Psychosis (hallucinations and delusions) occurring in the context of Parkinson's disease may be primary, reflecting a progression of the underlying disease process, or secondary to the use of dopaminomimetic drugs. Psychotic symptoms can develop in up to 40% of Parkinson's disease patients. Reference Aarsland, Larsen, Cummins and Laakek1,Reference Fénelon, Mahieux, Huon and Ziégler2 Visual hallucinations, of animals or people, and paranoid or persecutory delusions (which may or may not be related to the hallucinations) are typically observed in Parkinson's disease. The onset of psychotic symptoms is associated with agitation, reduced quality of life and significant increases in caregiver burden, often heralding transfer to nursing homes. Reference Aarsland, Larsen, Tandberg and Laake3 Increasing age, cognitive decline and depression are risk factors for the development of psychotic symptoms. Reference Goetz and Stebbins4 The management of these distressing symptoms has proven to be difficult. Attempts to reduce dopaminomimetic drugs or the initiation of antipsychotic medication often results in unacceptable deterioration in motor function. Reference Cummings5 A number of open-label trials have been conducted of the atypical antipsychotics quetiapine, Reference Fernandez, Trieschmann, Burke, Jacques and Friedman6,Reference Targum and Abbott7 olanzapine, Reference Aarsland, Larsen, Lim and Tandberg8 risperidone Reference Ford, Lynch and Greene9,Reference Meco, Alessandri, Giustini and Bonifati10 and clozapine. Reference Friedman and Factor11 The results have been mixed. Antipsychotics exert their effect by antagonising dopaminergic neurotransmission, which also exacerbates parkinsonian motor deficits. Nevertheless, antipsychotics which exhibit fast dissociation from the dopamine receptor, such as quetiapine, or those that have preferential activity at dopamine-4 receptors (clozapine) may help alleviate psychotic symptoms without compromising motor function. There are now a number of randomised controlled trials and in this report we set out to review and analyse the efficacy and tolerability of antipsychotics in treating Parkinson's disease psychosis. This systematic review and meta-analysis will be reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (www.prisma-statement.org).
Method
Criteria for considering studies for this review
Double-blind randomised controlled trials (RCTs).
Types of participants
Patients of any age and of both genders suffering from Parkinson's disease and psychosis that emerged after the diagnosis and treatment of Parkinson's disease. Cognitive impairment can occur in the context of Parkinson's disease as the disease progresses (Parkinson's disease dementia) but other parkinsonian syndromes such as dementia with Lewy body patients are excluded. Patients with drug or alcohol misuse or pre-existing psychosis or affective disorders are also excluded.
Types of intervention
Trials of typical antipsychotics and atypical antipsychotics at any dose and formulation compared to placebo or no treatment.
Types of outcome measure
-
1. Psychiatric symptoms – standardised psychiatric rating scales assessing psychopathology such as the Brief Psychiatric Rating Scale (BPRS) or Clinical Global Impression Scale (CGI). The BPRS is the most commonly used scale in Parkinson's disease psychosis trials. Reference Fernandez, Aarsland, Fénelon, Friedman, Marsh and Tröster12
-
2. Motor symptomatology – standardised assessment scales such as the Unified Parkinson's Disease Rating Scale – Motor Subscale (UPDRSM).
-
3. Reporting of events precipitating trial withdrawal.
Search methods for identification of studies
Cochrane Library (terms: ‘parkinson’, ‘psychosis’, ‘hallucinosis’, ‘hallucination’, ‘delusion’, ‘antipsychotic’, ‘neuroleptic’), MEDLINE, EMBASE and PsycINFO. Titles, keywords and abstracts of citations from electronic databases retrieved and full copies of potentially suitable trials assessed further.
The following search terms used (MEDLINE, EMBASE, PsyINFO): [Exp Parkinson's Disease AND Exp Psychosis] AND Exp Antipsychoic. The following limits applied: English language, Humans and Randomised Controlled Trials.
Other sources: reference lists of located trials, proceedings and abstracts from International Congress on Parkinson Disease and Movement Disorders, review of articles published in specific journals (Age & Aging, International Journal of Geriatric Psychiatry) and personal communication with other researchers in the field.
The selection of studies is summarised in the PRISMA flow diagram (Fig. 1).
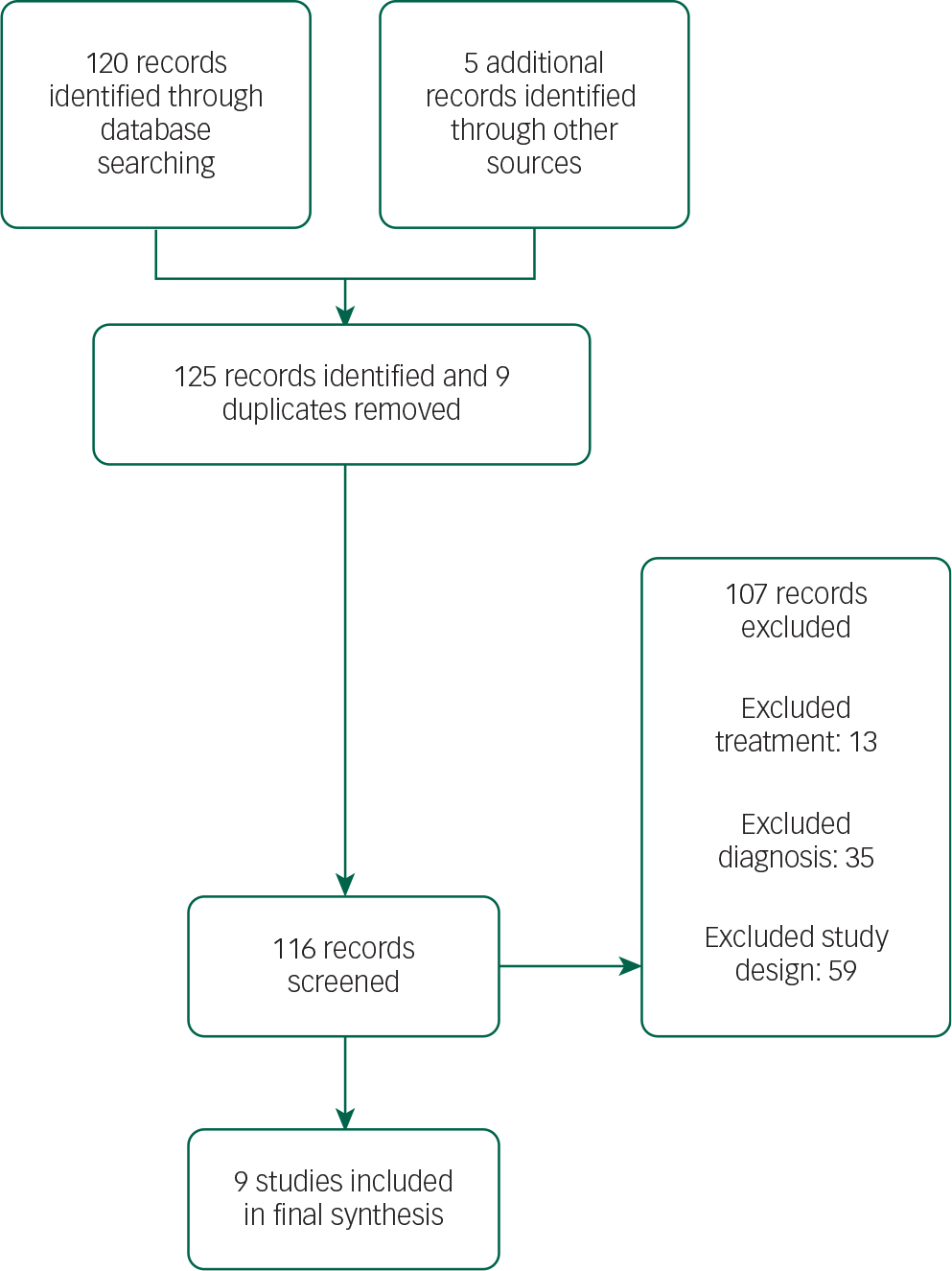
Fig. 1 PRISMA flow diagram.
The search retrieved 125 publications. The final selection list consisted of nine papers with a total sample size of 517 patients. Three hundred and forty-nine patients received antipsychotic treatment. There were three quetiapine studies, two clozapine studies, three olanzapine studies and one pimavanserin study. The selection process was undertaken jointly by two authors. Disagreements were resolved by discussion. The search was performed in April 2015.
Data management
A narrative synthesis for each agent is presented. Where a quantitative analysis can be performed measures of differences in psychotic symptoms or extrapyramidal side-effects are expressed as mean differences (with 95% confidence intervals). Statistical analysis used the generic inverse model on statistical software provided by the Cochrane Collaboration (RevMan 5.3). The data were treated as continuous. Post-intervention scores, change-from-baseline standard deviations (s.d.) and samples sizes were input. Baseline scores (with s.d.) and change-from-baseline scores (with s.d.) were used to calculate post-intervention scores if these were not reported explicitly in the papers. Reference Higgins, Altman, Gøtzsche, Jüni, Moher and Oxman13 Outcomes were assessed by a random effects model as this takes into account any differences between studies even if there is no statistically significant heterogeneity. Reference Higgins, Altman, Gøtzsche, Jüni, Moher and Oxman13 When relevant data were not included in the papers published, authors were contacted.
Assessment of risk of bias in included studies
The Cochrane risk of bias tool was used. The following sources of bias were assessed: selection bias, performance bias, attrition bias, detection bias and selective reporting of studies. Reference Higgins, Altman, Gøtzsche, Jüni, Moher and Oxman13
Assessment of heterogeneity
Heterogeneity between studies was assessed using the I 2 statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I 2 estimate is greater than or equal to 50% this is interpreted as indicating high levels of heterogeneity. Statistical significance of heterogeneity was additionally tested with χ2 tests, using a threshold of P<0.20 as the threshold for statistical significance, because the power of this test is known to be low if the number of studies included is small. Reference Higgins, Altman, Gøtzsche, Jüni, Moher and Oxman13
Results
Characteristics of included studies
Table 1 presents the characteristics of included studies. Adverse events precipitating patient withdrawal from a trial are shown in Table 2. The Breier et al Reference Friedman19 study incorporated two trials, one in the USA and the other in Europe. Baseline clinical and demographic data did not differ between the two groups in this study and both trials met the criteria for inclusion in this review.
Table 1 Characteristics of included studies.
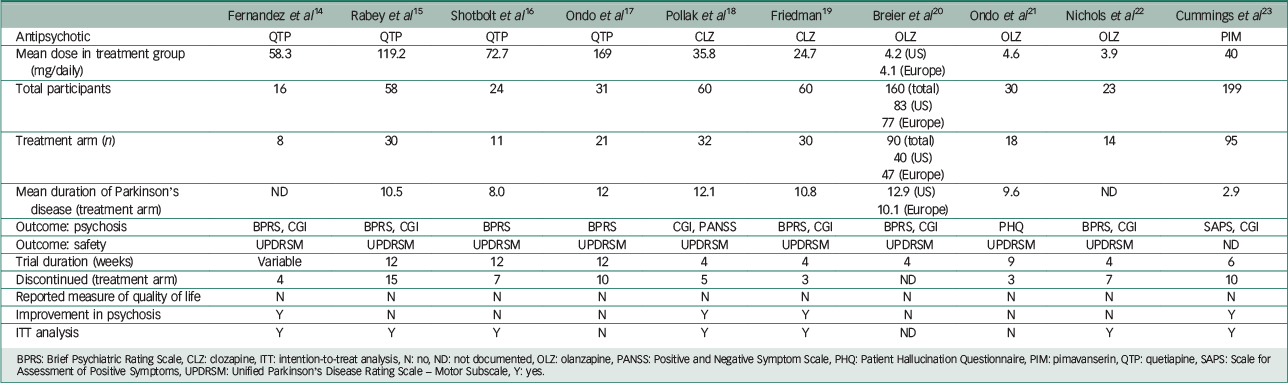
| Fernandez et al Reference Fernandez, Okun, Rodriguez, Malaty, Romrell and Sun14 | Rabey et al Reference Rabey, Prokhorov, Miniovitz, Dobronevsky and Klein15 | Shotbolt et al Reference Shotbolt, Samuel, Fox and David16 | Ondo et al Reference Ondo, Tintner, Voung, Lai and Ringholz17 | Pollak et al Reference Pollak, Tison, Rascol, Destée, Péré and Senard18 | Friedman Reference Friedman19 | Breier et al Reference Breier, Sutton, Feldman, Kadam, Ferchland, Wright and Friedman20 | Ondo et al Reference Ondo, Levy, Vuong, Hunter and Jankovic21 | Nichols et al Reference Nichols, Hartlein, Eicken, Racette and Black22 | Cummings et al Reference Cummings, Isaacson, Mills, Williams, Chi-Burns and Corbett23 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Antipsychotic | QTP | QTP | QTP | QTP | CLZ | CLZ | OLZ | OLZ | OLZ | PIM |
| Mean dose in treatment group (mg/daily) | 58.3 | 119.2 | 72.7 | 169 | 35.8 | 24.7 | 4.2 (US) 4.1 (Europe) |
4.6 | 3.9 | 40 |
| Total participants | 16 | 58 | 24 | 31 | 60 | 60 | 160 (total) 83 (US) 77 (Europe) |
30 | 23 | 199 |
| Treatment arm (n) | 8 | 30 | 11 | 21 | 32 | 30 | 90 (total) 40 (US) 47 (Europe) |
18 | 14 | 95 |
| Mean duration of Parkinson's disease (treatment arm) | ND | 10.5 | 8.0 | 12 | 12.1 | 10.8 | 12.9 (US) 10.1 (Europe) |
9.6 | ND | 2.9 |
| Outcome: psychosis | BPRS, CGI | BPRS, CGI | BPRS | BPRS | CGI, PANSS | BPRS, CGI | BPRS, CGI | PHQ | BPRS, CGI | SAPS, CGI |
| Outcome: safety | UPDRSM | UPDRSM | UPDRSM | UPDRSM | UPDRSM | UPDRSM | UPDRSM | UPDRSM | UPDRSM | ND |
| Trial duration (weeks) | Variable | 12 | 12 | 12 | 4 | 4 | 4 | 9 | 4 | 6 |
| Discontinued (treatment arm) | 4 | 15 | 7 | 10 | 5 | 3 | ND | 3 | 7 | 10 |
| Reported measure of quality of life | N | N | N | N | N | N | N | N | N | N |
| Improvement in psychosis | Y | N | N | N | Y | Y | N | N | N | Y |
| ITT analysis | Y | Y | Y | N | Y | Y | ND | N | Y | Y |
BPRS: Brief Psychiatric Rating Scale, CLZ: clozapine, ITT: intention-to-treat analysis, N: no, ND: not documented, OLZ: olanzapine, PANSS: Positive and Negative Symptom Scale, PHQ: Patient Hallucination Questionnaire, PIM: pimavanserin, QTP: quetiapine, SAPS: Scale for Assessment of Positive Symptoms, UPDRSM: Unified Parkinson's Disease Rating Scale – Motor Subscale, Y: yes.
All patients in studies included were on concomitant dopaminergic agents; a risk of bias graph and summary are presented (Figs 2 and 3).
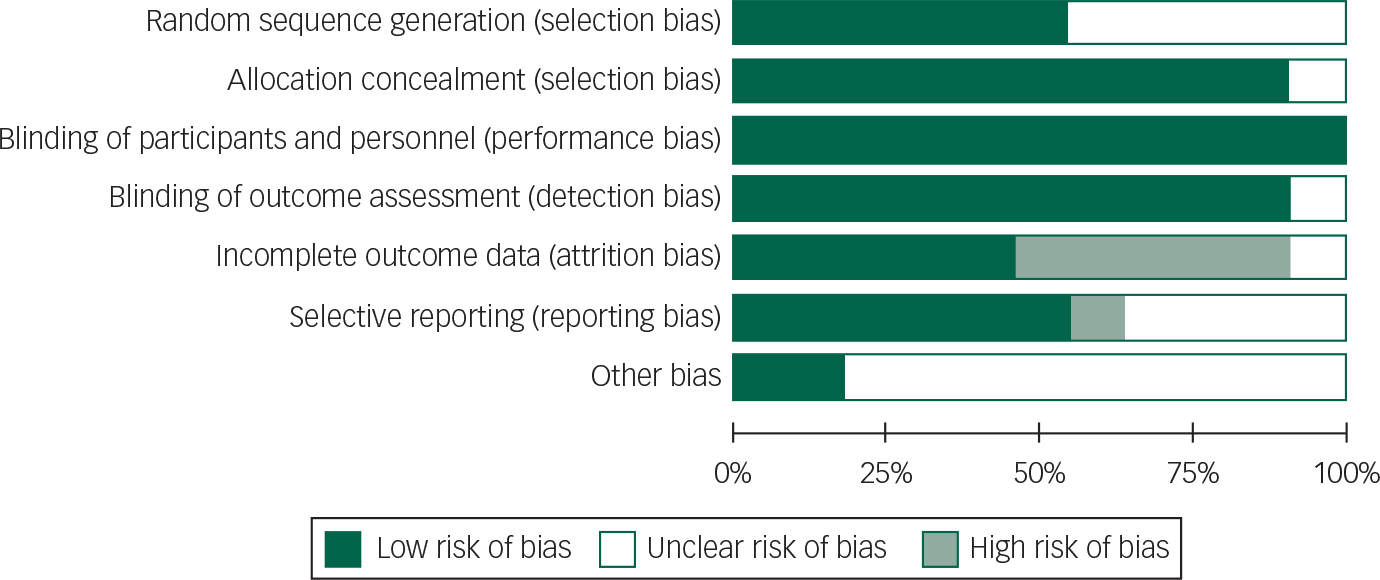
Fig. 2 Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
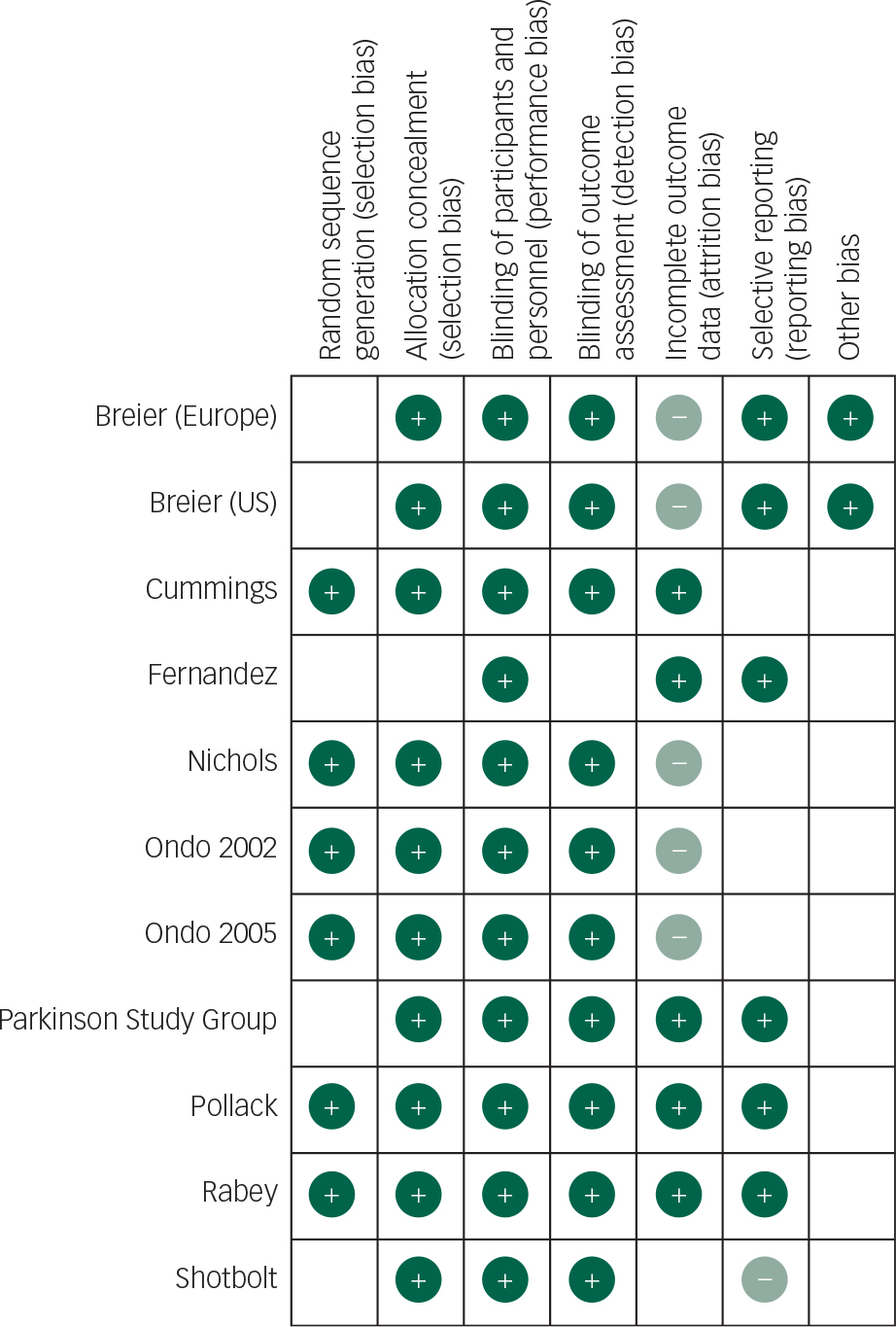
Fig. 3 Risk of bias summary: review of authors’ judgements about risk of each bias item for each included study.
Table 2 Safety profile: adverse events precipitating patient withdrawals in treatment groups
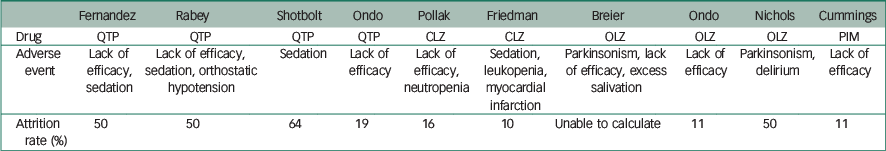
| Fernandez | Rabey | Shotbolt | Ondo | Pollak | Friedman | Breier | Ondo | Nichols | Cummings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | QTP | QTP | QTP | QTP | CLZ | CLZ | OLZ | OLZ | OLZ | PIM |
| Adverse event | Lack of efficacy, sedation | Lack of efficacy, sedation, orthostatic hypotension | Sedation | Lack of efficacy | Lack of efficacy, neutropenia | Sedation, leukopenia, myocardial infarction | Parkinsonism, lack of efficacy, excess salivation | Lack of efficacy | Parkinsonism, delirium | Lack of efficacy |
| Attrition rate (%) | 50 | 50 | 64 | 19 | 16 | 10 | Unable to calculate | 11 | 50 | 11 |
Main findings
Quetiapine
Data from the Fernandez, Rabey and Shotbolt trials were combined in a meta-analysis below (Figs 4 and 5). Although all the quetiapine trials used BPRS and UPDRSM as efficacy and safety measures respectively, change-from-baseline or post-intervention scores were not explicitly stated in the Ondo trial and the author was unavailable to provide raw data.
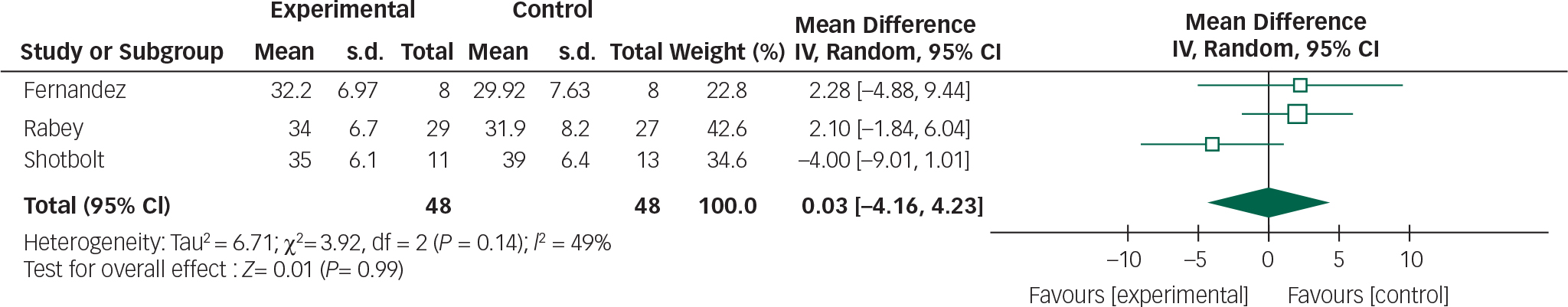
Fig. 4 Random effects meta-analysis of the use of quetiapine in the management of Parkinson's disease psychosis, efficacy measure: BPRS.
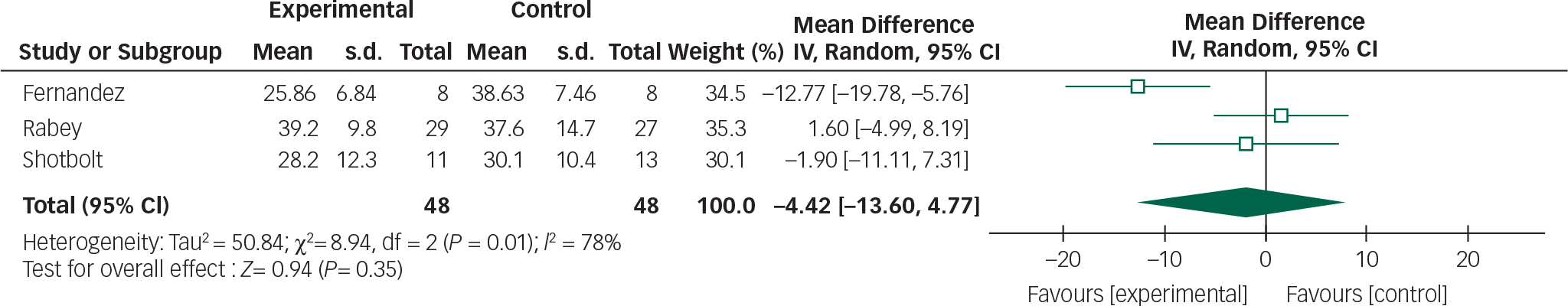
Fig. 5 Random effects meta-analysis of the use of quetiapine in the management of Parkinson's disease psychosis, safety measure: UPDRSM.
Based on the present meta-analysis quetiapine does not appear to significantly improve psychotic symptoms in Parkinson's disease. In addition, the overall mean difference for both outcomes crosses the null value therefore any reduction in BPRS scores or stability in UPDRSM scores is not statistically significant.
The use of quetiapine was also associated with high drop-out rates ranging between 19 and 64%. A lack of efficacy and sedation were the most common adverse events precipitating withdrawal. The latter is particularly important given the role this may have on increasing falls risk Reference Rigler, Shireman, Cook-Wiens, Ellerbeck, Whittle and Mehr24 and reducing quality of life.
These findings, however, need to be interpreted with caution. The pooled analysis of quetiapine studies still contains significant levels of heterogeneity and the overall effect size did not reach statistical significance. This probably reflects the small study sample sizes and high drop-out rates. The only quetiapine study to include a power calculation Reference Rabey, Prokhorov, Miniovitz, Dobronevsky and Klein15 stated that 24 patients would be required in each arm for 80% power (5% significance level).
Olanzapine
Data from the three olanzapine trials could not be combined owing to heterogeneous reporting of outcomes, non-standardised assessment scales Reference Breier, Sutton, Feldman, Kadam, Ferchland, Wright and Friedman20 and incomplete outcome data. Reference Ondo, Levy, Vuong, Hunter and Jankovic21 However, one study incorporated two RCTs so a meta-analysis was performed of these trials (Figs 6 and 7). Reference Friedman19
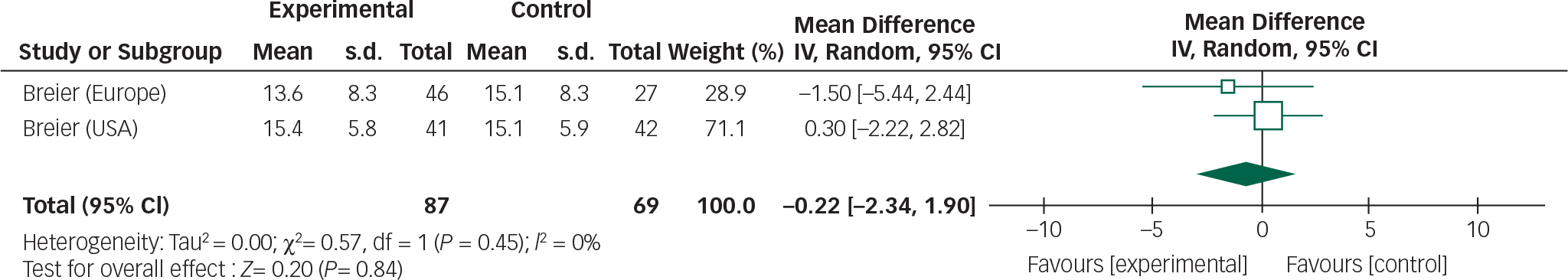
Fig. 6 Random effects meta-analysis of the use of olanzapine in the management of Parkinson's disease psychosis, efficacy measure: BPRS.
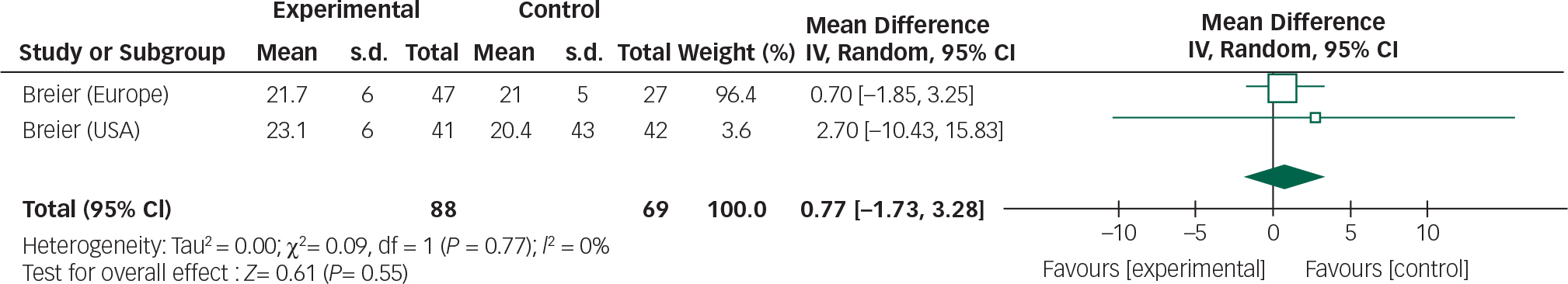
Fig. 7 Random effects meta-analysis of the use of olanzapine in the management of Parkinson's disease psychosis, safety measure: UPDRSM.
The overall mean difference for both efficacy and safety outcomes crosses the null value; therefore, any reduction in BPRS scores or stability in UPDRSM scores is not statistically significant.
In addition, incomplete reporting of adverse events and patient withdrawals make assessing the safety profile of the drug difficult. Based on the three trials included in this review olanzapine does not appear to improve psychotic symptoms, but may cause a deterioration in motor function. Olanzapine use in the elderly is also associated with an increased risk of cerebrovascular accidents and therefore should be used with caution. Reference Citrome, Collins, Nordstrom, Rosen, Baker and Nadkarni25
Clozapine
Data from the two clozapine trials can be combined in a meta-analysis by the CGI and UPDRSM as measures of efficacy and safety, respectively (Figs 8 and 9).
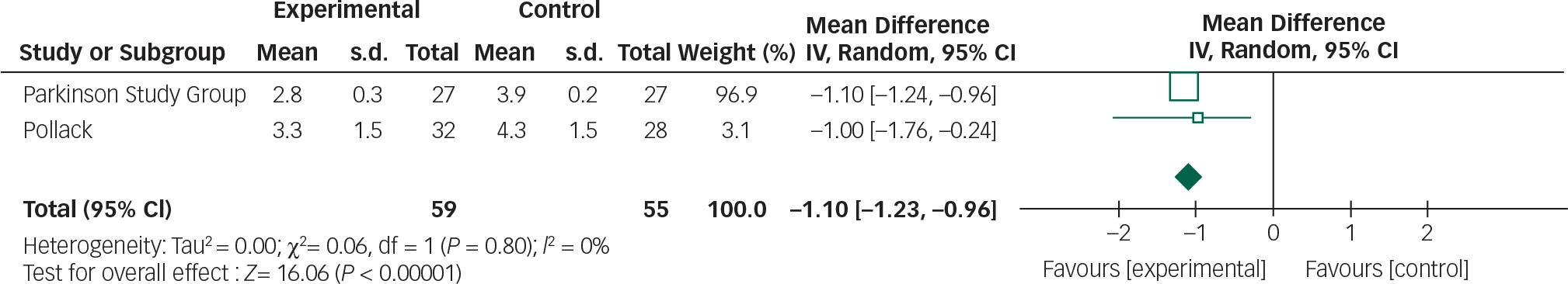
Fig. 8 Random effects meta-analysis of the use of clozapine in the management of Parkinson's disease psychosis, efficacy measure: CGI.
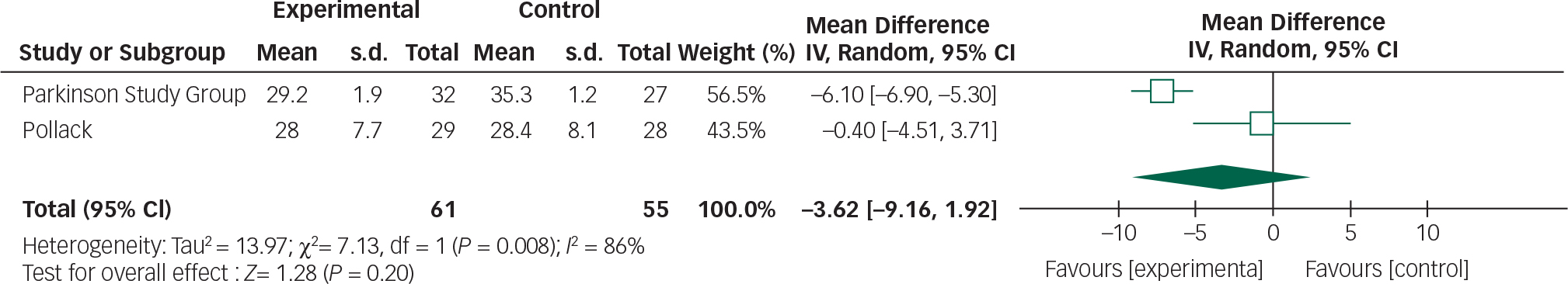
Fig. 9 Random effects meta-analysis of the use of clozapine in the management of Parkinson's disease psychosis, safety measure: UPDRSM.
Clozapine appears to result in an improvement in CGI scores (95% CI −1.23 to −0.96). Further research is required to replicate these findings in larger samples and assess the clinical relevance of this CGI score improvement. Though clozapine demonstrated some efficacy over placebo in alleviating psychotic symptoms, a direct comparison cannot be made with other studies employing the BPRS. Approximations between CGI and BPRS are theoretically possible; however, the absolute improvements reported in the clozapine studies cannot be reliably translated. Reference Leucht, Kane, Etschel, Kissling, Hamann and Engel26 The subjectivity of rater-assessed changes in mental state assessed by the CGI is a disadvantage in comparison to BPRS which examines specific symptoms.
Clozapine had no significant effect on UPDRSM scores. The following values have been suggested to describe minimal, moderate and large clinically important changes in UPDRSM scores: 2.5, 5.2 and 10.8, respectively. Reference Shulman, Gruber-Baldini, Anderson, Fishman, Reich and Weiner31
There was 0% heterogeneity between the two studies analysed and this was statistically significant.
The clozapine trials were associated with the lowest attrition rates. Overall 3% (2/64) of the patients exposed to clozapine experienced leukopenia/neutropenia requiring withdrawal from the study. Clozapine requires close blood and physical monitoring given the risk of dose-independent leukopenia/neutropenia. There were no significant differences in non-haematological adverse events between treatment and placebo groups in both studies. However, the two papers reviewed did not explicitly give the details of the physical background of the patients who withdrew from the studies.
Clozapine is recommended by NICE in the UK, 27 though it is recognised that few specialists caring for Parkinson's disease patients have experience with or access to clozapine services. This requirement for robust monitoring is echoed by a small study Reference Wolters, Hurwitz, Mak, Teal, Peppard and Remick28 (n=6) that investigated the efficacy of clozapine in the management of psychosis in Parkinson's disease dementia and other functional psychiatric illnesses comorbid with Parkinson's disease. In that study Reference Wolters, Hurwitz, Mak, Teal, Peppard and Remick28 psychosis improved with clozapine, but patients experienced sedation, delirium and worsening motor symptoms.
Pimavanserin
There is evidence that neocortical 5-HT2A upregulation may play a role in the pathogenesis of Parkinson's disease psychosis. Reference Ballanger, Strafella and van Eimeren29 The role of pimavanserin, a novel selective 5-HT2A inverse agonist without dopaminergic affinity, has been investigated in the management of Parkinson's disease psychosis. In a six-week double-blind RCT (n=199), 95 patients were exposed to the drug. Reference Nichols, Hartlein, Eicken, Racette and Black22 The mean change-from-baseline for CGI was −0.58 (baseline 4.27 in treatment arm) and −4.34 for caregiver burden. This is the biggest Parkinson's disease psychosis trial to date. Pimavanserin was associated with a significant improvement in psychotic symptoms compared with placebo. However, further trials are required to replicate these findings and determine the clinical significance of these improved scores.
Patients were followed up for adverse events (including electrocardiogram and physical examination) though there was no formal assessment of motor function. Eleven per cent of patients in the treatment group withdrew from the trial because of worsening psychotic symptoms. Subclinical QTc prolongation was also noted in the treatment group.
Methodologically this study was sound with appropriate random sequence generation and blinding. However, patients were given brief psychosocial therapy 2 weeks before the commencement of treatment which potentially introduces the confounding influence of additional psychotherapeutic intervention. In addition, the patients included in this trial had a shorter Parkinson's disease duration and 17% of those in the treatment had previously been treated with quetiapine 1 month before the trial. The short trial duration restricts our ability to assess the duration of the antipsychotic response and its impact on longer-terms outcomes including caregiver burden.
Discussion
We have presented an up-to-date systematic review and meta-analysis of randomised controlled trials investigating the role of antipsychotics in the management of Parkinson's disease psychosis.
Limitations
Specific limitations of individual studies in each antipsychotic group have been highlighted above. More generally, the number of trials included in these meta-analyses, despite adopting a random effects model, is still small. This is particularly relevant when considering the effect of individual antipsychotics and caution should be exercised in interpreting the results of this review. There is promising evidence for the efficacy and safety of clozapine, but further research is needed. In addition, the clozapine trials were much shorter than the quetiapine trials which may potentially account for the differences in the reported incidence of adverse events. As with all systematic reviews publication bias is a source of error. As fewer than 10 studies were reviewed it was deemed inappropriate to assess publication bias using a funnel plot. Only published trials were assessed in this review.
Implications for clinical practice and future research
In this review, quetiapine has not demonstrated statistically significant efficacy or tolerability, but is associated with troublesome side-effects and high drop-out rates. It is, however, important to note that these studies were small and that there is anecdotal evidence of efficacy. Quetiapine should therefore be used with caution. Olanzapine also requires caution due to an increased risk of parkinsonism and cerebrovascular accidents. Clozapine is associated with an antipsychotic effect. To facilitate its use clinically services need to be developed and integrated to ensure that patients can be appropriately followed-up and monitored. A retrospective chart review of a clozapine clinic for patients with Parkinson's disease found a 66% response rate to clozapine. Reference Hack, Fayad, Monari, Akbar, Hardwick and Rodriguez30 However, there was a 41% retention rate to the service due to the inconvenience associated with frequent blood monitoring. Pimavanserin is novel treatment that warrants further investigation.
No solid recommendations can based on the current evidence owing to the limitations discussed above. Further research is needed, including adequately powered RCTs of various antipsychotics using uniform rating scales. There is also a need for further studies investigating the side-effects, adherence, barriers to engagement, cost-effectiveness, quality of life and transition to nursing home placement. These questions can be tackled utilising both quantitative and qualitative methods. For example, a mixed-methods approach could be used incorporating an RCT to assess treatment and safety outcomes, using standardised rating scales, and interviews or patient reported outcome measures to investigate the functional impact of the therapeutic effect (or side-effect).















eLetters
No eLetters have been published for this article.