LEARNING OBJECTIVES
After reading this article you will be able to:
• understand the scope of the potential psychiatric manifestations of Parkinson's disease, including those that may be precipitated by treatments
• recognise assessment scales that are available to aid diagnoses of neuropsychiatric conditions
• identify some of the current treatments for the mental health problems that are encountered in people with Parkinson's and the areas in which a strong evidence base is lacking.
Idiopathic Parkinson's disease is the second most common neurodegenerative disorder after Alzheimer's disease. Motorically it is characterised by tremor, rigidity, bradykinesia and postural instability. Although it was historically considered to be a movement disorder, the motor symptoms are now increasingly recognised as representing the ‘tip of the iceberg’. The significant underlying burden of non-motor symptoms spans neuropsychiatric, sleep, somatosensory and autonomic domains. These can often precede the motor symptoms by years or even decades (Klingelhoefer Reference Klingelhoefer and Reichmann2015) and have a negative effect on quality of life.
Parkinson's has been described as the ‘quintessential neuropsychiatric disorder’ (Weintraub Reference Weintraub and Burn2011), which reflects both the scope and commonality of neuropsychiatric symptoms (illustrated in Fig. 1) that can be encountered. Although these symptoms are common and frequently debilitating, they are underrecognised and invariably have a profoundly negative impact on patients’ social functioning and ability to work (Perepezko Reference Perepezko, Hinkle and Shepard2019). Neuropsychiatric symptoms are associated with overall higher carer burden and, potentially, higher care costs while being frequently undeclared by patients (Chaudhuri Reference Chaudhuri, Prieto-Jurcynska and Naidu2010; Politis Reference Politis, Wu and Molloy2010).
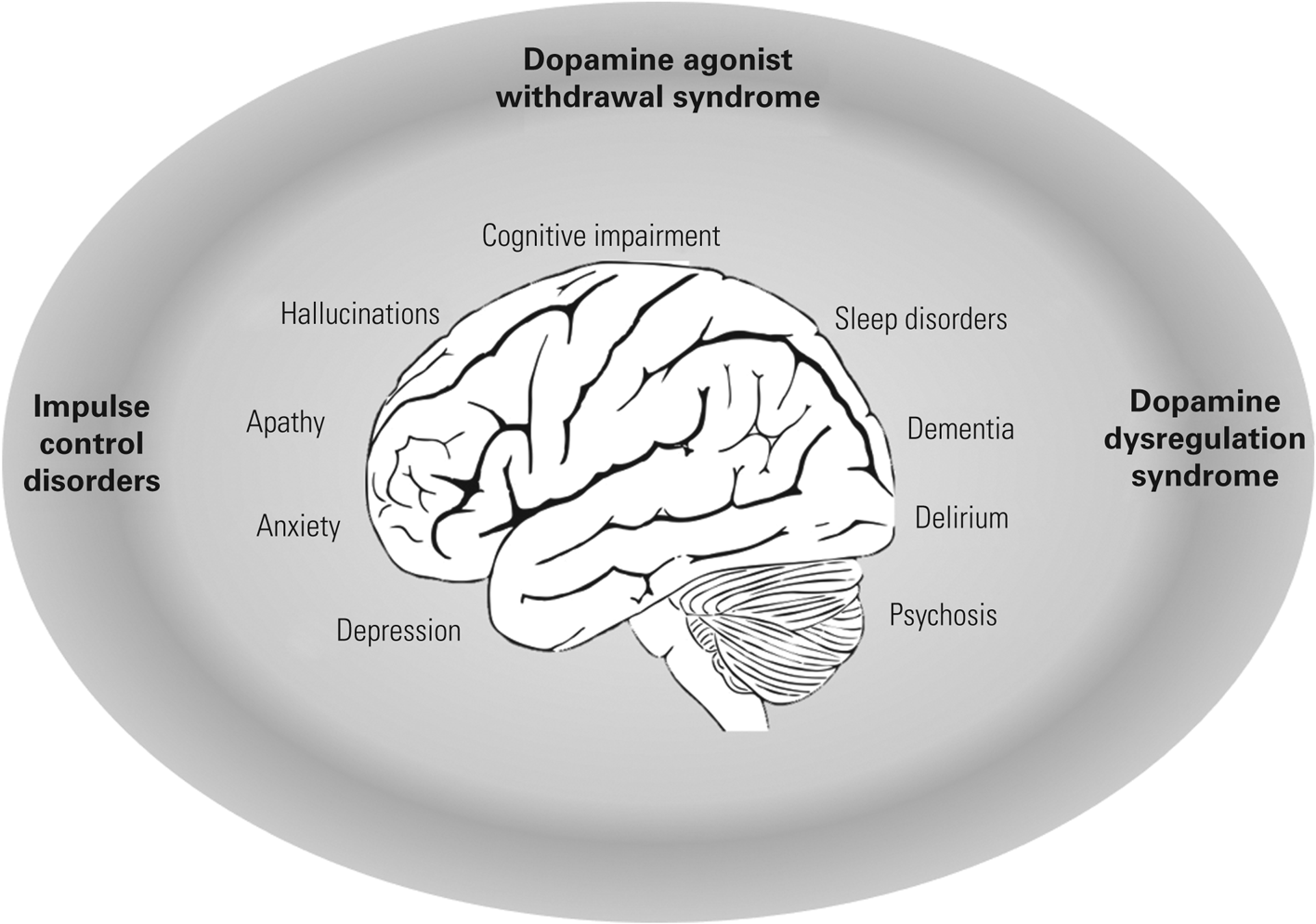
FIG 1 The spectrum of neuropsychiatric disorders in Parkinson's disease. Treatment-related syndromes are indicated in bold text.
In this article we describe the spectrum of neuropsychiatric symptoms encountered in Parkinson's, necessarily limiting the scope to cover manifestations in idiopathic Parkinson's disease rather than the full spectrum of Parkinsonian syndromes. We describe where the underlying aetiology is understood to be alpha-synuclein pathology and disruption of neurochemical pathways and where adverse effects of treatments precipitate manifestations such as impulse control disorders and psychosis. Where assessment scales can be usefully utilised to aid diagnosis and assessment of symptom severity these are included, along with the relative strengths and limitations. Although a strong evidence base for interventions in some domains is lacking, it is encouraging that literature in the field is growing year on year (Fig. 2). We have sought to provide a pragmatic approach to managing neuropsychiatric problems to help clinicians tackle the unmet need for better recognition and treatment of these often underrecognised and frequently inadequately managed symptoms.

FIG 2 Number of articles published with neuropsychiatric domain in the article title between 1970 and 2019.
The PubMed search terms used were: (Parkinson*[Title]) AND (dementia[Title] OR cognitive impairment[Title]); parkinson*“[Title] AND ”depression“[Title]; parkinson*”[Title] AND (“psychosis”[Title] OR “hallucination”[Title]); (Parkinson*[Title]) AND (impulse control disorder[Title] OR dopamine dysregulation syndrome[Title]); parkinson*“[Title] AND impulse control disorder”[Title] OR “dopamine dysregulation syndrome”[Title]; (Parkinson*[Title]) AND (impulse control disorder[Title] OR dopamine dysregulation syndrome[Title]); (Parkinson*[Title]) AND (anxiety[Title]); (Parkinson*[Title]) AND (apathy[Title]); (Parkinson*[Title]) AND (insomnia[Title] OR sleepiness[Title] OR fatigue[Title] OR REM[Title]) filtered from 1970–2019.
Neuropsychiatric symptoms of the disease
Depression
In his original essay on ‘the shaking palsy’, James Parkinson noted that ‘A more melancholy object I never beheld’ (Parkinson Reference Parkinson1817). Nowadays, depression is still recognised as a common non-motor feature of Parkinson's disease, and the prevalence of clinically significant symptoms has been estimated at 35% (Reijnders Reference Reijnders, Ehrt and Weber2008). A multicentre study demonstrated that half the study participants were depressed according to the Beck Depression Inventory whereas only 1% had self-reported symptoms (Findley Reference Findley, Eichhorn and Janca2002; Huse Reference Huse, Schulman and Orsini2005). Particular diagnostic challenges arise from individuals’ underreporting of their depressive symptoms (Dissanayaka Reference Dissanayaka, Sellbach and Silburn2011), hypomimia (lack of facial expression), concurrent mood disturbances such as anxiety or apathy (Pagonabarraga Reference Pagonabarraga, Kulisevsky and Strafella2015) and psychomotor slowing, and symptoms are under-recognised by clinicians (Shulman Reference Shulman, Taback and Rabinstein2002).
Screening/rating scales
There are a number of validated screening tools available for identifying depression in people with Parkinson's disease, including the Hamilton Rating Scale for Depression (HRSD), Beck Depression Inventory (BDI), Hospital Anxiety and Depression Scale (HADS), Montgomery–Åsberg Depression Rating Scale (MADRS) and the Geriatric Depression Scale (GDS), all of which are recommended by the International Parkinson and Movement Disorder Society. A working group on depression in Parkinson's recommended that an inclusive approach be taken to symptoms (i.e. regardless of the aetiology) and that the motoric ‘on’ or ‘off’ state should be considered when making a diagnosis (Marsh Reference Marsh, McDonald and Cummings2006).
Aetiology
The underlying pathophysiology of depression in Parkinson's disease is poorly understood and likely to be multifactorial. A study of almost 1500 out-patients with the disease concluded that higher rates of depression were seen in individuals with greater severity of motor symptoms (Riedel Reference Riedel, Klotsche and Spottke2010). Although there may be a reactive element to living with a neurodegenerative condition, there is substantial evidence of a neurotransmitter deficit. This biochemical basis is likely mediated through alteration in serotonin, noradrenaline and acetylcholine neurotransmitters. These changes may have a cumulative effect, with the hypodopaminergic state with depressive symptoms being more prominent in the motoric ‘off’ state (van der Velden Reference van der Velden, Broen and Kuijf2018). Pharmacological trials have demonstrated an antidepressant effect of the dopamine agonists pramipexole and ropinirole (Barone Reference Barone, Poewe and Albrecht2010).
Treatment
The choice of pharmacological therapy is often driven by determination of symptom severity and effect on the individual's quality of life. The options in treating depression in Parkinson's disease are similar to those for depression in any chronic disease state and include selective serotonin reuptake inhibitors (SSRIs), serotonin–noradrenaline reuptake inhibitors (SNRIs), monoamine oxidase type B (MAO-B) inhibitors and tricyclic antidepressants (TCAs). However, there is a paucity of robust randomised controlled trial evidence. SSRIs are most commonly prescribed because of their more favourable side-effect profile and lower potential for drug–drug and drug–disease interactions. The use of MAO-B inhibitors for the motor treatment of Parkinson's disease increases the risk of developing serotonin syndrome through the addition of an antidepressant, although this is rare in clinical practice (Richard Reference Richard, Kurlan and Tanner1997). Although the use of TCAs has a potential advantage in promoting sleep in individuals who suffer insomnia, the anticholinergic effects of this drug class and daytime somnolence should be considered. Treatment of depression in Parkinson's disease should ideally be delivered within the framework of a multidisciplinary team, who can initiate and monitor pharmacological therapy, providing specific support and expertise.
Apathy
Apathy is one of the most common non-motor features of Parkinson's disease (den Brok Reference den Brok, van Dalen and van Gool2015). Increasing efforts have been made within the past decade to distinguish apathy as an isolated mood disorder from apathy as a ‘by-product’ of depression, although the two frequently coexist (den Brok Reference den Brok, van Dalen and van Gool2015). Apathy may be defined as a ‘state of reduced motivation that manifests as a reduction in goal-directed behaviour’ (Velakoulis Reference Velakoulis, Walterfang, Schapira and Byrne2007). It may result in low levels of activity, reduced interests and a loss of socialisation. The overlap in symptoms between apathy and depression can make diagnosing isolated apathy challenging. There are, however, certain characteristic thoughts and symptoms that are specific to apathy and the presence or absence of these help to confirm the diagnosis. They include emotional indifference and reactivity, reduced activity and interest in the world, with a lack of concern for others (Pagonabarraga Reference Pagonabarraga, Kulisevsky and Strafella2015). Depending on the diagnostic criteria used and the concurrent symptoms of depression and cognitive impairment, the prevalence of apathy ranges from 17–70% in Parkinson's disease (Aarsland Reference Aarsland, Marsh and Schrag2009) and, as with depression, the symptoms may precede the motor symptoms of Parkinson's. The pathophysiology is poorly understood, but may be associated with a neuronal disruption in areas that regulate goal-directed behaviour: mainly dopaminergic projections between the frontal cortex and the ventral tegmentum (Marin Reference Marin1991; Carlson Reference Carlson, Foti and Mujica-Parodi2011).
Rating scales
The development of diagnostic criteria for apathy has advanced significantly with the creation of a task force, commissioned in 2008 by the International Parkinson's and Movement Disorder Society, to assess the clinimetrics of the available rating scales (Shulman Reference Shulman, Armstrong and Ellis2016). Subsequent review of the clinimetric properties of 13 scales suggested that the five item WHO Well-being Index (WHO-5) and the Neurasthenia Scale detect apathy severity, and the 33-item Lille Apathy Rating Scale (LARS) is valid in the diagnosis of its 33 items, while the Starkstein Apathy Scale (SAS) has utility in the exclusion of apathy (Carrozzino Reference Carrozzino2019).
Treatment
There are several therapeutic approaches to treating apathy, including dopaminergic medications (Marsh Reference Marsh, McDonald and Cummings2006; Chaudhuri Reference Chaudhuri, Martinez-Martin and Antonini2013). A double-blind randomised controlled trial comparing the cholinesterase inhibitor rivastigmine with placebo showed a significant improvement with rivastigmine in the symptoms of apathy at 6 months (Devos Reference Devos, Moreau and Maltête2014).
Anxiety
It is estimated that one-third of people with Parkinson's disease experience symptoms of anxiety, which is considered to be a group of disorders consisting of generalised anxiety disorder, obsessive–compulsive disorder, agoraphobia, social phobia and panic disorder. It can be difficult to distinguish symptoms of anxiety from those of depression or even somatic Parkinson's disease (e.g. sleep disturbances, apathy), which can be present simultaneously; so, the identification of one should lower the threshold of clinical suspicion for the other.
Classification of these conditions in studies varies, therefore the reported prevalence rates vary, ranging from 3.6 to 55% (Dissanayaka Reference Dissanayaka, Sellbach and Matheson2010; Broen Reference Broen, Narayen and Kuijf2016). A systematic review reported the average prevalence of all anxiety disorders in Parkinson's disease to be 31%, with generalised anxiety disorder, panic disorder and social phobia being the most common (Broen Reference Broen, Narayen and Kuijf2016). This wide difference in prevalence may be accounted for by the underreporting of symptoms by patients and underrecognition by clinicians.
Diagnostic criteria and scales
Researchers will often rely on established criteria, for example from the DSM-IV, to make a diagnosis; however, diagnosis in a clinical setting can remain challenging. A study of 42 patients with Parkinson's disease in a university-based movement disorders clinic found that 29% had DSM-III-R anxiety disorder diagnoses, but an additional 40% had anxiety symptoms that did not meet the diagnostic threshold (Menza Reference Menza, Robertson-Hoffman and Bonapace1993). Confirmation of the diagnosis can be challenging because of the overlap between somatic features such as sleep disturbance. Self-reported anxiety scales have been tested in Parkinson's disease, but their clinical utility is uncertain owing to the lack of consensus regarding appropriate cut-off scores (Leentjens Reference Leentjens, Dujardin and Marsh2008). A detailed patient history and a collateral history are essential but often omitted because of time constraints in clinic. Furthermore, identification can be complicated by the presence of an underlying cognitive impairment. Specific symptoms of anxiety in Parkinson's disease include panic attacks during motoric ‘off’ periods, excessive worry and increased subjective motor symptoms. The timing of anxiety symptoms can mirror motor fluctuations or manifest in an unrelated pattern (Aarsland Reference Aarsland, Marsh and Schrag2009).
Aetiology
As with depression, the aetiology of anxiety in Parkinson's disease is multifactorial. There may well be underlying neurobiological changes that cause anxiety; but it is also likely that there are psychosocial causes in reaction to the burden of having Parkinson's disease. Social anxiety is especially common in the context of the reactive model, and people with Parkinson's express concern over being negatively perceived in public, which leads to social withdrawal. They may also fear the progression of the disease, disability, institutionalisation and issues surrounding their death. With the high risk of falls in the disease, an associated fear of falling can be very prominent (Prediger Reference Prediger, Matheus and Schwarzbold2012).
The role of dopaminergic neurotransmission in the context of anxiety is poorly understood but has been linked to both social phobia and anxiety symptoms in animal models (Prediger Reference Prediger, Matheus and Schwarzbold2012). Serotonergic and noradrenergic systems also have a role in Parkinson's disease anxiety owing to their widespread distribution in the structures involved in emotion modulation. Research demonstrates that people with Parkinson's disease who score higher on anxiety questionnaires have a shorter serotonin transporter allele, highlighting the potential role of neuropsychiatric genetics in this group (Menza Reference Menza, Sage and Marshall1990).
Treatment
Anxiety in Parkinson's disease has a huge impact on quality of life, not only for the patient but also for their family and carers. Social anxiety can lead to isolation in the community, which further exacerbates anxiety, creating a self-perpetuating cycle. It can also precipitate loneliness, particularly in older people, which can contribute to the subsequent development of depression. This can in turn affect treatment plans, hindering motivation and engagement in rehabilitation and healthcare services (Prediger Reference Prediger, Matheus and Schwarzbold2012).
There is a paucity of evidence on the management of anxiety in Parkinson's disease. We have found no robust clinical trials of either pharmacological or psychological strategies. Anxiety has been assessed as a secondary outcome in depression clinical trials but because of the selection criteria used, any therapeutic effects on anxiety were diluted (Koychev Reference Koychev and Okai2017).
Guidance for managing anxiety in older adults with chronic physical health conditions (Koychev Reference Koychev and Okai2017) can be extrapolated, which places initial emphasis on lifestyle modification, focusing on optimal sleep, exercise, nutrition and socialising, in addition to eliminating any exacerbating medical causes of anxiety, such as metabolic anomalies, nutritional deficiencies and drug reactions. Pharmacological approaches are then advised, with the introduction of SSRIs and SNRIs. The use of benzodiazepines is discouraged because of their side-effect profile. Behavioural therapies in the form of cognitive–behavioural therapy, mindfulness and desensitisation are also of benefit but are reliant on adequate motivation and adherence (Koychev Reference Koychev and Okai2017).
Psychosis
Psychosis in Parkinson's disease is a spectrum of neuropsychiatric manifestations consisting of ‘positive’ symptoms, namely illusions, hallucinations and delusions (Ravina Reference Ravina, Marder and Fernandez2007). Definitions of psychotic symptoms in the context of Parkinson's disease are consistent with definitions in the wider psychiatric literature. The prevalence of psychosis varies depending on the diagnostic tool used. In 2007, the combined National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Mental Health (NIMH) work group proposed unifying diagnostic criterion for Parkinson's disease psychosis which includes the presence of at least one psychotic symptom (Ravina Reference Ravina, Marder and Fernandez2007). Longitudinally, prevalence increases with disease duration, (Gibson Reference Gibson, Mottram and Burn2013) and at 12 years 60% of patients from a community cohort reported hallucinations or delusions (Forsaa Reference Forsaa, Larsen and Wentzel-Larsen2010).
Aetiology: risk factors
Proposed risk factors for the development of psychosis in Parkinson's disease include the duration and severity of illness, and cognitive impairment. The development of these symptoms at a time when demands associated with caring for someone with Parkinson's disease are already high results in a greater sense of burden for the carer in comparison with caring for someone with psychosis-free Parkinson's, and is also a predictive factor for nursing home placement (Marsh Reference Marsh, Williams and Rocco2004). Additional risk factors for psychosis include treatment with dopaminergic and anticholinergic medications. The relationship between the use of Parkinson's disease medication and development of Parkinson's disease psychosis remains controversial. Anecdotally, an association between Parkinson's disease psychosis and the use of dopaminergic therapies and duration of treatment has been observed. Psychotic symptoms can develop on initiation of medication, with a subsequent improvement on reduction or withdrawal, and this is most potently seen with the use of dopamine agonists. Nonetheless, a causal effect of dopaminergic medications has not been established and psychotic symptoms have been described in recently diagnosed patients who have not yet started treatment (Aarsland Reference Aarsland, Marsh and Schrag2009).
Treatment
The initial management of Parkinson's disease psychosis involves the identification and treatment of any precipitating factors. Psychosis may be the manifestation of an acute illness or a change in medication, and a thorough clinical evaluation is imperative. If the patient is tolerating the symptoms of psychosis with no adverse features, the best approach may be to watch and wait. Many people with Parkinson's disease do not find hallucinations distressing and retain insight.
Where intervention is warranted, there are a number of approaches. In patients with significant cognitive impairment, rivastigmine has good efficacy (Burn Reference Burn, Emre and McKeith2006). Clozapine and pimavanserin (a 5-HT2A inverse agonist, not yet licensed for use in the UK) are efficacious (Cummings Reference Cummings, Isaacson and Mills2014). There are barriers for the routine use of clozapine in the clinical setting, namely the regular blood tests required to monitor for agranulocytosis and the registration required for prescribing the drug. Other atypical antipsychotics used in clinical practice include quetiapine, olanzapine and risperidone, but these can cause worsening of motor symptoms and other side-effects, including QT prolongation, sedative effects, metabolic syndrome and a potential deterioration in cognition. The most recent National Institute for Health and Care Excellence (NICE) guidance advocates low-dose quetiapine and clozapine as the most appropriate medications in Parkinson's disease psychosis (National Institute for Health and Care Excellence 2017) and in clinical practice at present, quetiapine is commonly used.
Cognitive impairment and dementia
Cognitive impairment is a common non-motor manifestation in Parkinson's disease and is heterogeneous in terms of its severity, rate of progression and the cognitive domains affected. It has a significant negative impact on patients and their carers and is associated with increased disability, mortality (Levy Reference Levy, Tang and Louis2002), carer burden (Aarsland Reference Aarsland, Larsen and Karlsen1999) and need for nursing home placement (Aarsland Reference Aarsland, Larsen and Tandberg2000). Although Lewy body dementia is beyond the scope of this article, it is important to mention that both Parkinson's disease dementia and Lewy body dementia share many clinical and neurochemical features despite being two distinct entities. Their diagnosis is based on an arbitrary distinction between the time of onset of dementia in relation to motor symptom onset. In Parkinson's dementia, cognitive impairment presents 1 or more years after the presentation of Parkinsonism, whereas in Lewy body dementia, cognitive impairment often precedes Parkinsonism (Aarsland Reference Aarsland2016). In idiopathic Parkinson's disease, cognitive impairment ranges from subjective cognitive decline, to mild cognitive impairment (MCI), to dementia, but does not necessarily progress linearly.
Aetiology
Longitudinal studies suggest that MCI in Parkinson's disease increases the risk of developing Parkinson's dementia. One study showed that (controlling for age, disease stage, education and gender) MCI was strongly associated with development of Parkinson's dementia (OR = 5.1; 95% CI 1.51–16.24, P = 0.005) over a 4-year follow-up (Janvin Reference Janvin, Larsen and Aarsland2006). However, this study included relatively small numbers. More recently, evidence suggests that MCI may not always progress to Parkinson's dementia, with some patients remaining stable on longitudinal assessments or even reverting to normal cognition (Lawson Reference Lawson, Yarnall and Duncan2017). The prevalence of MCI in Parkinson's disease ranges from 15 to 53% (Yarnall Reference Yarnall, Rochester and Burn2013) but this large variation is likely caused by differences in study settings (hospital versus community patients), variable clinical characteristics and inconsistent definitions of MCI. The Movement Disorder Society published a unifying set of diagnostic measures in 2012 to standardise practice across clinical trials (Litvan Reference Litvan, Aarsland and Adler2011). From a clinical perspective, MCI and Parkinson's dementia can be best differentiated by determining whether the cognitive impairment significantly affects activities of daily living.
Several longitudinal studies have demonstrated that approximately 50% of patients develop Parkinson's dementia 10 years after initial Parkinson's disease diagnosis (Auyeung Reference Auyeung, Tsoi and Mok2012; Williams-Gray Reference Williams-Gray, Mason and Evans2013). The Sydney Multicenter Study, the largest follow-up study of newly diagnosed patients, showed that 83% of individuals with Parkinson's disease had developed Parkinson's dementia at 20 years post-diagnosis and 75% developed Parkinson's dementia before death (Hely Reference Hely, Reid and Adena2008). Additionally, not only does the progression of cognitive impairment in Parkinson's disease vary, so too does the pattern of cognitive deficits. Cognitive deficits may affect executive function, attention, processing speed and/or visuospatial function (Williams-Gray Reference Williams-Gray, Foltynie and Brayne2007). The Cambridgeshire Parkinson's Incidence from GP to Neurologist (CamPaIGN) cohort study assessed cognition at 3.5 and 5.5 years post-diagnosis and found a decline in mini-Mental State Examination (MMSE) score at a rate of 0.3 (s.d. = 0.1) points per year over 5.2 years (Williams-Gray Reference Williams-Gray, Evans and Goris2009). In addition, it showed that deficits in semantic fluency and visuospatial function at baseline were associated with a greater risk for developing Parkinson's dementia, whereas deficits in executive function were not (Williams-Gray Reference Williams-Gray, Foltynie and Brayne2007, Reference Williams-Gray, Evans and Goris2009). This finding, in addition to other studies, led to the ‘dual syndrome hypothesis’, which suggests that patients with primarily executive dysfunction, driven by changes in dopaminergic pathways, are less likely to develop Parkinson's dementia, whereas those with memory and visuospatial functional deficits, caused predominantly by deficits in acetylcholine, are more prone to rapid cognitive decline and progression to Parkinson's dementia.
The heterogeneity of both the presentation and progression of cognitive impairment in Parkinson's disease is not fully understood and is clearly a complex interplay between genetic and environmental factors. Risk factors associated with an increased risk for Parkinson's dementia include the patient's age, older age at onset of Parkinson's disease and the presence of hallucinations. In addition, the Parkinson's phenotype with more predominant gait dysfunction is associated with a more rapid cognitive decline than the tremor-dominant phenotype (Williams-Gray Reference Williams-Gray, Evans and Goris2009). Genetic factors also play a role, with mutations in the alpha-synuclein (SNCA) gene (Waxman Reference Waxman and Giasson2009) and mutation in the glucocerebrosidase (GBA1) gene (Cilia Reference Cilia, Tunesi and Marotta2016) being associated with a more rapid cognitive decline, in addition to an earlier onset of Parkinson's dementia. One retrospective longitudinal study found that that 56% of GBA1 mutation carriers had dementia at age 70, compared with 15% of non-carriers (Cilia Reference Cilia, Tunesi and Marotta2016). Interestingly, individuals with a Parkin mutation, which accounts for 50% of autosomal recessive Parkinson's disease, are less likely to develop Parkinson's dementia, with one review finding Parkinson's dementia in fewer than 3% of cases (Grünewald Reference Grünewald, Kasten and Ziegler2013).
Treatment
The only drug currently licensed for Parkinson's dementia is rivastigmine, an acetylcholinesterase inhibitor, with memantine, an N-methyl-d-Aspartate (NMDA) receptor antagonist used as second line if the patient is intolerant to rivastigmine (National Institute for Health and Care Excellence 2017). However, a meta-analysis including three randomised controlled trials comparing memantine 20 mg daily with placebo concluded that the drug showed only a mildly beneficial effect on the Clinicians’ Global Impression of Change (CGIC) assessment, with no significant change on cognitive function assessed using the MMSE (Wang Reference Wang, Yu and Tang2015). There is currently no successful treatment for MCI and, given the limited pharmacological options, it is important to consider non-pharmacological options such as physical exercise programmes and cognitive training/stimulation.
A systematic review of eight studies including 158 patients suggested that physical exercise had beneficial effects on global cognition (Murray Reference Murray, Sacheli and Eng2014). Physical activity intervention studies have historically recruited small numbers of participants and utilised heterogeneous interventions with varying degrees of exercise intensity, mode and duration.
Cognitive training is a structured teaching programme designed to target specific cognitive domains. A meta-analysis involving seven studies (272 patients) showed a small but statistically significant improvement compared with controls (Leung Reference Leung, Walton and Hallock2015).
Overall, given the heterogeneity of cognitive impairment in Parkinson's disease and the impact it has on the individual's quality of life and on carer burden, the primary aim of management should be to take an individualised approach addressing patient and carer concerns and expectations.
Delirium
Given the liability to develop dementia and the high degree of cognitive vulnerability it is proposed that people with Parkinson's disease are at increased risk of developing delirium in acute illness (Vardy Reference Vardy, Teodorczuk and Yarnall2015). Features of delirium such as cognitive fluctuation, somnolence and hallucinations can overlap with those seen particularly in Parkinson's dementia, which can confound the diagnosis. There is a lack of evidence as to the long-term motor and cognitive outcomes for people with Parkinson's disease who have experienced delirium, although there is a suggestion that both domains can worsen, albeit the studies were relatively small (Serrano-Dueñas Reference Serrano-Dueñas and Bleda2005; Umemura Reference Umemura, Oeda and Tomita2014). Although antipsychotics can worsen motor symptoms, clinically quetiapine is most commonly used where pharmacological strategies are required in addition to treatment for underlying precipitants and using conservative strategies such as one-to-one nursing and environmental optimisation.
Treatment-related disorders
Impulse control disorders
Impulse control disorders are addictive behaviours manifesting as binge eating, pathological gambling, compulsive shopping or abnormal sexual behaviours (Weintraub Reference Weintraub, Koester and Potenza2010). These can occur in isolation or together. In Parkinson's disease they are associated with dopamine agonist therapy, and all patients initiated on this treatment should be counselled as to the risk and proactive enquiry at subsequent appointments should be used to ascertain whether these symptoms are arising. Left unrecognised and untreated, impulse control disorders can have very significant ramifications for personal relationships and finances. Individuals at higher risk of developing impulse control disorders in Parkinson's disease are men, those with certain personality traits or psychiatric disturbance (such as impulsivity, novelty-seeking, anxiety and depression), younger patients, smokers and those taking higher doses of dopamine agonists for a longer duration. Management consists of down-titration of dopamine agonist therapy, supported by cognitive–behavioural therapy and careful management of worsening motor symptoms (Okai Reference Okai, Askey-Jones and Samuel2013).
Dopamine agonist withdrawal syndrome
Dopamine agonist withdrawal syndrome results from the rapid down-titration or withdrawal of a dopamine agonist drug. Clinically it is recognised by a cluster of symptoms, including autonomic instability (fatigue, orthostatic hypotension, nausea, vomiting, diaphoresis), psychological symptoms (anxiety, panic attacks, dysphoria, depression, fatigue, agitation, irritability, suicidal ideation) and generalised pain, as well as drug cravings (Nirenberg Reference Nirenberg2013). The cessation or rapid reduction of dopamine agonist drugs is a major factor in the development of the syndrome and patients should be monitored closely in case this occurs.
Dopamine dysregulation syndrome
Dopamine dysregulation syndrome refers to the compulsive (mis)use of dopaminergic therapy. The combination of dopamine replacement therapy, coupled with predisposing individual risk factors and Parkinson's disease pathology, yields an addictive syndrome (Béreau Reference Béreau, Fleury and Bouthour2018). This manifests as mood fluctuations and addictive behaviour. Management consists of careful down-titration of dopamine agonist therapy and fractionation of levodopa therapy. Intrajejunal levodopa infusion therapy or deep brain stimulation can be considered in refractory cases.
Conclusions
Neuropsychiatric manifestations of Parkinson's disease are common and have a devastating effect on patients’ quality of life and cause significant carer strain. Assessment and active management of the neuropsychiatric symptoms encountered in people with Parkinson's disease is essential to mitigate these very distressing sequelae and the resultant negative impact that these conditions can have on functioning. The overlap of symptoms can make the diagnosis of a mental disorder in the context of Parkinson's disease challenging, but proactive enquiry is the first step in better defining and treating these syndromes. In this brief review we have sought to give an overview of the spectrum of conditions, current understanding of pathophysiology, criteria for diagnosis and management strategies.
Funding
No specific funding supported this work. K.T. is supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Campus. E.J.H. has held grants from Parkinson's UK, the NIHR, the British Geriatrics Society and The Gatsby Foundation.
Author contributions
S.J. wrote the first draft with support from K.T. L.S. contributed to the section on anxiety and K.B. to the background review and figures. E.J.H. supervised the writing and all authors approved the final manuscript.
MCQs
Select the single best option for each question stem
1 When considering depression in Parkinson's disease:
a there is no relationship between rates of depression and severity of motor symptoms
b validated screening tools include the Hospital Apathy and Depression scale (HADS), Starkstein Apathy Scale (SAS) and Geriatric Depression Scale (GDS)
c motor ‘on’ or ‘off’ state should be considered when making a diagnosis of depression
d dopamine agonists should be avoided in Parkinson's disease depression
e serotonin syndrome is commonly encountered clinically in people with Parkinson's disease depression.
2 Impulse control disorders in Parkinson's disease are most commonly associated with:
a levodopa
b dopamine agonists
c monoamine oxidase inhibitors
d catechol-O-methyltransferase (COMT) inhibitors
e deep-brain stimulation.
3 With regard to anxiety in Parkinson's disease:
a obsessive–compulsive disorder is the most common anxiety disorder seen in people with Parkinson's disease
b there are no gold standard diagnostic criteria
c a longer serotonin transporter allele is associated with higher levels of anxiety in Parkinson's disease
d depression and anxiety are easily differentiated
e benzodiazepines are the first-line treatment.
4 People with Parkinson's disease:
a are more likely to develop rapid cognitive decline if they are of tremor-dominant phenotype
b are more likely to develop Parkinson's disease dementia if they possess the Parkin mutation
c should be offered memantine as first-line therapy
d are unlikely to benefit from physical therapy with regard to their cognition
e who have visuospatial dysfunction are more likely to develop Parkinson's disease dementia.
5 With regard to psychosis in Parkinson's disease:
a psychosis exclusively presents late in the course of Parkinson's disease
b treatment with quetiapine or risperidone does not affect motor symptoms
c prevalence increases with disease duration
d treatment of psychotic symptoms should be initiated regardless of the impact the symptoms are having on the patient
e there is strong evidence of association between Parkinson's disease medication and psychosis.
MCQ answers
1 c 2 b 3 b 4 e 5 c






eLetters
No eLetters have been published for this article.