The highest prevalence of cigarette smoking is reported in people with schizophrenia (Box 1): it is nearly three times that in the general population and higher than in those with other mental disorders (de Leon Reference de Leon and Diaz2005). A meta-analysis of 42 studies involving 7593 people with schizophrenia from 20 nations revealed that 62% were smokers (de Leon Reference de Leon and Diaz2005). The World Health Organization has pointed out that the prevalence of smoking has declined over the years (World Health Organization 2015), but a corresponding decline has not been seen in people with mental illnesses (Berg Reference Berg, Sentir and Cooley2014). In the USA, 50% of total cigarette production is consumed by people with a mental illness (Berg Reference Berg, Sentir and Cooley2014).
BOX 1 Statistics for smoking and schizophrenia
• A meta-analysis that looked at data from 22 countries revealed the prevalence of smoking in people with schizophrenia to be 62%
• Prevalence of smoking in people with schizophrenia is three times that in the general population
• Prevalence of smoking in people with schizophrenia is higher than in people with other mental illnesses
Mortality among people with schizophrenia is high, with a standardised mortality ratio of two (Brown Reference Brown, Inskip and Barraclough2000). Their life expectancy is about 20 years less than that of the general population. Tobacco smoking (which we shall refer to as smoking, for short) is an independent risk factor for cardiovascular diseases (Brown Reference Brown, Inskip and Barraclough2000; Goff Reference Goff, Cather and Evins2005), which account for two-thirds of natural causes of premature deaths in people with schizophrenia (Goff Reference Goff, Cather and Evins2005). Therefore, smoking prevention is of paramount importance for this group.
Apart from the recognised factors that affect substance use disorders in general, examination of the particular association between smoking and schizophrenia raises three main possible hypotheses:
• first, patients smoke at an increased frequency because smoking ameliorates some of the symptoms of schizophrenia and side-effects of medication
• second, smoking causes an increased predisposition to develop psychosis
• third, common genetic risk factors make some individuals more vulnerable to developing schizophrenia and nicotine dependence.
The self-medication hypothesis takes precedence over the two other hypotheses (Chambers Reference Chambers2009). The two latter hypotheses have not gained proper attention from clinicians and policy makers, despite being supported by evidence (Gurillo Reference Gurillo, Jauhar and Murray2015). Thus, examination of these two hypotheses and critical appraisal of the self-medication hypothesis are timely and pertinent.
The self-medication hypothesis
The self-medication hypothesis was initially described by Khantzian on the basis of his observation that people with mental illnesses take cocaine, in his opinion, to self-medicate their symptoms (Khantzian Reference Khantzian1997). Later, this finding was employed to explain excessive smoking rates among people with mental illnesses (Manzella Reference Manzella, Maloney and Taylor2015).
The strongest evidence for the self-medication hypothesis comes from the neurophysiological findings of sensory processing deficits in pre-pulse inhibition and suppression of P50-evoked potential, and abnormalities in anti-saccadic eye movement tests (Kumari Reference Kumari and Postma2005). These deficits and abnormalities, frequently encountered in people with schizophrenia, are linked to impairment in the filtering of extraneous information. Furthermore, these abnormalities correlate with the degree of cognitive impairment and thought disorder. Several neuroscientific studies support the premise that smoking improves these abnormalities (Hong Reference Hong, Wonodi and Lewis2008; Kumari Reference Kumari, Antonova and Geyer2008; Drusch Reference Drusch, Lowe and Fisahn2013; Awad Reference Awad and Voruganti2015).
Hypofunction of central nicotinic acetylcholine receptors is well described in the scientific literature in people with schizophrenia. It results in reduced dopamine and glutamate levels in the prefrontal cortex, leading to negative and cognitive symptoms. Nicotine is hypothesised to reverse this deficit and normalise frontal dopamine and glutamate levels, resulting in an improvement in these symptoms (Parikh Reference Parikh, Kutlu and Gould2016). Working memory and sustained attention are also improved by nicotine use, according to some reports (Dépatie Reference Dépatie, O'Driscoll and Holahan2002).
More recent research findings suggest that patients may smoke to alleviate some of the extrapyramidal side-effects of antipsychotic medications (Goff Reference Goff, Henderson and Amico1992). The given neurobiological explanation is that nicotine stimulates acetylcholine receptors on the cell bodies situated in the ventral tegmental area of the midbrain. Through this mechanism, nicotine is thought to increase dopamine levels in the striatum via the mesolimbic pathway, which reduces the dopamine receptor blockade caused by antipsychotics and results in an improvement of extrapyramidal side-effects (Laruelle Reference Laruelle2014).
It has been reported that the management of nicotine dependence in schizophrenia is challenging and people with the disorder are less likely to consider stopping smoking (Dalack Reference Dalack, Becks and Hill1999). Moreover, there are reports that deterioration of their mental state may occur on nicotine withdrawal (Dalack Reference Dalack, Becks and Hill1999). Some research shows evidence of fewer negative and cognitive symptoms in people with schizophrenia who smoke, which helps them to socialise better (Dalack Reference Dalack, Healy and Meador-Woodruff1998).
Critical appraisal of the self-medication hypothesis
Medical professionals and the general public seem to accept the self-medication hypothesis without much critique. However, acceptance of this assumption for more than three decades may have led to inadequate interventions and reduced concordance with the treatment of nicotine dependence in people with schizophrenia (Manzella Reference Manzella, Maloney and Taylor2015).
Numerous studies counter the impression that smoking improves the symptoms of schizophrenia. People with severe nicotine dependence are reported to have experienced more severe positive and negative symptoms and therefore receive higher doses of antipsychotics than those without (Krishnadas Reference Krishnadas, Jauhar and Telfer2012). These results should be understood in the context of potentially important limitations. In these studies, self-reported smoking status has been taken as a reliable measure to diagnose nicotine use disorder. Concordance with medication has not been measured by more objective methods. Another limitation is that the cross-sectional nature of the study design makes ascertaining of the direction of causality impossible. A systematic review highlighted the fact that smoking and being overweight are linked with increased severity of symptoms and poorer outcome in both schizophrenia and bipolar affective disorder (Dickerson Reference Dickerson, Stallings and Origoni2013). A prospective study from China of 374 individuals with schizophrenia revealed that smoking was independently associated with more frequent hospital admissions, more severe negative symptoms and deterioration of thought disorder over 1–2 years (Wang Reference Wang, Xiang and Weng2010). Both the systematic review and the prospective study also illustrated that symptom severity and the number of relapses are higher in patients with schizophrenia who smoke, contradicting the self-medication hypothesis.
Aside from ameliorating symptoms or side-effects, another reported benefit of smoking in schizophrenia is that nicotine tends to improve attention and other cognitive functions (Kumari Reference Kumari and Postma2005). One study compared attention and task performance of 17 individuals with schizophrenia and 17 healthy individuals, all of whom were smokers, before and after administration of a nicotine or placebo patch. Nicotine improved attention and concentration levels to a similar degree in both groups and there were no greater benefits in attention and task performance among those with schizophrenia. Interestingly, self-reported ability to concentrate improved only in the control group (Hahn Reference Hahn, Harvey and Concheiro-Guisan2013). However, generalisation of these findings is limited owing to the small sample size. Interestingly, a recent study involving 61 individuals with treatment-resistant schizophrenia who smoked reported that they performed worse than non-smoking counterparts on cognitive tasks, and had more negative symptoms and poorer social adjustment (Iasevoli Reference Iasevoli, Balletta and Gilardi2013).
It is important to note that symptoms of nicotine withdrawal, which simulate some of the symptoms of mental illnesses such as dysphoria, irritability and insomnia, may last up to 3 weeks following smoking cessation (Hall Reference Hall, Der-Avakian and Gould2015). In addition, polycyclic aromatic hydrocarbons, a constituent of cigarette smoke, lower the plasma levels of most antipsychotics, owing to the induction of cytochrome P-450 metabolism. Hence, plasma levels of antipsychotics tend to increase if patients stop smoking, resulting in the appearance of dose-related side-effects such as extra-pyramidal symptoms (Desai Reference Desai, Seabolt and Jann2001). Thus, it is possible that symptoms of nicotine withdrawal, along with side-effects owing to increased plasma levels of medication, will erroneously be attributed to worsening of symptoms of mental illness following smoking cessation.
A naturalistic study in people with schizophrenia found no significant difference in severity of symptoms between smokers and those who had quit smoking (Krishnadas Reference Krishnadas, Jauhar and Telfer2012). It also reported that 25% of those with a lifetime history of smoking in the study sample had quit smoking, indicating that people with schizophrenia can overcome tobacco addiction (Krishnadas Reference Krishnadas, Jauhar and Telfer2012). In addition, evidence confirms that there is no worsening of symptomatology of schizophrenia on reduction or cessation of smoking (Dome Reference Dome, Lazary and Kalapos2010). Furthermore, a recent randomised controlled study involving 140 centres in 16 countries with more than 8000 participants found that there was no difference in odds ratios of the effectiveness of smoking cessation interventions among people with a mental illness and without (Anthenelli Reference Anthenelli, Benowitz and West2016).
These findings give a new insight into understanding tobacco dependence in people with schizophrenia. The overall picture seems to be that the challenge in smoking cessation is unlikely to be worsening in any of the domains of cognitive deficit or other symptoms of schizophrenia, but is likely to be that of overcoming an addiction (Anthenelli Reference Anthenelli, Benowitz and West2016).
The tobacco industry and the self-medication hypothesis
The tobacco industry in the USA had to sign a master settlement agreement in 1998, in response to multiple cases of litigation against it. The result was around 70 million pages of tobacco industry documents and nearly 20 000 other media materials becoming accessible to the public (Prochaska Reference Prochaska, Hall and Bero2008; Hurt Reference Hurt, Ebbert and Muggli2009). Scrutiny of these documents clearly shows how the industry deceived the general public by advertising and other methods and suppressing opposing research (Prochaska Reference Prochaska, Hall and Bero2008). One study analysing these documents (Prochaska Reference Prochaska, Hall and Bero2008) pointed out that the tobacco industry had directly funded research on the hazards of stopping smoking that supports the self-medication hypothesis. Notably, the industry promulgated the image of nicotine as a therapeutic agent rather than an addictive agent. The most scandalous revelation is, perhaps, that the tobacco industry funded research studies that showed the benefits of smoking in correcting defects in pre-pulse inhibition and suppression of P50 in schizophrenia, which had provided one of the strongest neurobiological evidence bases for the self-medication theory. Furthermore, the tobacco industry persistently attempted to promote and maintain smoking among psychiatric patients.
Smoking causes schizophrenia: an alternative hypothesis
The risk factors for schizophrenia spectrum disorders are well established. In the recent past, there has been emerging interest among researchers in smoking as a risk factor for developing schizophrenia spectrum disorder – the ‘smoking causes schizophrenia’ hypothesis (Box 2). A high prevalence of smoking in the first episode of psychosis is well established (Myles Reference Myles, Newall and Curtis2012). This observation raises the possibility of smoking as a risk factor for schizophrenia. However, this association is not as straightforward as it appears.
BOX 2 Factors suggesting that smoking plays a causative role in psychosis
The association between smoking and schizophrenia fulfils four of the main Bradford Hill criteria for causation:
• the strength of association: prospective studies clearly show that smoking increases the risk of schizophrenia; this association is dose–dependent and it remains significant even after adjusting for confounding factors
• temporal relationship: smoking has been prospectively associated with the occurrence of schizophrenia and this association remains significant after adjusting for the prodromal period of the illness
• consistency: the association has been shown to be present across many countries, over time and in a variety of settings
• plausible mechanism: neurobiological research supports the hypothesis of stimulation of nicotinic receptors on dopaminergic neurons in the midbrain, which induces increased dopamine release in the nucleus accumbens.
Hence, we can propose that smoking may have a role in the causation of schizophrenia.
Individual studies
An extensive prospective Israeli study surveyed 14 248 military recruits devoid of major psychopathology to examine onset of smoking and subsequent risk for schizophrenia. The cohort, all adolescents, was initially screened for smoking and it was found that 28.4% smoked at least one cigarette per day. Subsequently, the cohort was followed up for 4–16 years. There was a significant association between daily smoking levels recorded at the initial screening and the risk of developing schizophrenia during the study period. Compared with not smoking, the adjusted relative risk of developing schizophrenia associated with smoking more than 10 cigarettes per day was 2.28 times higher (95% CI = 1.19–4.34) and was statistically significant (Weiser Reference Weiser, Reichenberg and Grotto2004). Two potential limitations can be found in this study. The first is that, since investigators assessed smoking status only at 18 years of age, and it was not possible to identify those who stopped or commenced smoking after the age of 18. The second is that this study did not take into account participants' cannabis use, a potential confounding factor in the association between smoking and schizophrenia.
An even larger prospective study from Sweden examined the smoking status of 1 413 849 women and 233 879 men. All the participants in both groups had no history of non-affective psychosis. The study explored the association between smoking and schizophrenia, including the dose–response relationship, the effect of confounding factors, including cannabis use, and the possibility of smoking being an epiphenomenon of the prodrome of schizophrenia. The average age at the end of the monitoring was 46 for women and 26 for men. The hazard ratios for developing non-affective psychosis in both men and women were significantly increased among the smokers (Kendler Reference Kendler, Lönn and Sundquist2015). The study researchers introduced a buffer period for the prodromal phase of illness, to nullify the hypothesis that patients smoke at a higher rate to self-medicate their symptoms during the prodromal phase. Even after this buffer time was built into the analysis, the hazard ratios remained significant. The effect of confounding factors did not seem to reverse or neutralise the significantly increased risk of developing schizophrenia in this population. Since the risk of schizophrenia was more strongly associated with heavier rather than lighter smoking, it is likely that there is a dose–response relationship, supporting the hypothesis that smoking causes schizophrenia. Temporal precedence of smoking over the prodromal period further strengthens this ‘smoking causes schizophrenia’ hypothesis.
It is worth noting that we came across one prospective study (Zammit Reference Zammit, Allebeck and Dalman2003; analysed in Gurillo Reference Gurillo, Jauhar and Murray2015) that reported that smoking was protective against future development of schizophrenia.
Meta-analyses
Four recent meta-analyses each concluded that smoking increases the risk of psychotic disorders (Myles Reference Myles, Newall and Curtis2012; Gurillo Reference Gurillo, Jauhar and Murray2015; Hunter Reference Hunter, Murray and Asher2018; Scott Reference Scott, Matuschka and Niemelä2018).
Myles et al (Reference Myles, Newall and Curtis2012) examined the prevalence and course of tobacco use during early psychosis in a meta-analysis of 58 studies. Individuals with first-episode psychosis tended to have been smokers before the onset of illness, to be heavy smokers at the time of presentation for treatment and were much more likely to smoke than aged-matched healthy controls.
Gurillo et al (Reference Gurillo, Jauhar and Murray2015) examined data from 61 cross-sectional case–control and prospective studies in which smoking rates were reported for people with psychotic disorders, again revealing that smoking increased the risk of psychosis (Fig. 1). They also found that individuals who developed psychosis started smoking at an earlier age than the healthy controls, but this result was non-significant.
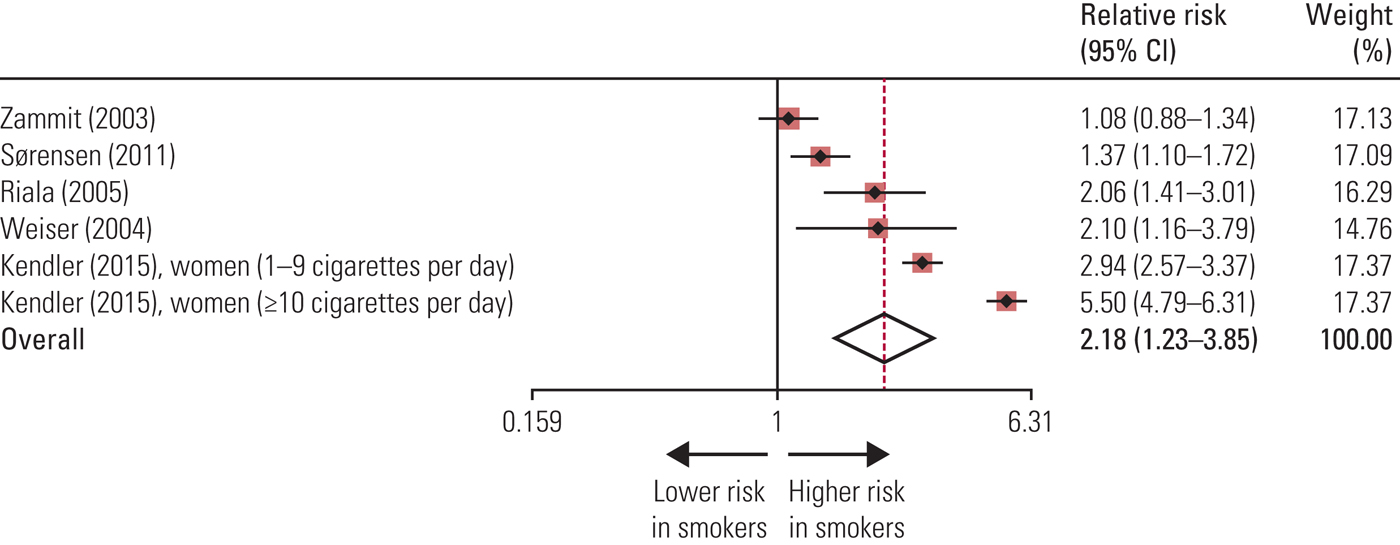
FIG 1 Risk of developing psychosis in prospective studies in daily smokers compared with non-smokers. Black diamonds represent relative risks; red squares represent weights (from random-effects analysis); horizontal lines represent 95% CIs; the white diamond represents overall relative risk (dotted line) and 95% CI. Source: Gurillo et al (Reference Gurillo, Jauhar and Murray2015). Reproduced with permission.
A meta-analysis by Hunter et al (Reference Hunter, Murray and Asher2018) is different from the previous two since it used specifically schizophrenia, rather than psychosis, as an outcome measure. Relative risk and hazard ratios of five prospective studies are pooled to ascertain the pooled relative risk. It was revealed that smokers had significantly increased the risk of developing schizophrenia compared with non-smokers (RR = 1.99, 95% CI 1.10–3.61).
Although these three meta-analyses included a number of prospective studies, they did not assess concurrent use of other psychoactive substances, a limitation addressed by Scott et al (Reference Scott, Matuschka and Niemelä2018). This team carried out a meta-analysis of seven cohort studies and one cross-sectional study. Importantly, it identified smoking as a significant risk factor for schizophrenia spectrum disorder even after adjusting for confounding factors such as comorbid cannabis use, socioeconomic status, and history of parental psychotic disorder and parental substance use disorder. Limitations include not considering childhood trauma as a confounding factor and determining smoking status from responders’ description of current tobacco use (which may not be a reliable measure). Of the eight studies, seven were shown to be compatible with the Bradford Hill criteria for causality. All seven cohort studies were rated as high quality on the Newcastle–Ottawa Scale.
Maternal prenatal cotinine levels and risk of schizophrenia in offspring
Use of serum cotinine, a reliable metabolite of nicotine, as an indicator of smoking precludes the risk of self-report bias that is present many studies. A Finnish study tried to elucidate the relationship between maternal prenatal cotinine levels and the offspring's risk of developing schizophrenia (Niemelä Reference Niemelä, Sourander and Surcel2016). The study team used the national registry of schizophrenia diagnoses to identify cases of schizophrenia in the offspring of mothers delivered between 1983 and 1998. They found 997 cases and matched them to controls on date of birth, gender and place of residence. They then analysed serum cotinine levels in the mothers’ early- to mid-gestation plasma samples archived in a national biobank. The study revealed that offspring born to mothers who had smoked during pregnancy were at an increased risk of developing schizophrenia, with a high odds ratio (3.41) and a clear 95% confifidence interval (1.86–6.24). The study team carefully addressed the possibility of covariates affecting this result and found that the risk of developing schizophrenia is 38% higher in mothers whose serum cotinine levels were high.
According to this study, nicotine appears to be the main cause of a disruption of the neurodevelopmental process that increased the risk of future schizophrenia. However, there are many toxic compounds that can affect the neurodevelopmental process, including other psychoactive substances and chemical compounds originating from the diet. Failure to assess these confounding variables is one of the major limitations of the study.
Is the association of smoking and schizophrenia due to concurrent use of cannabis and other psychoactive substances?
As already mentioned, one limitation of many of these studies is the failure to objectively measure specific details of consumption of other psychoactive substances. Although there is an ever-growing body of evidence to suggest a causative role of tobacco smoking in schizophrenia, it is noted that cannabis has already been identified as a major risk factor (Gurillo Reference Gurillo, Jauhar and Murray2015). The effect of smoking on schizophrenia may operate through cannabis misuse since tobacco (particularly cigarette) smoking is identified as a gateway to cannabis use (Tullis Reference Tullis, Dupont and Frost-Pineda2003). Therefore, caution needs to be exercised before drawing conclusions here.
However, some studies have objectively measured consumption of psychoactive substances other than tobacco. The aforementioned Swedish study (Kendler Reference Kendler, Lönn and Sundquist2015), which was a prospective study involving 1.6 million individuals from Swedish registry data, reported that smoking was prospectively associated with non-affective psychosis risk, and the association remained significant even after adjusting for a range of confounders, including other psychoactive substance use. The Northern Finland birth cohort study (Stahl Reference Stahl2013), involving 6081 candidates, is another example where cannabis use was measured. It reported that heavy smoking is an independent risk factor for psychosis even after other confounding variables, including psychoactive substances, were considered (OR = 2.87; 95% CI 1.76–4.86). Furthermore, the risk of having psychotic episodes increased with the number of cigarettes smoked daily in a dose–response manner (OR = 1.05; 95% CI 1.01–1.08).
The biological explanation for the ‘smoking causes schizophrenia’ hypothesis
It is a well-accepted theory that psychosis is associated with a hyperdopaminergic state in the mesolimbic pathway. Nicotine binds to nicotinic receptors on dopaminergic neurons in the mesolimbic pathway, stimulating dopamine release in the nucleus accumbens (Stahl Reference Stahl2013). It has also been identified that nicotine receptor activation in dopaminergic neurons causes hypersensitivity of dopamine D2 receptors. Both mechanisms, in theory, support the higher occurrence of positive symptoms of schizophrenia in nicotine users (Prochaska Reference Prochaska, Hall and Bero2008; Stahl Reference Stahl2013).
Shared genetic vulnerability: a second alternative hypothesis
We have identified 14 research papers, including epidemiological and genetic studies, that looked at the degree to which shared genetic risk factors elucidate the association between smoking and schizophrenia.
An epidemiological study comparing the association between nicotine dependence and schizotypal personality traits in first-degree relatives of people with schizophrenia and a control group without a family history of the disorder showed a higher prevalence of smoking in first-degree relatives who had more schizotypal features (Esterberg Reference Esterberg, Jones and Compton2007). This indicates the possibility of a genetic link between smoking and schizophrenia.
However, a Swedish study contests the shared genetic vulnerability hypothesis (Kendler Reference Kendler, Lönn and Sundquist2015). Comparing pairs of monozygotic female twins, full sisters, half sisters and female cousins selected on the basis of discordance for smoking, the research team hypothesised that the strength of the association between smoking and non-affective psychosis should decline with pairs of increasing genetic relationship. In other words, if the association between smoking and non-affective psychosis is to be explained by shared genetic vulnerability, a significant decline across decreasing relatedness of categories of relatives would be observed. However, results showed only a modest decline in hazard ratios across relatives of increasing affinity. Co-relative analytical hazard ratio results did not show an appreciable difference between the general population and cousins, and only a moderate association was found in half and full sisters. Heavy tobacco smokers in the monozygotic twin pairs were around 1.7 times more likely to develop psychosis than their non-smoking twins, indicating that genetic factors do not entirely explain the relationship between smoking and later psychosis.
Another study that looked into the genetic variants linked to smoking initiation and schizophrenia using data from genome-wide association studies (GWAS) by the Tobacco and Genomics Consortium and the Psychiatric Genomics Consortium found a causal effect of smoking initiation on schizophrenia (OR = 1.73, 95% CI 1.30–2.25; P < 0.001). However, when the impact of pleiotropy of the genetic variant and the effect of variants from other genes were taken into consideration, the association become non-significant (OR = 1.01, 95% CI 0.98–1.04; P = 0.32) (Gage Reference Gage, Jones and Taylor2017).
A meta-analysis (Chen Reference Chen, Bacanu and Yu2016) identified several genes common to both schizophrenia and nicotine dependence. These shared genes are involved in calcium signalling, long-term potentiation and neuroactive ligand-receptor interaction pathways, which are important in cognitive functions and neuronal plasticity. Some genetic studies have shown that genetic polymorphism in the region of the α7 subunits of nicotine receptors increases the risk of both schizophrenia and nicotine dependence, and there is some evidence of hypofunction of α7 nicotinic receptors in both groups. Furthermore, GWAS identified CHRNA3, CHRNA4, CHRNA5 as common genes for both conditions (Gurillo Reference Gurillo, Jauhar and Murray2015; Anthenelli Reference Anthenelli, Benowitz and West2016). It has also been found that genes that code for dopamine receptors D2 and D3 and brain-derived neurotrophic factor (BDNF) are involved in the smoking behaviour of people with schizophrenia (Novak Reference Novak, LeBlanc and Zai2010; Kendler Reference Kendler, Lönn and Sundquist2015). Furthermore, recent genetic studies have identified other genes associated with increased tobacco use in those with schizophrenia. These include the functional polymorphism Val66Met in the BDNF gene (Zhang Reference Zhang, Chen and Xiu2012; Gurillo Reference Gurillo, Jauhar and Murray2015) and functional polymorphisms in the dopamine β-hydroxylase gene DBH (Zhang Reference Zhang, Chen and Xiu2012; Hirasawa-Fujita Reference Hirasawa-Fujita, Bly and Ellingrod2017), the μ-opioid receptor gene OPRM1 and the dopamine-2 receptor gene DRD2 (Hirasawa-Fujita Reference Hirasawa-Fujita, Bly and Ellingrod2017).
However, the shared genetic vulnerability hypothesis does not provide a way of identifying the direction of the association between smoking and schizophrenia (Chen Reference Chen, Bacanu and Yu2016).
Schizophrenia and nicotine dependence are multifactorial in aetiology (Fig. 2), and multiple susceptibility genes have been identified for each of these conditions. None of these genes is necessary and/or sufficient for the manifestation of the illness. Moreover, these studies have not been consistently replicated (Gorwood Reference Gorwood2014).
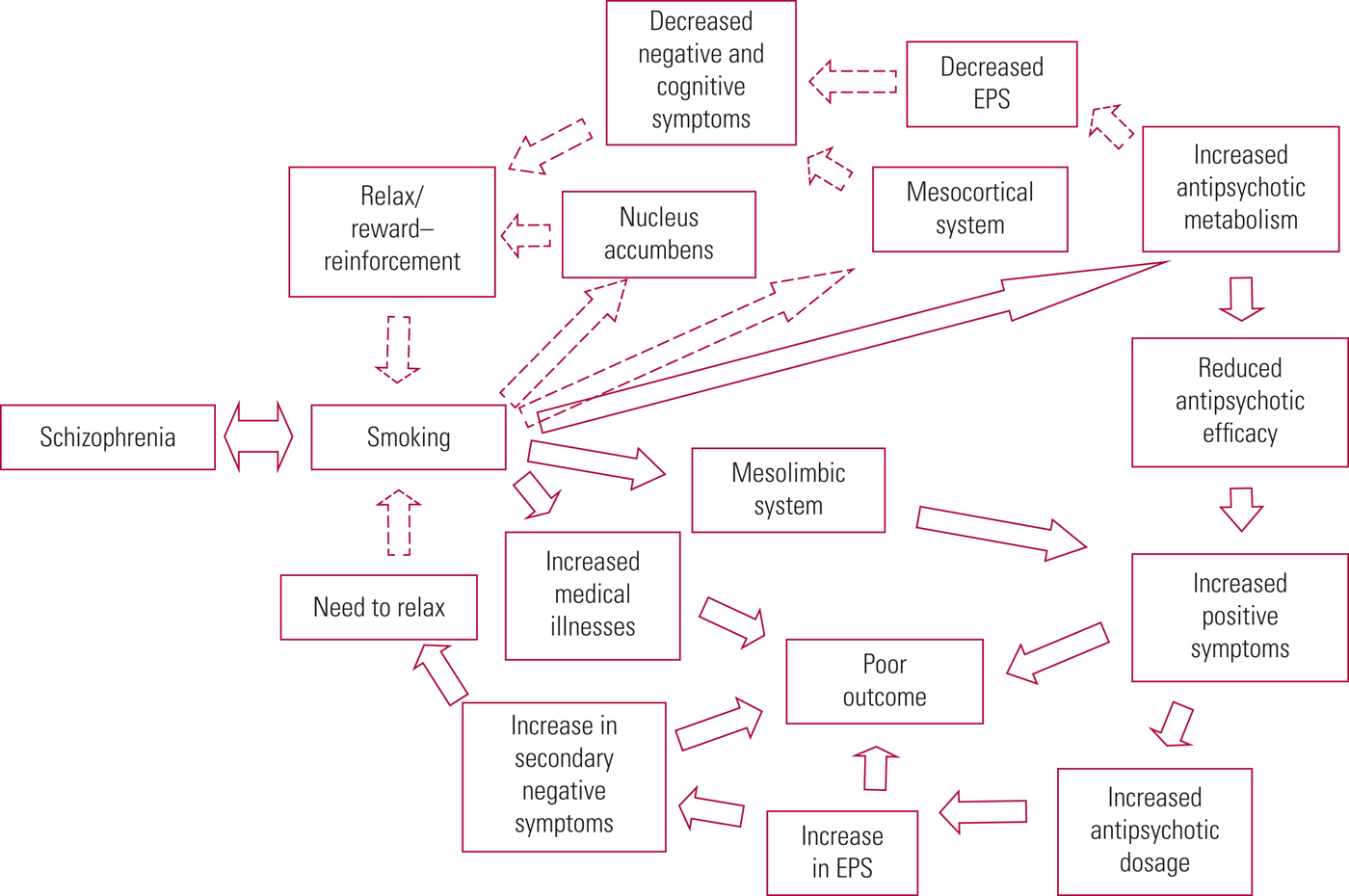
FIG 2 Possible reasons for nicotine dependence in people with schizophrenia. EPS, extrapyramidal symptoms; dashed arrows indicate factors supporting the self-medication hypothesis; double-sided arrows suggest a shared vulnerability; continuous arrows depict pathways that may contribute to poor outcomes. Source: Krishnadas et al (Reference Krishnadas, Jauhar and Telfer2012). Reproduced with permission.
The environment makes up a more substantial component of nicotine dependence risk, and we are far from identifying specific causative common genes that explain the association between schizophrenia and smoking.
The contribution of psychosocial factors to smoking in schizophrenia
Published research evidence identifying psychosocial risk factors contributing to the high prevalence of smoking among people with schizophrenia is limited. Tobacco use is essentially a substance use disorder caused by an array of aetiological factors, including psychosocial risk factors. People with schizophrenia have many of these psychosocial risk factors, such as poor education, low income, unemployment, perceived positive effects of smoking and lack of support for smoking cessation, putting them at a higher risk of becoming addicted to smoking (Ziedonis Reference Ziedonis, Hitsman and Beckham2008).
Most hospitals around the world have banned smoking on hospital premises in the recent past (McKee Reference McKee, Gilmore and Novotny2003; Lawn Reference Lawn and Pols2005). However, mental health services are known to have adopted ways of reinforcing smoking behaviour among psychiatric patients, such as handing out tobacco tickets to people with schizophrenia as a reward for enhanced activities of daily living in psychiatric in-patient facilities and rehabilitation units (Ziedonis Reference Ziedonis, Hitsman and Beckham2008). Another study illustrated the effectiveness of cigarettes used in a token economy to improve activities of daily living in patients on a psychogeriatric ward (Gustafson Reference Gustafson1992). Such approaches are likely to increase the prevalence of smoking among patients with schizophrenia.
Further psychological factors that may contribute to the high rate of smoking in schizophrenia can be identified in the scientific literature. For example, a qualitative study on individuals with schizophrenia undergoing rehabilitation reported that they smoked out of boredom (Lucksted Reference Lucksted, Dixon and Sembly2000). The prevalence of smoking among people with schizophrenia varies widely with gender, culture, religion and economic status (de Leon Reference de Leon and Diaz2005; Manzella Reference Manzella, Maloney and Taylor2015). For example, it is lower in China and some South Asian countries than in European countries (de Leon Reference de Leon and Diaz2005; Edrisinghe Reference Edrisinghe, Wijesinghe and Williams2014). Researchers believe that restrictions imposed by cultural, familial and religious practices and norms and lack of financial independence may play a role in the varied prevalence of smoking among people with schizophrenia (Edrisinghe Reference Edrisinghe, Wijesinghe and Williams2014). Hence, it can be argued that psychosocial factors do play a role in causing a higher prevalence of smoking in schizophrenia.
Conclusions
Well-conducted prospective studies indicate that smoking is a risk factor for the development of schizophrenia. A more rigorous examination of the role of smoking as a causal factor (Box 3) is required. Prospective well-designed studies with adequate samples, the measurement of appropriate biological markers and adjustment for the effect of concurrent use of other psychoactive substances will tell us more about the direction of the association between smoking and schizophrenia. The findings will have deeper implications for our understanding of the aetiology of schizophrenia.
BOX 3 Current hypotheses to explain the high prevalence of smoking in patients with schizophrenia
• The self-medication hypothesis proposes that people with schizophrenia experience an improvement in symptoms of psychosis and in the side-effects of antipsychotic medication following smoking; consequently, they tend to smoke more heavily
• The ‘smoking causes schizophrenia’ hypothesis proposes that smoking is a risk factor for developing psychosis
• The shared genetic vulnerability hypothesis suggests that common genes increase the risk of developing both schizophrenia and nicotine dependence
It is evident that the self-medication hypothesis, the ‘smoking causes schizophrenia’ hypothesis and the shared genetic vulnerability hypothesis are not mutually exclusive, and that all three may contribute to the observed higher prevalence of smoking in people with schizophrenia.
Further exploration of the ‘smoking causes schizophrenia’ hypothesis will not only provide a better platform for reducing mortality in schizophrenia, but may also create scientific knowledge that will potentially help to reduce the morbidity of the disorder or even prevent its development.
A steadily growing body of evidence shows the presence of more severe symptoms of schizophrenia in tobacco smokers. These observations call into question the self-medication hypothesis. This knowledge can be used to point out the relationship between nicotine dependence and symptom severity, with the aim of promoting smoking cessation among people with schizophrenia.
Acknowledgements
The authors are grateful to Tanya McLaven, Deputy Librarian, University Hospital Leicester, UK for the comprehensive literature review, and Dr Neshantha Harishchandra, PhD, Senior Lecturer, Department of English and Linguistics, the University of Ruhuna for proofreading the manuscript of this article.
MCQs
Select the single best option for each question stem
1 Smoking among people with schizophrenia:
a accounts for consumption of 50% of the cigarettes produced in the USA
b has a higher prevalence than among people with other mental illnesses and the general population
c does not explain the high mortality rate in that population
d is a protective factor against the initiation of cannabis use
e has declined in parallel with the decline of smoking prevalence in the general population.
2 The main hypotheses suggested to explain the high prevalence of smoking among patients with schizophrenia are:
a the self-medication hypothesis and the shared genetic vulnerability hypothesis
b the shared genetic vulnerability hypothesis and the ‘schizophrenia causes smoking’ hypothesis
c the self-medication hypothesis, the ‘smoking causes schizophrenia’ hypothesis and the shared genetic vulnerability hypothesis
d the ‘biopsychosocial factors cause schizophrenia and smoking’ hypothesis
e the ‘nicotine improves attention’ hypothesis, the ‘smoking causes schizophrenia’ hypothesis and the shared genetic vulnerability hypothesis.
3 The self-medication hypothesis:
a was first described by Khantzian to explain cocaine use by people with mental illnesses
b has already been discarded by clinicians and policy makers
c proposes that smoking reduces mortality rates in people with schizophrenia
d proposes that people with schizophrenia tend to substitute antipsychotics with nicotine
e is weakened by the finding that nicotine improves attention levels in people with schizophrenia to the same degree as in healthy controls.
4 The ‘smoking causes schizophrenia’ hypothesis is weakened by:
a the dose–response relationship between smoking and risk of having schizophrenia
b worse clinical outcomes for people with schizophrenia who smoke
c earlier onset of schizophrenia among people who smoke every day
d higher prevalence of smoking in people with the first episode of psychosis
e paucity of controlling for cannabis use in studies exploring the smoking–schizophrenia association.
5 The shared genetic vulnerability hypothesis:
a helps to identify the direction of the association between smoking and schizophrenia
b is strengthened by consistent replication of genetic studies
c implicates CHRNA3, CHRNA4 and CHRNA5 as common genes in the development of nicotine dependence and schizophrenia
d implicates the BDNF, DBH, OPRM1 and DRD2 genes in developing resistance against tobacco use in people with schizophrenia
e opposes the ‘smoking causes schizophrenia’ hypothesis.
MCQ answers
1 b 2 c 3 a 4 e 5 c





eLetters
No eLetters have been published for this article.