There are approximately 33 million people with problematic opioid use worldwide.1 The misuse of opioids is associated with a number of adverse health outcomes, including infections, chronic disease and mortality.Reference Degenhardt and Hall2 Methadone maintenance treatment (MMT) is a commonly prescribed medication for opioid use disorder (OUD).Reference Mattick, Breen, Kimber, Davoli, Richard and Breen3 Methadone is a synthetic opioid agonist that acts on opioid receptors to prevent withdrawal symptoms and reduce cravings in an effort to help patients abstain from opioid use. However, this rather idealistic goal is seldom achieved, as 66% of individuals on MMT continue to use illicit opioids.Reference Rosic, Naji, Bawor, Dennis, Plater and Marsh4
Patients in MMT often use other substances like cocaine, amphetamines, benzodiazepines, and most commonly, cannabis.Reference Rosic, Naji, Bawor, Dennis, Plater and Marsh4 Although cannabis is not typically a direct cause of premature death,Reference Degenhardt, Whiteford, Ferrari, Baxter, Charlson and Hall5 prolonged use has adverse consequences on both physical and mental health. Studies have shown chronic cannabis use to be associated with increased risk for substance use disordersReference Blanco, Hasin, Wall, Flórez-Salamanca, Hoertel and Wang6 and non-substance use psychiatric disorders.Reference Wittchen, Fröhlich, Behrendt, Günther, Rehm and Zimmermann7,Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones and Burke8 A well-established association exists between cannabis use and the risk of psychotic disorders.Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones and Burke8 There is less conclusive evidence for its association with affective disorders,Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones and Burke8 with mood disorders showing a more reliable association than anxiety disorders.Reference Wittchen, Fröhlich, Behrendt, Günther, Rehm and Zimmermann7 Furthermore, most of this literature on cannabis use and its associated health outcomes is from the general population, which is not reflective of high-risk patients, such as those receiving treatment for OUD.
There is an increasingly common notion that cannabis might serve as a substitution drug for opioids among patients in MMT.Reference Lucas9 Some studies suggest cannabis is associated with treatment retentionReference Raby, Carpenter, Rothenberg, Brooks, Jiang and Sullivan10 and a reduction in opioid withdrawal symptoms.Reference Scavone, Sterling, Weinstein and Van Bockstaele11 Conversely, others found that cannabis is associated with an increased risk for non-medical prescription opioid use and OUD,Reference Olfson, Wall, Liu and Blanco12 and is a sex-specific predictor for poor response to MMT in women.Reference Zielinski, Bhatt, Sanger, Plater, Worster and Varenbut13 Despite these contrasting findings, few studies have investigated the overall health consequences of cannabis in patients with OUD. This means that we are promoting a ‘replacement’ drug in the absence of adequate research on its potential adversities. This raises the pertinent question of whether cannabis use among patients with OUD in MMT is associated with comorbidities like concurrent psychiatric disorders or patterns of other substance use, as is seen in the general population.
Objectives
The primary objective of this study is to assess the association between health conditions and cannabis use in a large, representative sample of patients with OUD receiving MMT. Our secondary objectives are first to determine if this association differs by sex, and second to investigate whether heaviness of cannabis use is associated with comorbid disorders in this population. The recent legalisation of cannabis in Canada, which came into effect October 2018 amid the ongoing opioid crisis, requires imminent investigation into the consequences of cannabis use for this population. This research can inform the clinical utility of screening for cannabis use in MMT models of care.
Method
Study design
The data used for this cross-sectional study were extracted from the Genetics of Opioid Addiction (GENOA) research programme, a prospective cohort study conducted in collaboration with the Population Genomics Program at McMaster University and the Canadian Addiction Treatment Centres (CATC).Reference Samaan, Bawor, Dennis, Plater, Varenbut and Daiter14 Patients were recruited from 14 out-patient CATC clinics across Southern Ontario. This study included patients recruited between May 2013 and March 2016.
Participant characteristics
We screened all eligible candidates for the following inclusion criteria in addition to the original study criteria: at least 18 years of age, satisfying DSM-IV15 criteria for opioid addiction (terminology in DSM-5 changed to opioid use disorder, which is used throughout this paper), receiving methadone treatment, able to provide informed written consent and completion of the Mini-International Neuropsychiatric Interview (M.I.N.I.) and the Maudsley Addiction Profile (MAP), as these measures were necessary for answering the study question. The exclusion criteria included receiving opioid substitution treatment other than methadone, or the inability to communicate in English (see Supplementary Fig. 1 https://doi.org/10.1192/bjo.2019.78). Less than 1% of participants in this study were receiving treatment with opioid substitution therapy other than methadone.
All eligible participants recruited for the GENOA study provided written informed consent before being interviewed by research personnel at the clinic where they received their methadone treatment. Upon completion of the baseline interview, participants were given a gift card to a coffee shop valued at Can$5. This study was approved by the Hamilton Integrated Research Ethics Board (study identifier 11-056) and all methods were performed in accordance with the relevant guidelines and regulations.
Baseline and outcome measures
During the structured face-to-face interview, we asked participants about sociodemographics, psychiatric and family history, physical and psychological health, and drug use. When asked to indicate their biological sex, all participants reported either male or female. The Brief Pain Inventory was used to ask about pain with specific questions about intensity, quality, the interference of pain with daily life and the extent of pain relief.Reference Cleeland and Ryan16 The Brief Pain Inventory has previously been used for patients receiving MMT, and is reported to have a high sensitivity in this population.Reference Dennis, Roshanov, Bawor, Paul, Varenbut and Daiter17 Our measure of pain in this study was participants’ self-reported estimate of how much their pain interfered with their general activity, reported on a scale of 0 (does not interfere) to 10 (completely interferes). Smoking status was dichotomised into current smoker or not based on participants’ self-reported use of cigarettes (i.e. tobacco). Body mass index (BMI) was calculated as a continuous variable, using participants’ self-reported weight and height data.
The M.I.N.I. was used by trained research personnel to establish psychiatric comorbidities.Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller18 Specifically, the M.I.N.I. provided information about all past and present diagnoses of the following psychiatric disorders: schizophrenia, major depressive disorder, bipolar disorder type 1, bipolar disorder type 2, bipolar disorder not otherwise specified, generalised anxiety disorder, panic disorder without agoraphobia, agoraphobia with panic disorder, agoraphobia without panic disorder, social anxiety, obsessive–compulsive disorder and post-traumatic stress disorder. We categorised all psychiatric disorders into three main groups: psychotic disorders (schizophrenia), mood disorders (major depressive disorder and bipolar disorders type 1, type 2 and not otherwise specified) and anxiety disorders (generalised anxiety disorder, panic disorder without agoraphobia, agoraphobia with panic disorder, agoraphobia without panic disorder, social anxiety, obsessive–compulsive disorder and post-traumatic stress disorder). We included both past and current psychiatric disorders and, if a participant had multiple psychiatric comorbidities, we considered only the primary diagnosis according to the DSM-IV.
As part of the CATC protocol, all patients underwent routine weekly or biweekly urine toxicology screens for the presence of opioids at the clinic where they received their methadone. Results of urine opioid screens were obtained from patients’ electronic medical records for 3 months before the baseline interview, which included an average of 16 screens. The cut-off concentration for opiates was 300 ng/mL. Illicit opioid use was dichotomised to reflect no positive screens versus any positive screens during a 3-month period.
The MAP was used to ascertain self-reported substance use in the past 30 days. This included the typical dose used, the route of administration and the number of days of substance use in the past 30 days for the following substances: alcohol, heroin, illicit methadone, illicit benzodiazepines, cocaine, crack cocaine, amphetamines and cannabis.Reference Marsden, Gossop, Stewart, Best, Farrell and Strang19 Self-reported drug use was dichotomised into no use in the past 30 days versus any use in the past 30 days for each respective substance included in the MAP.
The primary risk variable of cannabis use was assessed by the MAP. In an earlier study, self-reported cannabis use from the MAP was validated by conducting sensitivity and specificity analysis for participants recruited through GENOA who had both urine drug screens for cannabis detection and MAP data available.Reference Zielinski, Bhatt, Sanger, Plater, Worster and Varenbut13 The sensitivity was 79.9% (95% CI 72.7–85.8) and the specificity was 80% (95% CI 73.6–85.4).Reference Zielinski, Bhatt, Sanger, Plater, Worster and Varenbut13 Since many CATC sites discontinued urine screens for cannabis, we deemed self-reported cannabis use as an appropriate measure.
All data obtained for the GENOA study were entered and stored on REDCap version 8.8.0 created at Vanderbilt University (https://www.project-redcap.org/), a secure electronic data capture tool.Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde20
Statistical analysis
We used descriptive statistics to compare demographic and clinical characteristics of cannabis users and non-users. Continuous measures were reported as means (s.d.), and categorical variables were expressed as n (%).
We performed a multivariable logistic regression to assess the relationship between adverse health conditions and cannabis use. The variables of interest included pain, smoking, BMI, substance use, opioid urine screens and comorbid psychiatric disorders. We dichotomised cannabis use as any cannabis use in the past 30 days versus no cannabis use in the past 30 days. We adjusted for important confounding variables, specifically sex, age, employment, marital status and education. Age was converted into a categorical variable with two levels: youth (18–25 years) and adults (≥26 years). This was done to reflect the known impact cannabis has on the developing brain. To examine biological sex differences, we performed a subgroup logistic regression analysis, while controlling for the same confounding variables.
We conducted a secondary multivariable linear regression to investigate the presence of a dose-dependent relationship between adverse health conditions and cannabis use among only those who reported using cannabis. We replaced cannabis use, as a dichotomised variable, with a continuous measure of the heaviness of use, while controlling for the same confounding variables. We quantified heaviness of cannabis use as the product of the number of days used in the past 30 by the typical amount administered per use (measured in grams). For participants who reported typical usage in values other than grams, we referred to Mariani et al for the quantification of common marijuana measurements.Reference Mariani, Brooks, Haney and Levin21 Some participants reported using ‘less than one joint’ or a ‘couple of puffs of a joint’, in which case we coded these and all other vague quantifications conservatively, as equal to half a joint (0.33 g).
For each logistic regression analysis, we reported the adjusted odds ratios, 95% confidence intervals and P-values. The level of significance for hypothesis testing was set at α = 0.05 for all analyses. For the linear regression analysis, we reported the unstandardised coefficient, 95% confidence intervals and P-values. We performed a test of collinearity, using the variance inflation factor to ensure that no variables with a variance inflation factor over ten were included in the analyses. All statistics were performed with IBM SPSS version 23 for Macintosh. This study is reported in adherence with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Sample size calculation
The general standard for regression analyses is to include a minimum of ten events per predictor variable. In our primary and secondary analyses, we had a total of 640 participants with each model and 19 covariates. For our subgroup analyses, we included 19 covariates for a sample of 291 women, and 349 men. As such, our sample was suitably sized to provide adequate stability for the multivariable regression models.
Data availability
The data-sets used and analysed during the study are available from the corresponding author upon request.
Results
We recruited a total of 773 participants for the larger GENOA project who were interviewed with the M.I.N.I. and potentially eligible for this study. Of these participants, two were excluded because they were on buprenorphine-naloxone treatment as opposed to methadone. Furthermore, ten participants were excluded for missing data pertaining to the primary dependent variable (cannabis use as measured by the MAP) and 89 were excluded for missing data pertaining to the primary explanatory variable of interest (incomplete psychiatric disorders as measured by the M.I.N.I.). A total of 672 participants met the eligibility criteria and were included in all further analyses (see Supplementary Fig. 1).
Demographic and clinical characteristics
Our sample of 672 participants included a comparable number of cannabis non-users (n = 337) to users (n = 335). Approximately half of all cannabis non-users were female (52.8%), whereas slightly over a third of all cannabis users were female (38.8%). The mean age of cannabis non-users and users was 40.42 (s.d. 10.90) and 37.10 (s.d. 11.07), respectively. The average BMI was similar among cannabis non-users (28.52, s.d. 9.94) and cannabis users (27.19, s.d. 7.20). Average ratings of pain (on a 10-point scale) among cannabis non-users and users were 4.96 (s.d. 3.25) and 5.12 (s.d. 3.52), respectively. A comprehensive summary of demographic and clinical characteristics comparing cannabis non-users and users is reported in Table 1.
Table 1 Demographic and clinical characteristics of cannabis users and non-users on methadone maintenance treatment

Among cannabis non-users, alcohol was consumed by 129 participants (38.3%), heroin was used by 29 (8.6%), illicit methadone by 3 (0.9%), illicit benzodiazepine by 18 (5.3%), cocaine by 43 (12.8%), crack cocaine by 19 (5.6%) and amphetamine by 9 (2.7%). Among cannabis users, alcohol was consumed by 171 (51%), heroin was used by 26 (7.8%), illicit methadone by 5 (1.5%), illicit benzodiazepine by 29 (8.7%), cocaine by 62 (18.5%), crack cocaine by 25 (7.5%) and amphetamine by 12 (3.6%). A summary of substance use among cannabis non-users and users are reported in Table 2.
Table 2 Substance use and psychiatric disorders summary

Substance use dichotomised as any use in the past 30 days versus no use in the past 30 days. For individuals with psychiatric comorbidities, only the primary diagnosis was considered.
MDD, major depressive disorder; NOS, not otherwise specified; GAD, generalised anxiety disorder; w/o, without; OCD, obsessive–compulsive disorder; PTSD post-traumatic stress disorder.
Although all participants were receiving treatment for OUD, there were 433 (64.4%) with an additional psychiatric disorder, representing 61.4% of cannabis non-users and 67.5% of cannabis users. Of the 207 cannabis non-users with a comorbid psychiatric disorder, 155 (46.0%) had a mood disorder, 39 (11.6%) had an anxiety disorder and 13 (3.9%) had a psychotic disorder. Among the 226 cannabis users with a comorbid psychiatric disorder, 150 (44.8%) had a mood disorder, 51 (15.2%) had an anxiety disorder and 25 (7.5%) had a psychotic disorder. A complete summary of psychiatric disorders among cannabis non-users and users are reported in Table 2.
Primary analysis
The primary logistic regression analysis revealed a significant association between cannabis use and a number of health factors as reported in Table 3. Cannabis users were more likely to consume alcohol (odds ratio 1.46, 95% CI 1.04–2.06, P = 0.029) and less likely to use heroin (odds ratio 0.45, 95% CI 0.24–0.86, P = 0.016). Of the psychiatric comorbidities, there was a significant association between cannabis use and the presence of an anxiety disorder (odds ratio 1.75, 95% CI 1.02–3.02, P = 0.043). No association between pain and cannabis use was seen in this study.
Table 3 Multivariable logistic regression with cannabis use as the dependent variable (n = 640)
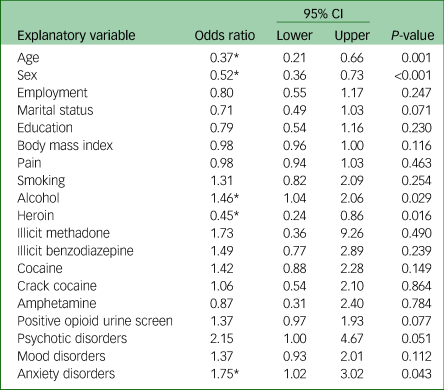
Age dichotomised as 18–25 years and ≥26 years. Body mass index and pain interpreted as one-point increase. Substance use dichotomised as any use in the past 30 days versus no use in the past 30 days.
* Significant at P < 0.05.
Females were less likely to be cannabis users (odds ratio 0.52, 95% CI 0.36–0.73, P < 0.001), as were patients older than 26 years of age (odds ratio 0.37, 95% CI 0.21–0.66, P = 0.001).
Stratified analysis by sex
A subgroup analysis identified sex-specific associations of cannabis use as reported in Table 4. Male cannabis users were more likely to have a comorbid anxiety disorder (odds ratio 2.59, 95% CI 1.21–5.53, P = 0.014), whereas female cannabis users were more likely to use alcohol (odds ratio 1.79, 95% CI 1.06–3.02, P = 0.028). Additionally, males who used cannabis were more likely to be between 18 and 25 years of age (odds ratio for older men 0.30, 95% CI 0.12–0.71, P = 0.007).
Table 4 Multivariable logistic regression on factors associated with cannabis use by sex
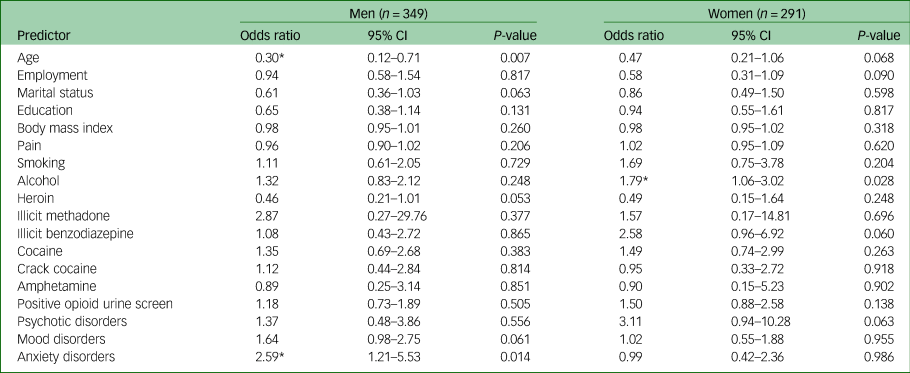
Age dichotomised as 18–25 years and ≥26 years. Body mass index and pain interpreted as one-point increase. Substance use dichotomised as any use in the past 30 days versus no use in the past 30 days.
* Significant at P < 0.05.
Secondary analysis by heaviness of cannabis use
The secondary multivariable linear regression was conducted to assess for a dose-dependent relationship between cannabis and health and social conditions, as reported in Supplementary Table 1. Males were heavy cannabis users as compared with females (unstandardised coefficient (B) = −11.11, 95% CI −21.08 to −1.14, P = 0.029) and heavy cannabis users were less likely to be employed ((B) = −11.18, 95% CI −21.76 to −0.60, P = 0.038).
Discussion
The present study sought to investigate the association between a multitude of health conditions and cannabis use in a well-characterised sample of patients with OUD receiving MMT. We found that nearly half of our sample (n = 335) reported using cannabis in the past 30 days, indicating that cannabis is very commonly used within the OUD population, even before legalisation of its recreational use in Canada. Cannabis users were younger, more likely to have anxiety disorders, more likely to use alcohol and less likely to use heroin. We did not identify an association between cannabis and pain. Heavy cannabis users were more likely to be male with an increased likelihood of being unemployed. It remains unknown as to how the prevalence of cannabis use will change following its legalisation in Canada in October 2018.
Cannabis and other substance use
Our results suggest that cannabis use is associated with patterns of other substance use. We found that cannabis users were 1.46 times more likely to use alcohol. Polysubstance use is a common clinical phenomenon, with a number of possible explanations. Environmental and genetic factors might contribute to a generalised risk for developing non-specific substance use behaviours.Reference Palmer, Young, Hopfer, Corley, Stallings and Crowley22 Another possibility is that specific combinations of drugs, when used together, might offer synergistic effects.Reference Pakula, Macdonald and Stockwell23 Based on our findings, use of cannabis and alcohol are correlated among individuals in MMT, consistent with an epidemiological study by Geels et al that found cannabis use to be among the factors that most strongly predicted heavy alcohol consumption.Reference Geels, Vink, van Beek, Bartels, Willemsen and Boomsma24 From our subgroup analysis, we found that women using cannabis were 1.79 times more likely to use alcohol. The literature has largely shown that alcohol use and the prevalence of alcohol use disorders is greater among men.Reference Wilsnack, Vogeltanz, Wilsnack, Harris, Ahlström and Bondy25 However, interestingly, some studies have found a diminishing sex gap in alcohol consumption, misuse and dependence.Reference Keyes, Grant and Hasin26 Because of lack of accurate data about the amount of alcohol use, it is not possible to infer the degree to which cannabis use is associated with problematic alcohol consumption among women in MMT. Nonetheless, this presents as a concerning combination of behaviours in an already vulnerable population.
Based on our results, cannabis users were 0.45 times less likely to be using heroin compared with cannabis non-users. Studies on cannabis use among patients in MMT have produced varied findings; some investigations have found cannabis users to be at greater risk for illicit opioid use,Reference Wasserman, Weinstein, Havassy and Hall27 whereas others found no significant association.Reference Epstein and Preston28 Zielinski et al Reference Zielinski, Bhatt, Sanger, Plater, Worster and Varenbut13 undertook the largest prospective investigation to date on the association between cannabis and illicit opioid use, using data collected from the greater GENOA project, as done in our study. It should be noted that the time frame of data collection and inclusion criteria varied between studies. resulting in somewhat different sample populations. Nonetheless, Zielinski et al found cannabis to be a sex-specific predictor for poor response to MMT in women.Reference Zielinski, Bhatt, Sanger, Plater, Worster and Varenbut13 Similarly, in our analysis we found a positive, albeit non-significant, trend in the association between cannabis use and positive opioid urine screens. Taken in conjunction with our findings, cannabis should not be considered as a substitute for heroin nor as a replacement for all opioid use. Although cannabis use might be associated with a decreased likelihood of heroin use, there is a trend toward increased illicit opioid use during treatment.
This seemingly contradictory observation may be related to two main points. The first is that self-reported heroin use is imprecise, as we do not have a validation of self-report versus objective measures of heroin use. The second is that although urine screens for opioids, which may include heroin, might suggest that one substance use is associated with other substance use, less heroin use does not equate to less opioid use as other forms of opioids may be consumed by those reporting less heroin use. Moreover, cannabis is not without considerable psychosocial and physical harms, as shown in this study and others. Further large studies are needed to assess the association between cannabis and heroin use before more definitive conclusions can be drawn.
Cannabis and psychiatric comorbidities
Approximately two-thirds (64.4%) of the patients in our sample had a concurrent psychiatric disorder, suggesting that OUD is commonly associated with significant comorbidities that may not be routinely screened for, nor managed in, opioid substitution therapy models of care. Specifically, we found that cannabis users were 1.75 times more likely to have a comorbid anxiety disorder. There has been considerable research into the mechanism by which cannabis is associated with psychiatric symptoms, particularly psychosis. Degenhardt and Hall reviewed longitudinal studies conducted worldwide to find that regular cannabis use consistently predicts an increased risk of a diagnosis of schizophrenia or psychosis, even after controlling for confounders like personal characteristics and other drug use.Reference Degenhardt and Hall29 The relationship between cannabis use and anxiety disorders is less understood, as evidence shows that chronic cannabis use is associated with a higher prevalence of anxiety disorders, and patients with anxiety disorders have comparably higher rates of cannabis use.Reference Crippa, Zuardi, Martín-Santos, Bhattacharyya, Atakan and McGuire30
From our subgroup analysis, we found that men using cannabis in MMT were 2.59 times more likely to have a comorbid anxiety disorder. To date, research suggests that women tend to exhibit more pronounced effects of cannabis use and are more prone to developing cannabis use disorder.Reference Cooper and Haney31 Animal studies using rodents have elucidated a sexually dimorphic endocannabinoid system, whereby females are more sensitive to the effects of cannabinoids.Reference Craft, Marusich and Wiley32 Yet, our study found cannabis use to be associated with a greater likelihood for anxiety disorders among men. In interpretation of these seemingly inconsistent findings, it is important to consider how gender modulates this relationship. Although sex refers to biological attributes, gender refers to socially constructed roles, behaviours and identities.33 When considering gender differences, women tend to more openly speak about using cannabis and more readily access healthcare services.Reference Fattore34 This increased proclivity for enrolling in treatment might explain why women tend to have shorter episodes of cannabis use disorder.Reference Khan, Secades-Villa, Okuda, Wang, Pérez-Fuentes and Kerridge35 Conversely, men are less likely to seek help in response to mental health concerns,Reference Möller-Leimkühler36,Reference Urbanoski, Inglis and Veldhuizen37 possibly because of social norms of masculinity that are incongruent with help-seeking decisions.Reference Heath, Brenner, Vogel, Lannin and Strass38 Among men in MMT, it is plausible that prolonged episodes of cannabis use and a reluctance to seek treatment for disorders such as anxiety are contributing to worsened mental health outcomes. Our results suggest that social barriers to accessing care might exist for men using cannabis in MMT.
Moreover, in an already vulnerable population of opioid users, it becomes relevant to consider how multiple psychiatric disorders affect treatment outcomes, including retention in therapy and abstinence of opioid use. Rosic et al found that having specific psychiatric comorbidities places patients in MMT at higher risk for poorer treatment outcomes.Reference Rosic, Naji, Bawor, Dennis, Plater and Marsh4 This means that cannabis may increase the risk for developing excess psychiatric comorbidities, which can ultimately contribute to poorer treatment outcomes.
Cannabis and pain
Despite the well-documented evidence on the harms of cannabis, it is often perceived as a harmless recreational drug, especially in comparison to opioids and other substances. It is used for ‘medicinal purposes’ in the USA and Canada, and has become an increasingly common alternative to opioids for chronic non-cancer pain conditions.Reference Nugent, Morasco, O'Neil, Freeman, Low and Kondo39 In this study we found no association between pain and cannabis use. So, although cannabis might have been initiated for its perceived analgesic effects, this did not translate into any correlation with measures of pain, especially when controlling for potentially contributing factors. Our findings are in keeping with a recent investigation by Campbell et al that found no evidence for opioid-sparing effects nor improvement in patient outcomes from the use of cannabis in people with chronic non-cancer pain who had been prescribed opioids.Reference Campbell, Hall, Peacock, Lintzeris, Bruno and Larance40 Moreover, there is a paucity of randomised controlled trials investigating the long-term consequences of cannabis use for the treatment of chronic non-cancer pain.Reference Deshpande, Mailis-Gagnon, Zoheiry and Lakha41
Heaviness of cannabis use
Our secondary objective was to investigate the association between adverse health conditions and heaviness of cannabis use. There is sufficient evidence to suggest that a dose-dependent relationship exists between cannabis and adverse psychiatric outcomes like psychosis,Reference Marconi, Di Forti, Lewis, Murray and Vassos42 with high potency cannabis carrying the greatest risk.Reference Di Forti, Marconi, Carra, Fraietta, Trotta and Bonomo43 We did not find such a relationship, likely because of the lack of data regarding the potency of cannabis. Furthermore, we did not ask participants about vaping, an increasingly common means of cannabis and other substance use, making it possible that the degree of cannabis use was underestimated for some participants.
In addition, there is some degree of overlap between symptoms of anxiety and cannabis-associated symptoms, which cannot be clarified based on this study data. The clinical and research challenges are to distinguish which substance contributes to which symptom, and if anxiety or other psychopathology symptoms were indeed independent from substance use; for example, when for example participants were asked, using the M.I.N.I., about the most problematic substance that they use, opioids were the most important and the substance of concern that they were all in treatment for. Given the degree of comorbid psychiatric and substance use in this sample, there are significant challenges (as in real-life clinical practice) to separate substance effects from psychopathology when they are concurrent.
We did, however, find that heavy cannabis users were less likely to be employed, further contributing to the social concerns associated with cannabis use. With the likelihood for increased cannabis use in the post-legalisation era in Canada, Uruguay, parts of the USA, and other countries, the effects of cannabis use pose both an individual and societal concern.
Clinical implications and future directions
By elucidating some of the clinical and social harms of cannabis use, this study, along with the pre-existing literature, demonstrates a need to reconsider the implications of ignoring and moreover promoting cannabis among patients on methadone for management of OUD. Its association with psychiatric comorbidities and further substance use behaviours comprises the health status of patients and poses potential risk factors for poor treatment outcomes. Future studies are needed to investigate potency and frequency of cannabis use among those in MMT to determine risk factors that might place certain individuals at greater risk for adverse health conditions.
Physicians are well positioned to obtain a comprehensive substance use history to identify cannabis users in MMT who might benefit from further interventions. Although there are currently no medication-assisted therapies to manage problematic cannabis use, other interventions such as psychosocial management can be utilised in clinical settings for addiction and mental health. Studies have found behavioural reinforcements, in the form of ‘take home’ privilegesReference Peles, Schreiber, Sason and Adelson44 or stepped-care interventions involving weekly counselling,Reference Kidorf, Neufeld, King, Clark and Brooner45 to be effective methods of reducing cannabis use in patients receiving MMT.
More specifically, we suggest the following:
(a) Implementing changes or improvements in practice: screening for cannabis use and associated comorbidities should be part of the standard of care for patients with OUD.
(b) Use of practical screening methods: asking patients about cannabis use can be done by simply asking the question and elaborating as per any clinical history-taking, or by a more structured approach, such as using a cannabis-related questionnaire or urine drug screen.
(c) Targeting specific groups of practitioners initially: healthcare providers working in the addiction field, including psychiatrists and addiction medicine specialists. Further groups may include pain management specialists and primary care providers.
Ultimately, ongoing research is needed to establish the best practices for addressing cannabis use among patients in MMT. Meanwhile, comprehensive assessment of patients with OUD, including systematic screening for cannabis use, may help improve OUD treatment outcomes.
Strengths and limitations
The cross-sectional data from our study cannot ascertain any type of causal relationship, as only a longitudinal study can establish whether cannabis use results in the onset of anxiety disorders or further alcohol use. Such a study would need to (a) find a significant association between cannabis use and the adverse health condition, (b) recruit a cohort of healthy participants that have neither substance use or psychiatric disorders and record whether cannabis use precedes the onset of psychiatric disorders or substance use or vice versa and (c) control for confounding variables that might otherwise explain the relationship. Such a study would be impractical, costly and involve a census conducted at the population level, likely yielding imprecise estimates. Our findings fulfilled the first criterion by elucidating a significant association between cannabis use and further substance use and psychiatric and social comorbidities. Given the lack of data on the age of initiation of cannabis use and incidence of psychiatric disorders and substance use, we were unable to establish temporal precedence. Although we did control for important confounders, the onset of psychiatric disorders and substance use is the result of a complex interplay of genetic and environmental factors, making it precipitous to suggest any sole factor to be the cause.
Despite these limitations, our study had numerous methodological strengths. Most importantly, we included a large representative sample of patients with OUD receiving MMT from multiple sites within Canada. Each participant in this study was personally interviewed by trained researchers. Finally, we used formal diagnostic interviews to assess psychiatric disorders, substance use and previously validated self-reported measures of cannabis use in reference to urine toxicology screens.
In conclusion, the recent legalisation of cannabis in Canada in light of the ongoing opioid epidemic requires that we empirically consider how increased accessibility to cannabis will affect a high-risk population, like those with OUD. Our findings suggest that there is a high prevalence of cannabis use among patients with OUD receiving MMT. We found that cannabis use is associated with increased odds for psychiatric comorbidities and patterns of substance use. This emphasises the need to revise current treatment models to screen and manage these comorbidities in addition to providing opioid substitution therapy.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2019.78.
Funding
This work was supported by the Canadian Institute for Health Research (CIHR; grant 126639, Drug Safety and Effectiveness Network, and catalyst grant 155404, Population Health Intervention Research on Legalization of Cannabis), the Hamilton Academic Health Sciences Organization and the Chanchlani Research Centre. The funding agencies had no role in the design of the study, collection or analysis of data or publication of results.
Acknowledgements
We would like to thank the GENOA and CATC clinical and research staff for their contributions to this project. We would like to also thank our senior project nurse, Sheelagh Rutherford, for her dedication in recruiting participants. Lastly, we would like to extend our sincere gratitude to all the participants who generously volunteered their time and samples to make this study possible.







eLetters
No eLetters have been published for this article.