Attention-deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder, which left untreated can have a significant impact on the lives of affected young people, their families and society. Reference Faraone, Biederman, Spencer, Wilens, Seidman and Mick1,Reference Wilens, McDermott, Biederman, Abrantes, Hahsey and Spencer2 In the UK, the National Institute for Health and Care Excellence (NICE) ADHD guidelines 3 provide the gold standard for the diagnosis and management of ADHD, advocating medication as the first-line treatment for severe or moderate ADHD. The landmark US Multimodal Treatment of ADHD (MTA) study 4 investigated the clinical effectiveness of a medication management strategy which involved a carefully monitored individualised titration period. This protocol resulted in significantly improved ADHD outcomes in the short to medium term. Reference Swanson, Kraemer, Hinshaw, Arnold, Conners and Abikoff5,Reference Jensen, Garcia, Glied, Crowe, Foster and Schlander6 The Texas Children's Medication Algorithm Project Reference Pliszka, Greenhill, Crismon, Sedillo, Carlson and Conners7,Reference Pliszka, Lopez, Crismon, Toprac, Hughes and Emslie8 was developed as a clinical tool to implement the MTA's medication protocol into routine clinical practice. Their findings indicated some deviance from the schedule, with a reluctance to titrate upwards and problems obtaining regular ratings from teachers and parents. Reference Pliszka, Lopez, Crismon, Toprac, Hughes and Emslie8 These findings indicate that despite the MTA medication protocol being considered by many as the US ‘gold standard’ in medication management, the Texas project found that some items were not feasible to implement in routine clinical practice. However, given cross-country differences in clinical practice, including differences in the delivery of ‘community care’ for ADHD and healthcare systems between the USA and UK, these findings may not translate to UK practice. Furthermore, since the MTA study which was conducted in the 1990s, prescribing practices have changed, with the introduction of long-acting ADHD medication.
In the UK, there is evidence of variable implementation of NICE clinical guidelines in ‘real-world’ settings Reference Sheldon, Cullum, Dawson, Lankshear, Lowson and Watt9 and the specific implementation challenges of the NICE ADHD guideline have been highlighted. Reference Atkinson and Hollis10 More recently, audit data collected within the East Midlands region of the UK have demonstrated poor adherence to NICE guidelines, particularly with regard to measuring blood pressure/heart rate, formal recording of side-effects and the use of rating scales to aid assessment and management. Reference Hall, Walker, Valentine, Guo, Kaylor-Hughes and James11 These findings indicate a gap between best practice guidelines and implementation of recommendations in ‘real-world’ practice that needs to be understood and addressed. Given the difficulties in implementing the Texas Algorithm (based on the MTA protocol) in the USA, variable implementation of NICE guidelines in general in the UK and the evidence of poor adherence to aspects of the NICE ADHD guideline, we decided to investigate how the MTA study-based practice parameters and NICE guideline recommendations are perceived by National Health Service (NHS) clinicians with respect to both importance and feasibly for implementation.
We adopted a multi-method approach to determine UK stakeholder (parent/carer and NHS healthcare professional) perceptions of current and best practice clinical guideline recommendations for assessment and medical management of ADHD. Data were collected via an online survey, a parent/carer focus group and a healthcare professional's workshop. These findings were used to generate a set of statements on ADHD medication management which were then rated for importance and feasibility using a Delphi survey method.
Method
Delphi survey development
The items for the Delphi survey were developed through four different methodological approaches. Initially a scoping literature review was conducted. Eight healthcare databases were searched (Allied and Complementary Medicine Database (AMED), British Nursing Index, Embase, HMIC (Health Management Information Consortium), MEDLINE, PsycINFO, CINAHL and Health Business Elite) for papers published in English, using the keywords ADHD and medication prescribing/monitoring/guidelines/titration. Informal searching, including hand-searching of article references and web search engines (including Google and Google Scholar) was also undertaken. In addition, colleagues who have contact with patients with ADHD were also consulted with regard to further literature that might be available. On the basis of this literature search, NICE guidelines were shown as the most prominent and ‘gold-standard’ approach to inform medication monitoring of ADHD in the UK. The MTA and Texas Algorithm were shown to be one of the most cited and influential sources for ADHD practice parameters in the USA. Many of the MTA principles are incorporated within the NICE guideline recommendations. However, the Texas Algorithm provides more specific details on aspects of titration and optimisation than do NICE guideline recommendations and hence we include statements from both sources. The findings from the literature search were used to inform questions for an online survey aimed at establishing healthcare professionals’ perceptions of medication prescribing and monitoring.
Online survey
Eighty-five healthcare professionals responsible for the treatment of children and young people with ADHD across four participating NHS trusts (health provider organisations) within the regional Collaboration for Leadership in Applied Health Research and Care(CLAHRC) footprint were invited to participate in the online survey. Consent to participate was implied by survey participation. Responses were obtained from 26 healthcare professionals (response rate 31%), 12 of whom were community paediatricians (46%) and 14 child and adolescent psychiatrists (54%) working in NHS trusts across three English counties (Nottinghamshire (n=12), Derbyshire (n=9) and Lincolnshire (n=5)). One respondent was excluded from analysis because of low question completion within the survey (1% completion), resulting in a sample of 25 healthcare professionals. Although this response rate was relatively low, it is similar to that found in other online surveys aimed at healthcare professionals working in ADHD. Reference Hall, Newell, Taylor, Sayal, Swift and Hollis12
The survey investigated healthcare professionals’ current practice in diagnosis, initiation of medication, titration, assessment of response to medication and maintenance, which were based on strategies derived from NICE guidelines, the MTA group and the Texas Children's Medication Algorithm Project.
The online survey questions and semi-structured focus groups were devised by drawing on the current literature and consultation within the study group, which included three consultant psychiatrists, one child and adolescent mental health nurse and two researchers with expertise in qualitative methods. Each member reviewed the questions to ascertain their appropriateness to the research question.
Parent/carer focus group
To assess parents/caregivers’ perceptions of medication management for children with ADHD, nine parents/carers of children currently receiving medication for ADHD attended a focus group, having read an information sheet and provided written consent prior to their participation.
The focus group was semi-structured and consisted of 12 questions pertaining to the families’ experiences of starting medication, how and when they were monitored, how information was fed back to them, the effect of the medication and their perceptions of the utility and feasibility of changes to medication management. Audio recordings from the focus group were anonymised, transcribed verbatim and thematically analysed in accordance with the guidelines of Braun & Clarke. Reference Braun and Clarke13 Themes were derived inductively and verified via interrater reliability measures. The researchers utilised an essentiality/realist paradigm Reference Braun and Clarke13 that sought to understand medication management through the words of the participants, as opposed to the researchers’ co-created meaning.
Healthcare professionals’ workshop
To further elucidate healthcare professionals’ views of medication, a workshop was conducted with 22 healthcare professionals. The sample comprised 11 consultant child and adolescent psychiatrists, 4 nurses/nurse specialists, 5 community paediatricians and 2 NHS managers. Consent to participate was implied by attendance. From this sample, five groups were created to discuss different issues which concernedthe decision to medicate and choice of drug, titration, medication maintenance, rating scales/outcomes, treatment adherence and engagement with the aim of identifying current and best practice. Verbal feedback from the healthcare professionals’ discussions were collated via written notes and summarised.
Delphi study
The Delphi method is an interactive method utilising an expert panel answering questions in two or more rounds to achieve consensus. Participants are given feedback on question responses after each round; the process terminates once a predefined stopping point is reached. Reference Dalkey and Helmer14,Reference Morrison and Barratt15 In this study, the aim of the Delphi process was to determine expert consensus on medication management strategies. The combination of multiple methodologies used to inform the Delphi survey ensured that items were comprehensive and relevant to aspects of medication management identified as relevant by multiple stakeholders.
Expert panel
The expert panel comprised 12 healthcare professionals working within the four participating NHS trusts: 7 child and adolescent psychiatrists, 3 consultant community paediatricians and 2 ADHD nurse prescribers, all experienced in the assessment and treatment of ADHD. Consent to participate was implied by acceptance of the invitation to form part of the expert group.
Procedure
Healthcare professionals were invited by email to become a member of the expert group. Statements for the Delphi study were drawn from the findings of the online survey, the parent/carer focus group, the healthcare professional workshop, the NICE guidelines, 3 the MTA Cooperative Group 4,Reference Greenhill, Abikoff, Arnold, Cantwell, Conners and Elliott16 and the Texas Children's Medication Algorithm Project. Reference Pliszka, Greenhill, Crismon, Sedillo, Carlson and Conners7,Reference Pliszka, Lopez, Crismon, Toprac, Hughes and Emslie8 Some additional statements were added by the study team to determine clinical opinion on treatment choices or less directive recommendations. For example, reasons to choose atomoxetine and titration doses should be increased as required up to British National Formulary (BNF) limits (see Table 1 for statement source). The final Delphi questionnaire comprised 103 statements, 92 of which were to be rated on both their importance and feasibility. A subset of statements (n=11) pertaining to clinician choice/preference and where issues of feasibility were not applicable were rated on importance alone. A 9-point Likert scale was used as a response option for each statement, with scores 7–9 denoting the statement is ‘important’ and/or ‘feasible’ and 1–3 denoting the statement is ‘unimportant’ and/or ‘unfeasible’.
Table 1 Category 1 Delphi statements (n=24) reaching consensus on both importance and feasibility
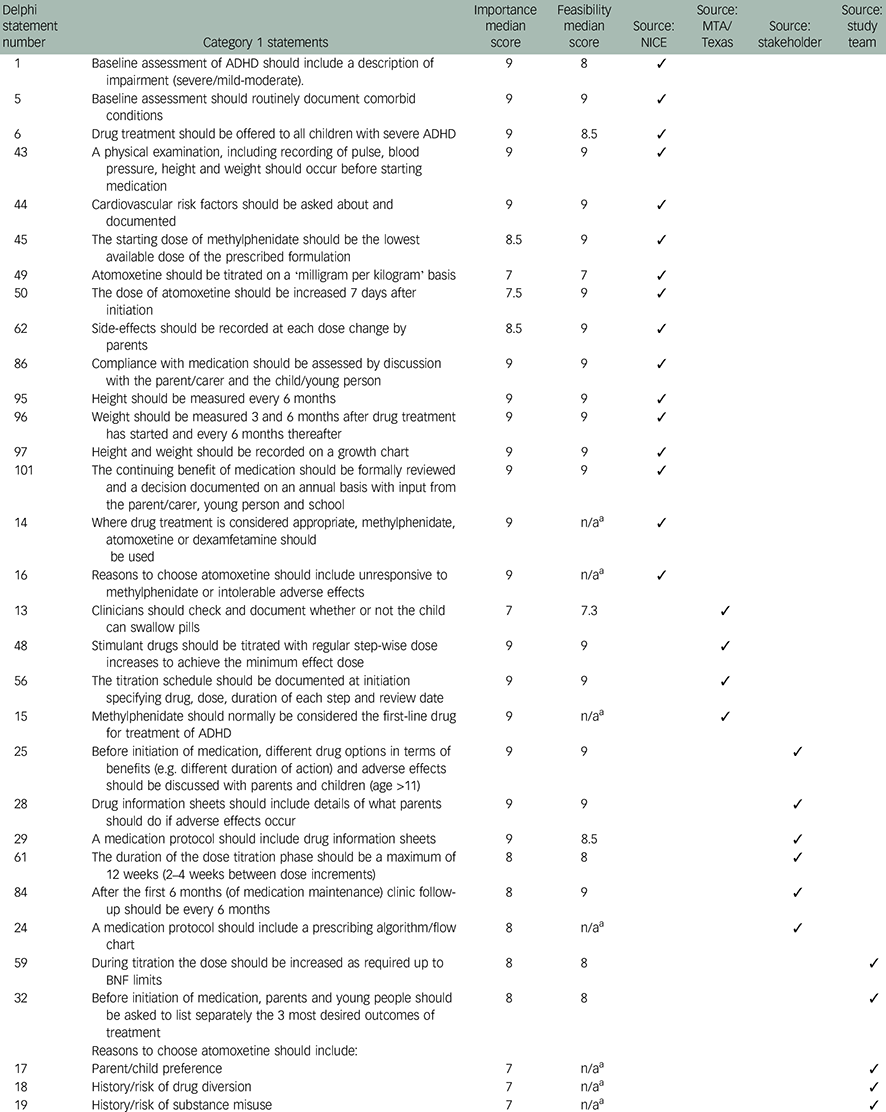
| Delphi statement number | Category 1 statements | Importance median score | Feasibility median score | Source: NICE | Source: MTA/Texas | Source: stakeholder | Source: study team |
|---|---|---|---|---|---|---|---|
| 1 | Baseline assessment of ADHD should include a description of impairment (severe/mild-moderate). | 9 | 8 | ✓ | |||
| 5 | Baseline assessment should routinely document comorbid conditions | 9 | 9 | ✓ | |||
| 6 | Drug treatment should be offered to all children with severe ADHD | 9 | 8.5 | ✓ | |||
| 43 | A physical examination, including recording of pulse, blood pressure, height and weight should occur before starting medication | 9 | 9 | ✓ | |||
| 44 | Cardiovascular risk factors should be asked about and documented | 9 | 9 | ✓ | |||
| 45 | The starting dose of methylphenidate should be the lowest available dose of the prescribed formulation | 8.5 | 9 | ✓ | |||
| 49 | Atomoxetine should be titrated on a ‘milligram per kilogram’ basis | 7 | 7 | ✓ | |||
| 50 | The dose of atomoxetine should be increased 7 days after initiation | 7.5 | 9 | ✓ | |||
| 62 | Side-effects should be recorded at each dose change by parents | 8.5 | 9 | ✓ | |||
| 86 | Compliance with medication should be assessed by discussion with the parent/carer and the child/young person | 9 | 9 | ✓ | |||
| 95 | Height should be measured every 6 months | 9 | 9 | ✓ | |||
| 96 | Weight should be measured 3 and 6 months after drug treatment has started and every 6 months thereafter | 9 | 9 | ✓ | |||
| 97 | Height and weight should be recorded on a growth chart | 9 | 9 | ✓ | |||
| 101 | The continuing benefit of medication should be formally reviewed and a decision documented on an annual basis with input from the parent/carer, young person and school | 9 | 9 | ✓ | |||
| 14 | Where drug treatment is considered appropriate, methylphenidate, atomoxetine or dexamfetamine should be used | 9 | n/a a | ✓ | |||
| 16 | Reasons to choose atomoxetine should include unresponsive to methylphenidate or intolerable adverse effects | 9 | n/a a | ✓ | |||
| 13 | Clinicians should check and document whether or not the child can swallow pills | 7 | 7.3 | ✓ | |||
| 48 | Stimulant drugs should be titrated with regular step-wise dose increases to achieve the minimum effect dose | 9 | 9 | ✓ | |||
| 56 | The titration schedule should be documented at initiation specifying drug, dose, duration of each step and review date | 9 | 9 | ✓ | |||
| 15 | Methylphenidate should normally be considered the first-line drug for treatment of ADHD | 9 | n/a a | ✓ | |||
| 25 | Before initiation of medication, different drug options in terms of benefits (e.g. different duration of action) and adverse effects should be discussed with parents and children (age >11) | 9 | 9 | ✓ | |||
| 28 | Drug information sheets should include details of what parents should do if adverse effects occur | 9 | 9 | ✓ | |||
| 29 | A medication protocol should include drug information sheets | 9 | 8.5 | ✓ | |||
| 61 | The duration of the dose titration phase should be a maximum of 12 weeks (2–4 weeks between dose increments) | 8 | 8 | ✓ | |||
| 84 | After the first 6 months (of medication maintenance) clinic follow-up should be every 6 months | 8 | 9 | ✓ | |||
| 24 | A medication protocol should include a prescribing algorithm/flow chart | 8 | n/a a | ✓ | |||
| 59 | During titration the dose should be increased as required up to BNF limits | 8 | 8 | ✓ | |||
| 32 | Before initiation of medication, parents and young people should be asked to list separately the 3 most desired outcomes of treatment | 8 | 8 | ✓ | |||
| Reasons to choose atomoxetine should include: | |||||||
| 17 | Parent/child preference | 7 | n/a a | ✓ | |||
| 18 | History/risk of drug diversion | 7 | n/a a | ✓ | |||
| 19 | History/risk of substance misuse | 7 | n/a a | ✓ |
ADHD, attention-deficit hyperactivity disorder; BNF, British National Formulary; MTA, Multimodal Treatment of ADHD; n/a, not applicable; NICE, National Institute for Health and Care Excellence; Texas, Texas Children's Medication Algorithm Project.
a Additional statements (n=7) rated on importance only.
The expert group completed round one of the questionnaire online using www.surveymonkey.com and individualised questionnaires were emailed to panelists for round two. In the second round, respondents were given the option to either retain their original score or modify it in light of the group median score. Similar to other studies, the survey was performed exclusively online. Reference Elwyn, O'Connor, Stacey, Volk, Edwards and Coulter17,Reference Herdman, Rajmil, Ravens-Sieberer, Bullinger, Power and Alonso18 In line with the guidance of Jones & Hunter, Reference Jones and Hunter19 all feedback was anonymous.
Data analysis methodology
Cut-off points for consensus drew on convention/standards adopted by Morrison & Barratt Reference Hall, Newell, Taylor, Sayal, Swift and Hollis15 and Langlands et al; 20 how ever a broader percentage gap was used to determine whether a statement should be re-rated, to minimise the influence of a smaller number of participants in this study. The following criteria were employed:
-
1. Items were considered to have metcrteria for consensus if 80% or more of the panel (10/12 members) rated the statement as 7, 8 or 9 (important/feasible) or as 1, 2 or 3 (unimportant/unfeasible).
-
2. Items were re-presented in the second round if 50–79% of the panel rated an item as 7–9 or 1–3.
-
3. Items were omitted from the second round if less than 50% of the panel rated an item as 7–9 or 1–3.
Round one resulted in 43 statements meeting criteria for consensus on importance and/or feasibility, four of which were rated unimportant and/or unfeasible. Fifty-five statements were re-rated on importance and/or feasibility during round two.
Ethical approval was granted by the local Research Ethics Committee and Research and Development Departments of Nottinghamshire Healthcare NHS Trust, Nottingham University Hospitals NHS Trust, Lincolnshire Partnership NHS Foundation Trust and Derbyshire Healthcare NHS Foundation Trust.
Results
Four different categories were used to cluster statements:
-
Category 1: statements rated as important and feasible
-
Category 2: statements rated as important but not feasible
-
Category 3: statements rated as feasible but not important
-
Category 4: statements rated as neither important nor feasible
For the purpose of this paper, we only report on statements in category 1 or 2 as these two categories allow an understanding of what clinicians consider to be important in ADHD practice as well as highlighting difficulties in implementing these aspects into routine care. At the end of the Delphi study, 24 statements reached consensus as being both ‘important’ (important/very important) and ‘feasible’ (feasible/very feasible), supporting the inclusion of these in ADHD medication management protocol (Table 1). It should be noted that there are some instances where the final median score for a statement was high, but criteria for consensus (≥80 agreement) were not met. For example, a median score of 8, whereby 9/12 panelists scored 7–9 (statement 60).
ADHD assessment
The Delphi study revealed a consensus on both the importance and feasibility of assessing and describing impairment level alongside the NICE recommendation to document comorbid conditions (Table 1, statements 1 and 5). Whereas 80% (20/25) of survey respondents agreed that it was important to distinguish between the NICE ADHD guideline descriptions of moderate and severe ADHD during assessment, only 12% (3/25) of respondents thought that this was an easy distinction to make in practice. The possible difficulty in assessing ADHD-related impairment may potentially be exacerbated by its poor construct definition, Reference Bell21–Reference Gordon, Antshel, Faraone, Barkley, Lewandowski and Hudziak23 the need to assess impairment across multiple domains and disentangle impairment related to comorbid conditions, as well as the weak association between symptom severity and severity of impairment. Reference Gathje, Lewandowski and Gordon22–Reference Yoshimasu, Barbaresi, Colligan, Voigt, Killian and Weaver24
Decision to medicate, drug choice and information provision to parents
The Delphi study revealed support for the NICE guideline that drug treatment should be offered to all children with severe ADHD (Table 1, statement 6) and that methylphenidate should normally be considered as the first line of drug treatment (Table 1, statement 15). However, whereas clinicians supported the clinical importance of the NICE guideline recommendation to offer behavioural advice alongside drug treatment (Table 2, statement 7) or to offer behavioural therapy prior to medication in cases of mild/moderate ADHD (Table 2, statement 9), there was no consensus on the feasibility of this. This may reflect a lack of resources within clinics; many NHS clinics are not commissioned or resourced to provide behavioural management advice or evidence-based parent programmes and so NHS healthcare professionals need to recommend that parents seek out this element of treatment from other agencies. This finding highlights the difficulty the NHS may have in implementing NICE ADHD guidance for access to behavioural interventions, suggesting that the recommendations were perhaps more aspirational than a reflection of what could currently be delivered in practice.
Table 2 Category 2 Delphi statements (n=19): rated as ‘important’ but ‘not feasible’

| Delphi statement number | Category 2 statements | Importance median score | Feasibility median score | Source: NICE | Source: MTA/Texas | Source: stakeholder | Source: study team |
|---|---|---|---|---|---|---|---|
| 7 | Drug treatment should only be offered to children together with behavioural management advice | 8.5 | 6.5 | ✓ | |||
| 9 | Children with mild/moderate ADHD should be offered behaviour therapy/parent training before a trial of medication is considered | 7.5 | 5 | ✓ | |||
| 54 | Monitoring of improvement/adverse-effects using telephone/SMS or email contact with the parent/young person should occur weekly during the titration phase | 7.5 | 4.5 | ✓ | ✓ | ||
| 83 | During the first 6 months (of medication maintenance) clinic follow-up should be every 3 months | 8.5 | 8 | ✓ | |||
| 98 | Heart rate and blood pressure should be monitored after each dose change | 9 | 9 | ✓ | |||
| 100 | Heart rate and blood pressure should be recorded on a centile chart | 8.5 | 6 | ✓ | |||
| 26 | Information sheets describing all available potential ADHD medications should be routinely provided to parents, young people (>11) and teachers | 8.5 | 6.5 | ✓ | |||
| 27 | An information sheet on the medication to be prescribed should be routinely provided to parents, young people (>11), GPs and teachers | 9 | 7.8 | ✓ | |||
| 30 | A designated individual (teacher/SENCO) within a school should be identified to provide feedback and liaison | 8 | 6 | ✓ | |||
| 31 | For children in secondary school, more than one teacher's view should be acquired | 7.8 | 5 | ✓ | |||
| 42 | A side-effect rating scale should be completed before initiation of medication | 7.3 | 6 | ✓ | |||
| 52 | During titration, the child should be seen in clinic on a monthly basis | 8 | 7 | ✓ | |||
| 10 | All pre-school children with ADHD should be offered behaviour therapy/parent training before a trial of medication is considered | 9 | 4 | ✓ | |||
| 12 | In children with comorbid ODD/CD or mood disorders, ADHD medication should be used together with psychological interventions | 8 | 7 | ✓ | |||
| 34 | The Conners short form (26 item) should be completed by parents at baseline (pre-medication) | 9 | 8 | ✓ | |||
| 37 | The Conners short form (26 item) should be completed by teachers at baseline (pre-medication) | 8.5 | 5.5 | ✓ | |||
| 40 | The Conners short form (26 item) self-report should be completed by young people (>11 years) at baseline (pre-medication) | 7 | 5 | ✓ | |||
| 73 | At the end of the titration phase the side-effect scale should be repeated by parents | 7 | 6 | ✓ | |||
| 76 | At the end of the titration phase the Conners short form (26 item)should be repeated by TEACHERS | 7 | 3 | ✓ |
ADHD, attention-deficit hyperactivity disorder; CD, conduct disorder; GP, general practitioner; MTA, Multimodal Treatment of ADHD; NICE, National Institute for Health and Care Excellence; ODD, oppositional defiant disorder; SENCO, special educational needs coordinator; Texas, Texas Children's Medication Algorithm Project.
The statements are based on information from their ascribed source; they have not been taken verbatim from each source.
The importance and feasibility of discussing medication benefits and adverse effects with families also obtained consensus within the Delphi study (Table 1, statement 25) which was also highlighted as important by parent/carers in the focus group.
Medication initiation and titration
A consensus was reached in the Delphi study on both the importance and feasibility of NICE recommendations to document physical parameters (blood pressure, heart rate, weight and height; Table 1, statement 43) and cardiac risk factors before starting medication (Table 1, statement 44), administering the lowest available dose when prescribing methylphenidate and titrating up to BNF limits (Table 1, statements 45 and 59). Documenting the titration schedule at initiation, specifying drug, dose, duration of each step and review date (Table 1, statement 56), and titrating with regular stepwise dose increases (Table 1, statement 48) also met consensus on importance and feasibility, suggesting acceptance of more protocol-driven, MTA-style, titration schedules. Consensus also emerged on the importance and feasibility of a maximum duration of 12 weeks for titration, with 2–4 weeks between dose increments (Table 1, statement 61), longer than the 4–6 weeks for titration recommended by NICE. Support in our study for a longer duration of titration may reflect clinician views on the challenges of assessing improvement within 4–6 weeks in the multiple outcomes recommended by NICE (symptom reduction, behaviour change, educational/relationship improvement) within a patient group typified by the presence of comorbidities Reference Bell21 and where teacher/parent feedback may conflict. Reference Mitsis, McKay, Schulz, Newcorn and Halperin25 Furthermore, healthcare professionals from the workshop reported difficulties with patients not attending follow-up appointments regularly, thus potentially delaying clinical decision-making.
Our findings suggested some conflict between the frequencies of contact deemed most beneficial as opposed to that deemed most feasible during titration. Consensus emerged on the importance of monthly clinic contact and weekly telephone/email contact with the family during titration and these schedules of contact were perceived as beneficial by 91% (20/22) and 82% (18/22) of online survey respondents respectively. However, there was no agreement on the feasibility of this approach (Table 2, statements 52 and 54).
Assessing response to medication: use of rating scales and assessment of side-effects
Support for the importance and feasibility of the NICE recommendation for parents to record side-effects at each dose change emerged from the Delphi study (Table 1, statement 62). However, 27% (6/22) of respondents from the online survey indicated that they did not use side-effect rating scales. Healthcare professionals commented at the workshop that assessment of side-effects was primarily achieved by monitoring physical health and via clinical enquiry; a lack of easily available and validated side-effect scales may be a barrier to their use.
Clinicians reached a consensus on the importance of the administration of the Conners’ short form (26 item) questionnaire Reference Conners26 with parents, teachers and the young person at baseline (Table 2, statements 34, 37 and 40). However, variation among healthcare professionals in the range and number of rating scales used in the UK has been previously reported Reference Tettenborn, Prasad, Poole, Steer, Coghill and Harpin27 and clinical enquiry may be a preferred source of information: all online survey respondents (22/22) placed value on the parents’ view, the child's view and the parents’ perception of progress at school when determining response to medication.
Medication maintenance: frequency of clinic contact
NICE recommendations for annual reviews of medication, assessment of concordance with medication via discussion with parents and patient, and monitoring of height and weight at fixed intervals after starting medication all reached consensus for both importance and feasibility in the Delphi study (Table 1, statements 101, 86, 95 and 96). Delphi study findings indicate consensus on the importance and feasibility of clinic follow-ups every 6 months, after the first 6 months of treatment (Table 1, statement 84). This aligns with results from the online survey, whereby biannual contact during maintenance was rated as beneficial and feasible by 77% (17) and 95% (21) of respondents respectively.
Delphi study responses met consensus on the importance of monitoring blood pressure and pulse after every dose change (Table 2, statement 98) and although the median score was high (9, very important) there was no consensus on the feasibility of this NICE recommendation, which healthcare professionals acknowledged as problematic to achieve in practice. This highlights an implementation challenge regarding obtaining recordings between clinic visits. This may be an area where home or ambulatory recordings sent electronically to the clinic using a telemedicine approach may help to bridge this implementation gap.
The greater frequency of contact observed in the MTA's medication management arm provided opportunity to identify and immediately adjust changes in medication needs during maintenance, indicating a need for long-term close monitoring. Reference Vitiello, Severe, Greenhill, Arnold, Abikoff and Bukstein28 Additional forms of communication (email, SMS, smart telephone apps) could help to ensure adequate assessment of medication response if intensive face-to-face clinic monitoring is not feasible and may help to maintain the lines of communication desired by parents/carers.
Acquisition of feedback from school and importance of school input
The Delphi study revealed consensus for both the importance of having a nominated individual teacher to provide feedback and in the case of secondary school-age children, gathering information from more than one teacher (Table 2, statements 30 and 31). However, there was no consensus on the feasibility of this. Healthcare professionals from the workshop highlighted difficulties in getting school information, citing it was ‘time-consuming’.
Obtaining teacher feedback relating to a child's response to treatment has been deemed ‘critical’ Reference Francke, Smit, de Veer and Mistiaen29 and is a NICE recommendation. However, our findings support previous research indicating that resource limitations are a barrier to guideline implementation. Reference Hall, Taylor, Moldavsky, Marriott, Pass and Newell30
Discussion
Summary of main findings
Half (50%) of the NICE ADHD guideline recommendations included in the Delphi study were endorsed as being both clinically important and feasible to implement. These included assessment of ADHD-related impairment, baseline physical examination, recording of height, weight and parental reported side-effects, communicating reasons to use atomoxetine and methylphenidate (see Table 1 for complete list). Meanwhile, a subset of NICE recommendations, although regarded as clinically important, was identified as difficult or challenging to implement in routine UK clinical practice. These included delivery of adjunctive behavioural interventions, weekly monitoring (telephone/SMS/email) during titration, measuring pulse and blood pressure after every dose change during titration and recording pulse and blood pressure on a centile chart (see Table 2 for complete list).
Study strengths, limitations and future research
To the best of our knowledge, this is the first study which has explicitly sought to understand current consensus on medication management strategies for ADHD within the constraints of real-world practice. Whereas the number of Delphi panelists (n=12) may be considered relatively small, this is sufficient to demonstrate consensus. There is no consensus in the literature on optimum sample size Reference Tettenborn, Prasad, Poole, Steer, Coghill and Harpin31 and panel sizes ranging from 10 to 1142 participants are apparent in published Delphi studies. Reference Vitiello, Severe, Greenhill, Arnold, Abikoff and Bukstein32 However, Jones & Hunter Reference Jones and Hunter19 recommend a Delphi panel size of between 9 and 12 participants. Although the sample size of the online survey, workshop and focus groups were also small, the combination of opinion of multiple stakeholders to inform the items of the Delphi survey enabled a more complete approach to understanding issues pertinent to clinicians, young people, parent and carers in relation to ADHD medication management. Given that the participant is often over-looked in determining ‘best practice’, our inclusion of this group is a real strength.
This study only elicited the views of participants located within the East Midlands region of England; thus, it is possible that healthcare professionals working in different healthcare systems may make different judgements about the importance and feasibility of ADHD practice recommendations. Furthermore, the characteristics of the patients being treated by the healthcare professionals completing the Delphi study were not determined: it is likely that these too will have influenced judgements about what is important and feasible. Additionally, the use of self-report to determine current practices may have been subject to biases such as overestimation of adherence to guidelines Reference Francke, Smit, de Veer and Mistiaen33 and triangulation with other objective assessments of patient management (e.g. case-note audits) would be a further method to characterise current practice.
Given the lack of understanding of current consensus in medication management in the UK, it was important to first establish current opinion on what constitutes ‘best practice’ within the constraints of everyday practice. Further research should establish the extent to which protocols are adhered to and factors that influence non-adherence by combining audit data with survey responses or qualitative interviews. Given the importance of regular contact during titration, future research could investigate whether utilisation of technology, such as Skype or apps, could provide a satisfactory interface for such monitoring. Although not specific to ADHD medication monitoring, a recent study did explore service user opinion of routine monitoring via an electronic questionnaire in mental health settings. Reference Hall, Taylor, Moldavsky, Marriott, Pass and Newell30 The findings revealed that parents and children were supportive of the use of technology to report progress to their healthcare professional.
Our study applied an iterative, mixed methods approach (literature review, online survey, stakeholder workshop and Delphi study) to determine previously unexplored perceptions of the implementation of national clinical guidelines and evidence-based recommendations for children and young people receiving medication for ADHD. Our findings indicate areas of agreement and discord with existing NICE 3 ADHD guidelines and conflict between the perceived importance of some best practice medication management strategies and the feasibility of their implementation. The study has identified a set of ADHD practice recommendations that are viewed by clinicians as important but currently not feasible to implement. These recommendations should be the focus of health service implementation strategies and research.
Clinician feedback indicated that the intensive monitoring schedules of the MTA protocol are currently regarded as impractical in routine UK practice and the time needed to titrate medication is likely to extend beyond that recommended by NICE guidelines. The use of technology to automate data collection and provide digital communication may be particularly relevant for aspects of practice viewed as important but currently unfeasible. The Delphi study findings have identified recommendations for practice where implementation challenges remain and clinical consensus is still to be reached. These findings could inform the update of the NICE ADHD clinical guideline and focus implementation strategies on those statements identified as important but not presently feasible to deliver in routine care. One potential area for development is the application of digital technologies to assist remote monitoring and follow-up during medication titration and optimisation.
Funding
This research was funded by the National Institute of Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East Midlands (CLAHRC EM) and NIHR MindTech Healthcare Technology Co-operative. The work has been funded by the National Institute for Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.





eLetters
No eLetters have been published for this article.