | 1.871 | (0.774, 4.522) | 2.264 | (0.937, 5.466) | 2.473 | (1.024, 5.969)* |
| Level (3) v. Level (4) | 1.723 | (0.701, 4.233) | 2.147 | (0.877, 5.257) | 2.366 | (0.967, 5.786) |
| Urbanisation level b | ||||||
| Level (1) v. Level (7) | 0.951 | (0.804, 1.123) | 0.953 | (0.813, 1.118) | 0.948 | (0.812, 1.108) |
| Level (2) v. Level (7) | 0.941 | (0.797, 1.111) | 0.956 | (0.816, 1.120) | 0.960 | (0.822, 1.120) |
| Level (3) v. Level (7) | 0.924 | (0.769, 1.112) | 0.947 | (0.795, 1.129) | 0.940 | (0.792, 1.115) |
| Level (4) v. Level (7) | 0.997 | (0.831, 1.197) | 0.995 | (0.836, 1.185) | 1.008 | (0.850, 1.195) |
| Level (5) v. Level (7) | 1.215 | (0.859, 1.720) | 1.262 | (0.909, 1.751) | 1.294 | (0.941, 1.778) |
| Level (6) v. Level (7) | 1.002 | (0.771, 1.302) | 1.019 | (0.793, 1.308) | 1.061 | (0.833, 1.351) |
| Clinical setting at index visit | ||||||
| OPD v. in-patient | 0.051 | (0.044, 0.058)** | 0.059 | (0.052, 0.067)** | 0.063 | (0.056, 0.072)** |
| ER v. in-patient | 0.842 | (0.718, 0.987)* | 0.813 | (0.696, 0.948)* | 0.797 | (0.683, 0.928)* |
| Physician type at index visit | ||||||
| Psychiatrist v. non-psychiatrist | 5.741 | (4.911, 6.711)** | 4.975 | (4.302, 5.753)** | 4.743 | (4.117, 5.463)** |
| Catastrophic card | ||||||
| Yes v. no | 1.456 | (1.318, 1.609)** | 1.531 | (1.393, 1.683)** | 1.563 | (1.425, 1.714)** |
| Initial diagnosis codes | ||||||
| 296.0 v. 296.7 | 1.575 | (1.329, 1.868)** | 1.640 | (1.399, 1.922)** | 1.652 | (1.415, 1.928)** |
| 296.1 v. 296.7 | 1.658 | (1.370, 2.007)** | 1.632 | (1.359, 1.958)** | 1.641 | (1.373, 1.961)** |
| 296.4 v. 296.7 | 1.848 | (1.594, 2.143)** | 1.885 | (1.638, 2.169)** | 1.911 | (1.666, 2.192)** |
| 296.5 v. 296.7 | 1.012 | (0.854, 1.200) | 0.998 | (0.851, 1.172) | 1.023 | (0.876, 1.195) |
| 296.6 v. 296.7 | 1.131 | (0.934, 1.368) | 1.144 | (0.958, 1.367) | 1.169 | (0.984, 1.389) |
| Total healthcare costs in the preceding year (in 1000 NTD) | 1.001 | (1.000, 1.001)** | 1.001 | (1.000, 1.001)** | 1.001 | (1.000, 1.001)** |
Note: Other adjusted variables (not shown here) in the Cox regressions included comorbid physical and mental illnesses over the 12-month pre-index period.
OPD, Out-patient department; ER, emergency room; CI, confidence interval; NTD, New Taiwan Dollar.
a Insurance premium was classified into four different levels: Level (1): Under 17 280 NTD; Level (2): Between 17 281 NTD and 36 300 NTD; Level (3): Between 36 301 NTD and 72 800 NTD; Level (4): Above 72 801 NTD.
b Urbanisation was classified into seven different levels: Level (1): Metropolitan city; Level (2): City; Level (3): Developing city; Level (4): Town; Level (5): Ageing population town; Level (6): Agricultural town; Level (7): Rural area.
* P<0.05, **P<0.001.
Baseline socioeconomic status in relation to future total healthcare costs
As revealed in Table 3, low-income household predicted higher healthcare costs in the second and third years respectively, whereas the individual's baseline premium levels did not significantly correlate with future healthcare costs. Urbanisation levels also correlated with future total healthcare costs in the second and third years. With regard to other factors, older age and male gender were shown to be associated with higher total healthcare costs in the second and third years (Table 3).
Table 3 Factors predicting total healthcare costs in the second and third year after index diagnosis

| Exp(β) | ||
|---|---|---|
| Second-year costs | Third-year costs | |
| First-year healthcare utilisation pattern a | ||
| Hospital treatment v. OPD 4–6 | 2.267 (2.068, 2.485)** | 2.192 (1.998, 2.406)** |
| OPD ≥7 v. OPD 4–6 | 1.242 (1.168, 1.32)** | 1.177 (1.106, 1.253)** |
| Age | 1.01 (1.008, 1.012)** | 1.009 (1.007, 1.011)** |
| Gender | ||
| Male v. female | 1.053 (1.001, 1.107)* | 1.095 (1.040, 1.153)* |
| Low-income household | ||
| Yes v. no | 1.883 (1.642, 2.160)** | 1.480 (1.285, 1.703)** |
| Insurance premium b | ||
| Level (1) v. Level (4) | 1.072 (0.822, 1.398) | 0.959 (0.729, 1.262) |
| Level (2) v. Level (4) | 0.936 (0.716, 1.222) | 0.878 (0.666, 1.157) |
| Level (3) v. Level (4) | 0.783 (0.594, 1.033) | 0.821 (0.617, 1.094) |
| Urbanisation level c | ||
| Level (1) v. Level (7) | 1.125 (1.021, 1.241)* | 1.108 (1.003, 1.224)* |
| Level (2) v. Level (7) | 1.143 (1.037, 1.260)* | 1.141 (1.033, 1.261)* |
| Level (3) v. Level (7) | 1.053 (0.946, 1.173) | 1.083 (0.970, 1.210) |
| Level (4) v. Level (7) | 1.108 (0.994, 1.236) | 1.162 (1.039, 1.299)* |
| Level (5) v. Level (7) | 1.082 (0.866, 1.350) | 1.363 (1.084, 1.713)* |
| Level (6) v. Level (7) | 1.015 (0.862, 1.195) | 1.101 (0.932, 1.301) |
| Clinical setting at index visit | ||
| OPD v. in-patient | 1.107 (1.003, 1.221)* | 1.238 (1.120, 1.368)** |
| ER v. in-patient | 1.334 (1.167, 1.526)** | 1.331 (1.160, 1.528)** |
| Physician type at index visit | ||
| Psychiatrist v. non-psychiatrist | 0.979 (0.914, 1.048) | 0.936 (0.871, 1.006) |
| Catastrophic card | ||
| Yes v. no | 1.534 (1.443, 1.630)** | 1.506 (1.415, 1.603)** |
| Initial diagnosis codes | ||
| 296.0 v. 296.7 | 1.041 (0.948, 1.143) | 1.021 (0.927, 1.124) |
| 296.1 v. 296.7 | 1.111 (0.991, 1.245) | 1.065 (0.947, 1.198) |
| 296.4 v. 296.7 | 1.094 (1.004, 1.191)* | 1.101 (1.009, 1.201)* |
| 296.5 v. 296.7 | 1.002 (0.921, 1.090) | 0.956 (0.877, 1.042) |
| 296.6 v. 296.7 | 1.045 (0.951, 1.150) | 1.016 (0.921, 1.121) |
| Total healthcare costs in the preceding year (in 1000 NTD) | 1.003 (1.003, 1.003)** | 1.003 (1.002, 1.003)** |
Note: Other adjusted variables (not shown here) in the cost models included comorbid physical and mental illnesses over the 12-month pre-index period.
OPD, Out-patient department; ER, emergency room; CI, confidence interval; NTD, New Taiwan Dollar.
a Study participants were grouped by first-year healthcare utilisation pattern for treatment of BPD: hospital treatment, participants who had been admitted at least once during the first year; OPD ≥7, participants who had not been admitted and had ≥7 out-patient visits during the first year; OPD 4–6, participants who had not been admitted and had 4–6 out-patient visits during the first year.
b Insurance premium was classified into four different levels: Level (1): Under 17 280 NTD; Level (2): Between 17 281 NTD and 36 300 NTD; Level (3): Between 36 301 NTD and 72 800 NTD; Level (4): Above 72 801 NTD.
c Urbanisation was classified into seven different levels: Level (1): Metropolitan city; Level (2): City; Level (3): Developing city; Level (4): Town; Level (5): Ageing population town; Level (6): Agricultural town; Level (7): Rural area.
* P<0.05, **P<0.001.
Baseline socioeconomic status in relation to mortality outcome
In this group of newly diagnosed BPD, a nearly threefold increase of relative risk of mortality was identified (SMR=2.97, 95% CI=2.71, 3.24) with age- and gender-standardisation. Men with newly diagnosed BPD had an SMR of 3.42 (95% CI=3.04, 3.82), whereas their female counterparts had an SMR of 2.45 (95% CI=2.12, 2.83). A sensitivity analysis for the control group of people without mental illness diagnoses yielded an SMR of 1.08 (95% CI=1.04, 1.12). With regard to baseline socioeconomic status factors, BPD individuals with the lowest premium level had an SMR of 3.00 (95% CI=2.68, 3.33). For participants whose premium levels were NTD 17 281–36 300, 36 301–72 800 and ≥72 801, the SMRs were 3.01 (95% CI=2.54, 3.54), 2.29 (95% CI=1.28, 3.77) and 2.07 (95% CI=0.25, 7.48) respectively. More notably, the mortality risk of bipolar disorder participants from low-income households was over five times higher than the general population (SMR for low-income males=5.51, 95% CI=3.21, 8.81; SMR for low-income females=6.47, 95% CI=2.96, 12.28; Table 4).
Table 4 Standardised mortality ratios

| Participant group | SMR
a
for death in 2008–2011 (95% CI) |
|---|---|
| All BPD individuals | 2.97 (2.71–3.24)* |
| Male | 3.42 (3.04–3.82)* |
| Female | 2.45 (2.12–2.83)* |
| BPD individuals from low-income households | 5.80 (3.79–8.51)* |
| Male | 5.51 (3.21–8.81)* |
| Female | 6.47 (2.96–12.28)* |
| Participants without mental illness (sensitive analysis) | 1.08 (1.04–1.12)* |
| Male | 1.02 (0.98–1.07) |
| Female | 1.18 (1.11–1.24)* |
CI, confidence interval.
a Compared with Taiwan's general population in 2009–2010.
* P<0.05.
Discussion
Based on a nationally representative sample of participants with newly diagnosed BPD, the current study has provided evidence of the negative impacts of socioeconomic deprivation on longitudinal outcomes of hospital treatment and mortality. It also provided the rarely available data concerning the associations between baseline socioeconomic status measurements and total healthcare costs over the consecutive 3 years. This is the first study specifically addressing the effects of baseline socioeconomic status measurements at both personal and household levels on outcomes of hospital treatment, excess mortality and total healthcare costs of participants with newly diagnosed BPD.
The current results indicated the presence of differential associations between BPD patients’ hospital treatment, healthcare costs and baseline socioeconomic status measurements at personal and household levels. Briefly, the individual's baseline premium level (as a proxy for personal socioeconomic status) predicted hospital treatment but not total healthcare costs in the following years. Contrarily, low-income household (as a proxy for family socioeconomic status) predicted both hospital treatment and total healthcare costs in the following years. In keeping with past studies showing that BPD participant's premorbid functioning best predicts future outcome, Reference Craig, Grossman, Mojtabai, Gibson, Lavelle and Carlson4 our results suggested that the higher baseline premium levels, roughly correlated with better premorbid occupational functioning of the affected individuals, the lower the likelihoods of being hospitalised in the second and third years after the index date. However, only household income (but not the affected individual's baseline premium level) predicted the individual's total healthcare costs, implying that the family level index may be the better socioeconomic status measurement in this context, which reflects the collective financial and social resources available to the affected individual to consume. From the perspective of healthcare providers, individuals from low-income households may place a greater burden on medical resources probably because of lack of alternative resources, which underlines the need to address inequalities and the importance of resources reallocation. If other family and social costs are taken into consideration, whether bipolar disorder individuals across socioeconomic groups differ in the costs to the society remains to be determined.
The higher rates of hospital treatment and the over five times higher SMR in bipolar disorder individuals from low-income households correspond with the poverty-related barriers to healthcare. 22 Although unaffordable insurance premiums or copayments have been considered as barriers to effective healthcare, this is not the major cause here because the exemptions of health insurance premiums and copayments make all health services and treatments in Taiwan essentially free for those from low-income households. On the other hand, the presence of other barriers – lack of knowledge or fear of stigma – may at least partly account for the poorer outcomes of hospital treatment and excess mortality. For instance, education levels and certain ethnic minorities have been reported to be associated with stigma and adherence to treatments in people with BPD. Reference Fleck, Keck, Corey and Strakowski23,Reference Johnson, Ozdemir, Manjunath, Hauber, Burch and Thompson24 Although lifestyle and health-related behaviours have been considered major determinants of the population distribution of health and disease, barriers such as material constraints, and limited opportunities to take up health promoting messages may further prohibit lower socioeconomic groups to adopt a healthy lifestyle. Reference Macintyre25–Reference Byberg, Melhus, Gedeborg, Sundstrom, Ahlbom and Zethelius28 Indeed, we found that those from low-income households had more physical and mental comorbidities in the current study cohort. Considering the well-recognised associations between socioeconomic deprivation and multimorbidity, Reference Violan, Foguet-Boreu, Roso-Llorach, Rodriguez-Blanco, Pons-Vigues and Pujol-Ribera29 these participants from low-income households may suffer from increased morbidities, thus leading to a more refractory disease course, poorer hospital treatment outcomes, higher treatment costs and ultimately higher mortality rates. Special care should be taken to address the unmet needs of de-stigmatisation and to enhance adherence as well as to reduce health-harming behaviours for those who are socioeconomically disadvantaged.
Another plausible explanation for the differential outcomes across socioeconomic groups with BPD may arise from potential differences in the disease subtypes. For instance, family income was shown to be associated with self-reported mood symptoms in bipolar disorder individuals. Reference Bauer, Glenn, Rasgon, Marsh, Sagduyu and Munoz30 More specifically, a subtype of BPD, the lithium-responsive form, was reported to be associated with higher socioeconomic status compared with those continuing to relapse. Reference Eid, Heim, Doucette, McCloskey, Duffy and Grof31 This subtype was linked to a strong family history Reference Calabrese, Fatemi, Kujawa and Woyshville32,Reference Bowden33 and more likely to present with a non-rapid cycling course with full remission between episodes. Reference Ruzickova, Turecki and Alda34 In accordance with the aforementioned study showing higher socioeconomic status of people with lithium-responsive BPD, Reference Eid, Heim, Doucette, McCloskey, Duffy and Grof31 offspring of lithium responder parents with BPD were also shown to be more socially successful than those of lithium non-responders. Reference Duffy, Alda, Kutcher, Cavazzoni, Robertson and Grof35 Because many of the earlier studies on socioeconomic status and risk for BPD examined samples of participants who were mainly lithium-responsive BPD – during the era when the diagnosis of BPD was used primarily for individuals with classical presentation, lithium was reported to be helpful for up to 80% of cases, Reference Schou and Thomsen36 whereas in recent studies using broad criteria, the benefit of lithium decreased tremendously, ranging from non-existent Reference Bowden, Calabrese, McElroy, Gyulai, Wassef and Petty37 to only 30%, Reference Garnham, Munro, Slaney, Macdougall, Passmore and Duffy38 the predominant findings between higher socioeconomic status and risk of BPD in earlier literature Reference Bebbington and Ramana13,Reference Hirschfeld and Cross39 may be due, in part, to the over-representation of lithium-responsive BPD. Future study to further elucidate the socioeconomic status effects on outcomes in bipolar individuals of different subtypes is warranted.
In people with BPD, younger age was ever reported to have negative effects on the odds of remission Reference Craig, Grossman, Mojtabai, Gibson, Lavelle and Carlson4 as well as re-hospital treatment at longitudinal follow-ups. Reference Perlick, Rosenheck, Clarkin, Sirey and Raue6,Reference Carlson, Kotov, Chang, Ruggero and Bromet7 In agreement with previous findings, we found that younger age was associated with higher odds of hospital treatment. We also found that male gender predicted higher likelihoods of being hospitalised as well as higher total healthcare costs over the following years. Indeed, existing evidence suggested that both young age and male gender negatively affected adherence to treatment in individuals with BPD. Reference Berk, Berk and Castle40 Additionally, we found a considerably higher SMR in female patients with BPD who were from low-income households. Although some evidence indicated a stronger association between low socioeconomic status and high probability of multimorbidity in women than in men, Reference Violan, Foguet-Boreu, Roso-Llorach, Rodriguez-Blanco, Pons-Vigues and Pujol-Ribera29 the extent to which the association between socioeconomic status and excess mortality in females with BPD could be attributed to this multimorbidity phenomenon remains to be determined.
Strengths and limitations
The strengths of the current study included whole country coverage, inclusion of bipolar disorder individuals diagnosed in all clinical settings, longitudinal follow-up for consecutive 3 years, examinations of socioeconomic status at both personal and household levels and provision of the rarely available data on the relationship between socioeconomic status, outcomes of hospital treatment and mortality, as well as future healthcare costs, which could be of great interest to both clinicians and policy makers. By including only newly diagnosed BPD individuals, the current study further minimised the bias in the baseline socioeconomic status because of illness-related social decline.
As service use data contained in the NHIRD includes only health services provided by the NHI system in Taiwan, the perspective of the current cost analysis was limited. In lack of exact income data, we performed analyses based on the individual's insurance premium level. Use of proxy definition of death was another limitation but the sensitivity analysis of SMR in a control group of people without mental illnesses yielded a reasonably similar death rate with that of Taiwan's general population. In effort to better assure for the validity of the BPD diagnoses, we only recruited participants who had at least four out-patient visits under diagnoses of BPD or at least one admission because of BPD treatment within the first year in this analysis. With the stringent criterion, we were able to identify a group of bipolar disorder individuals with more definite clinical diagnoses but the generalisability to those with a better illness course or milder form of bipolar spectrum disorders is consequently limited. Given the mean age of 44 years old, the study participants might not be fresh cases who had never been diagnosed or treated for BPD. It should be also borne in mind that sample sizes of certain comparison groups were small, for instance, only 264 BPD individuals were from low-income households, which was a limitation.
In conclusion, the current study, with the design of exploratory nature, suggests that socioeconomic deprivation in people with BPD may be associated with poorer treatment outcomes, excess mortalities and higher total healthcare costs based on a large national database. Special care should be given to those with lower socioeconomic status to improve participants’ health outcomes with potential benefits of cost-savings in the following years.
Funding
This study was supported by grants from the National Science Council of Taiwan (NSC-101-2314-B-418-008) and Far Eastern Memorial Hospital, Taiwan (FEMH-101-2314-B-418-008; FEMH-2015-C-011). The funding bodies played no role in study design, analysis or interpretation of data in this paper.
Acknowledgements
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes, Taiwan. The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.








eLetters
No eLetters have been published for this article.