LEARNING OBJECTIVES
After reading this article you will be able to:
• identify specific examples of neurological conditions that can present to the psychiatrist
• understand the value a neurological examination can add to a psychiatric assessment
• begin to plan relevant investigations to help distinguish differential diagnoses in patients with altered mental status.
Psychiatric practice loses a lot without some knowledge of Neurology. Indeed, the two specialties were once a unified field of clinical endeavour. If we see only the distinctive elements of both disciplines, we can lose those ways in which psychiatric practice is enhanced by a knowledge of Neurology and vice versa.
In this article we aim to explore common ground shared by both disciplines and to show how incorporation of some neurological knowledge can improve the practice of psychiatry. We will look at this through the lens of four fictional case vignettes of altered mental status which will serve to draw out the learning objectives.
Our individual case vignettes will focus on aspects ranging from cognitive impairment to movement disorders, any of which may present first to a psychiatrist. These vignettes are informed by clinical experiences but are fictitious and any resemblance to specific cases is unintentional. In each case we will highlight key aspects from the history and examination which can signpost particular differential diagnoses. This dissection of the key points from a case presentation is a fundamental aim. However complex a case may at first appear, by defining the individual characteristics, it is possible to build a sound differential. We will show how to strike a solid balance between precision in diagnostics and still casting the net wide enough to capture important neurological differential diagnoses. We would recommend reading the Case vignettes and then trying to think of your own list of differential diagnoses before proceeding with the case expositions.
Acute cognitive decline
Case vignette 1
A 38-year-old male is brought to the accident and emergency department (A&E) by his wife. For 3 days he has had several ‘odd spells’ in which he is reported to be confused. The confusion initially improved intermittently but has not resolved. He has a sense that he is ‘not himself’ and he complains of not knowing what is going on. On certain occasions he has failed to recognise familiar surroundings and people. He has been heard to wonder whether people are trying to ‘trick’ him, although this seems to be more of an attempt to explain things rather than a delusional belief. As the duty psychiatrist you are contacted to review the patient.
The patient is vague and confused. He complains of poor concentration. He was previously well. Apart from recently starting a selective serotonin reuptake inhibitor (SSRI) for depression he takes no other regular medicines. As a child he had ‘fits’ and this was investigated with an electroencephalogram (EEG) at the time. His wife is clear that he has not exhibited any convulsive symptoms in the 10 years she has known him. She reports he has had a low mood for around 6 weeks after being made redundant. He does not smoke, seldom drinks alcohol and does not use any illicit substances.
His physical observations are normal. His examination (systems and neurological) is normal, although he scores 14/30 on a cognitive screening measure. He particularly loses points on tasks of recall, attention and concentration. He manages well with the orientation tasks. A magnetic resonance imaging (MRI) scan of the head and a lumbar puncture are normal.
Acute infection and general medical considerations
From first principles we would want to know more about the history. However, we could already try to work up a provisional differential thinking along psychiatric and neurological lines from what we have. There are a large number of general medical differentials to be considered here also. See Box 1 for a general overview.
BOX 1 Some general medical differentials of confusion
Infection – such as urinary or respiratory tract infections or COVID-19
Endocrine or metabolic – such as hypo- or hyperglycaemia, thyroid disease, hypoxia, hepatic encephalopathy
Iatrogenic – such as medication-related effects of opiates, tricyclic antidepressants, antihistamines
Drug of misuse – such as hallucinogens, stimulants, benzodiazepines, opiates, alcohol
Environmental – such as occupational exposure to noxious chemicals or heavy metals
Inflammation – systemic inflammation, for example in autoimmune conditions, or CNS inflammation such as demyelinating plaques can cause confusion but do not have strong pointers in vignette 1 so far
Congenital, degenerative, neoplastic – there do not seem to be strong risk factors for these so far in this case and the presentation seems to be more acute
Vascular – stroke or vasculitis can present with acute confusion but there are no clear risks in vignette 1
Infections of the central nervous system (CNS) are important differentials here not to be missed. It could be dangerous to assume, for example, a urinary tract infection as the cause of confusion in an otherwise young and healthy person.
In this vignette we especially need to consider encephalitis. The term encephalopathy is a broader one which has been defined as ‘altered consciousness that persisted for longer than 24 h, including lethargy, irritability, or a change in personality and behaviour’ (Granerod Reference Granerod, Ambrose and Davies2010). Encephalitis can be considered to be encephalopathy plus at least two extra features, such as fever, seizures, cerebrospinal fluid (CSF) pleocytosis, EEG evidence for encephalitis or imaging evidence for encephalitis (Granerod Reference Granerod, Ambrose and Davies2010). Because of the potentially grave consequences of missing encephalitis, a strong clinical suspicion should be used even if some features are missing.
At the forefront of the possible causes of encephalitis would be herpes simplex encephalitis (Solomon Reference Solomon, Michael and Smith2012). This is the most common cause of encephalitis, accounting for approximately 19% of cases (Solomon Reference Solomon, Hart and Beeching2007). Prompt diagnosis is important as mortality can be as high as 70% in untreated cases and as low as 8–20% if early treatment is initiated (Solomon Reference Solomon, Hart and Beeching2007; Singh Reference Singh, Fugate and Hocker2016). In practice, this means treating with intravenous (IV) aciclovir if there is significant clinical suspicion and having a very low threshold for performing a prompt lumbar puncture if there are no contraindications. Confusion, headache, fever and behavioural change are the cardinal features to look out for, although not all features are required for the diagnosis (Sili Reference Sili, Kaya and Mert2014).
Similarly, with what we know so far of this case, bacterial meningitis is a further entity not to miss. Two organisms cause 80–90% of meningitis in UK adults, namely Streptococcus pneumoniae and Neisseria meningitidis. The presentation can be non-specific. This is illustrated when we consider that of the classic symptom triad – fever, neck stiffness and altered mental state – only 44% of patients exhibit all three (Brouwer Reference Brouwer, Tunkel and van de Beek2010). Where a significant concern of meningitis or encephalitis is present, prompt empirical treatment of the most likely causes should be strongly considered (see Box 2 for a more extensive range of possible aetiologies) (Ellul Reference Ellul and Solomon2018).
BOX 2 Some infective causes of encephalitis
Viral
Herpes simplex virus types 1 and 2
Varicella zoster virus
Enteroviruses
Adenovirus
Measles virus
HIV
Bacterial
Mycoplasma
Tuberculosis
Borrelia
Listeria
Opportunistic (immuno-compromised)
Cryptococcus
Toxoplasma
Cytomegalovirus
We have given priority here to clinical entities that are associated with high mortality in the acute phase. Autoimmune encephalitis, less likely here, will be discussed in vignette 2.
Psychiatric differentials
Turning now to psychiatric diagnoses, the history thus far would be atypical for a primary psychosis, but it should not be dismissed outright. The onset, patient age and reported confusion are certainly not typical for schizophrenia. The term ‘confusion’ is sometimes mistakenly used to describe the phenomenon of the delusional mood (Stanghellini Reference Stanghellini, Raballo and Broome2019). Additionally, catatonia would be another differential that should not be excluded at this point and the possibility of a somatoform disorder is also still conceivable.
Other factors, such as illicit drug or alcohol use, migraine with aura or even transient global amnesia, might be considered, but these are not well supported by the history in this case.
We have a picture of altered mental status and behavioural change. The process appears to have started acutely but is ongoing. There is no other neurological abnormality on examination. This does not discount the previously discussed infective causes but, coupled with the normal physical observations, it is important to think about alternative explanations as well.
Epilepsy, seizures and altered mental status
One clue that may direct our thinking is the collateral history, which referenced ‘fits’ as a child. His wife is clear that he has not displayed any recent convulsive symptoms. Importantly, not all seizure disorders manifest convulsive symptoms.
If we start to consider seizures and epilepsy in this case, then we might think about temporal lobe epilepsy, which can cause altered mental status and is the most common cause of adult epilepsy in the UK (Smith Reference Smith2008). Epilepsy classification by the International League Against Epilepsy (ILAE) has recently undergone a major change (Fisher Reference Fisher, Cross and French2017). Some important new terms resulting from this are highlighted in Table 1.
TABLE 1 Comparison of some common older terms for epileptic seizures with the newer terminology based on the International League Against Epilepsy 2017 classification
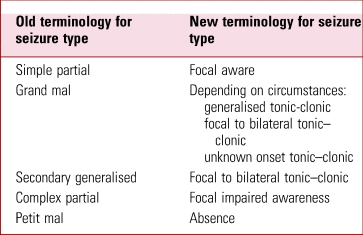
Source: Fisher (Reference Fisher, Cross and French2017).
Focal epilepsies originate in one particular part of the brain (temporal lobe epilepsy, for example). In contrast, generalised onset epilepsy reflects a process apparently originating in both hemispheres. This is the type associated with the classic generalised tonic–clonic semiology. Focal seizures can progress to become generalised seizures. These are now referred to as focal to bilateral tonic–clonic seizures.
An aura may occur before the main seizure and is itself a seizure type (focal aware seizure). Auras are commonly associated with temporal lobe seizures. These can often involve psychic phenomena such as déjà vu and jamais vu, which can be thought of as focal cognitive seizures. The most common aura in temporal lobe epilepsy is an ascending visceral sensation often starting from the epigastric area, although others occur. These can include unpleasant smells and tastes (typically these are stereotyped), or auditory phenomena. In our case it would be important to ask the patient and his wife about these features, as they will not necessarily be volunteered by the patient, who may think they are irrelevant or even be embarrassed by them.
There can also be a number of post-ictal phenomena in temporal lobe epilepsy. Commonly, these are seen as confusion and headache, which can last for hours, although agitation and aggression can occur, as can post-ictal psychosis. This psychosis occurs after the last seizure and typically happens after a lucid period which often lasts 1–6 days (Adachi Reference Adachi, Ito and Kanemoto2007; Hilger Reference Hilger, Zimprich and Pataraia2016). In terms of psychopathology, there is often an abnormal mood component, paranoid delusions and confusion, which can persist for the duration of the episode. The psychotic symptoms often spontaneously resolve within several days (Agrawal Reference Agrawal, Faruqui and Bodani2020).
Non-convulsive status epilepticus
In our case there does not seem to be evidence of frank psychosis. The patient's talk of ‘being tricked’ appears to be an attempt to explain his memory and concentration difficulties, as opposed to a delusional belief. There is a sense from the case details that this started suddenly, may have initially been intermittent and is now a continuing process. In the context of seizure disorders this should lead one to consider non-convulsive status epilepticus (NCSE), an underdiagnosed condition. This is the diagnosis in our patient. Specifically, this is a case of focal non-convulsive status without coma.
NCSE refers to prolonged seizure activity without significant convulsive symptoms. It is caused by continuous focal seizures or focal seizures with incomplete interictal recovery. The symptoms of memory and general cognitive decline in our patient suggest that the epileptic focus may lie in the temporal lobe. The clinician must have a high index of suspicion to make the diagnosis of NCSE, especially in older patients and in those who have suffered an acute stroke. A history of seizures may not be present. Although NCSE can have protean presentations, it is particularly important to consider this diagnosis in the presence of new, sustained, altered consciousness and abnormal eye movements such as nystagmus or repeated blinking (Husain Reference Husain, Horn and Jacobson2003). The investigation of choice is the EEG and when this reveals an ictal pattern the diagnosis can be clear cut. However, often there is a non-specific pattern which is similar to that seen in encephalopathy. In this scenario the EEG and clinical response to treatment may provide a clue as to whether NCSE is the aetiology (Sutter Reference Sutter, Semmlack and Kaplan2016). Neuroimaging should be undertaken for patients in whom NCSE is the suspected diagnosis, as there may be an underlying structural lesion serving as the focus of seizure activity.
The patient in this case had been recently started on an SSRI for depression. This is a potentially important clue in the overall diagnosis, as this may have precipitated the seizure. The patient does appear to have a seizure history as a child. Antidepressants can lower the seizure threshold and can worsen seizure control in people with epilepsy (Johannessen Reference Johannessen, Henning and Johannessen2016). This may be a direct effect, although there is some evidence to dispute this (Alper Reference Alper, Schwartz and Kolts2007; Okazaki Reference Okazaki, Adachi and Ito2011). Additionally, in people who are already on anticonvulsant medication, antidepressants can interact and alter plasma levels. There is significant comorbidity between epilepsy and psychiatric conditions and both antipsychotics and antidepressants may alter seizure threshold. However, this is mainly relevant at higher serum doses and so seizures are not an absolute contraindication to their use if needed (Habibi Reference Habibi, Hart and Bainbridge2016).
Where NCSE is suspected, input from the neurology team would be ideal to help guide management. Initiation of an anticonvulsant medication and serial EEG recordings are typically required. There is little direct evidence to suggest that NCSE itself causes neuronal damage, although harm could result from accidents or hypoxia.
Case vignette 2
A 24-year-old female is referred to the duty psychiatry team having presented to A&E with police in attendance. Her partner explains that she started behaving bizarrely 10 days before and this has been worsening since. She has no psychiatric history but had experienced some headache and fatigue a few weeks before.
The police had detained her at a local barber shop after she entered the premises and began shouting about a conspiracy that implicated the owners as the operators of a device which was sending out radiation and damaging the brains of local residents. She was aggressive, agitated and had to be physically restrained after causing some damage inside the shop.
When seen in A&E she is haughty, dismissive and argumentative. She claims that the police and doctors are preventing the ‘truth’ coming to light about the radiation-emitting device. On several occasions it is clear that she is responding to auditory hallucinations. When asked about this she seems convinced that someone known to her is in the next room and talking through the wall.
She is tachycardic but otherwise her observations are normal. She agrees to a computed tomography (CT) scan of the head, which is normal. Routine blood tests are normal, including inflammatory markers. A urine drug screen does not confirm the presence of any illicit substances. Physical examination, undertaken somewhat opportunistically, has not revealed any abnormalities. She is detained under the Mental Health Act and conveyed to the local psychiatric hospital.
The patient struggles with navigating the acute admissions psychiatric ward and appears confused. Over the first 3 days on the ward she is noted to be holding her arms in odd, fixed positions and occasionally displaying some writhing movements of her limbs. Her presentation gradually changes, in that while previously agitated, she is now largely akinetic and mute. She is being treated with an antipsychotic and is receiving intravenous fluids.
On the fifth day as an in-patient she has a generalised tonic–clonic seizure witnessed by staff. Autonomic dysfunction is also noted, in that she has gone into urinary retention and has been pyrexial.
Neuropsychiatric differential diagnosis in psychosis
This patient is presenting with a psychotic episode. There is an acute onset and no prior psychiatric history is mentioned. As in case vignette 1, a CNS infection needs to be considered.
Illicit drug use should be considered, and a direct and collateral history can help as a urine drug screen will not detect all potential substances. Some prescription medications, such as steroids, can precipitate psychosis and should be asked for in the history (Agrawal Reference Agrawal, Faruqui and Bodani2020). A number of systemic medical conditions can be associated with an organic psychosis, for example systemic lupus erythematous (SLE). Psychosis is only seen in 1.5% of people with SLE but it often presents pre-diagnosis or early in the illness (Hanly Reference Hanly, Li and Su2019). This occurrence in early disease will mean that onset of psychosis will often be in the early to mid-20s. This is also the typical period of onset in schizophrenia and therefore makes SLE an important consideration in individuals, particularly females, who present with a first episode of psychosis. There are further neuropsychiatric manifestations of SLE, from cognitive dysfunction to mood disorders, that make it an important condition to consider generally (Mak Reference Mak, Ho and Lau2009).
Other causes of vasculitis could also be considered. Although here we are told that the patient's inflammatory markers are normal, offering some degree of reassurance, this does not discount the possibility entirely. Additional features may emerge and, as with any good differential, it should be responsive and incorporate new information when this comes to light.
Infectious diseases such as HIV and syphilis should be considered, particularly in the light of any suggestive history such as recent prodromal flu-like illness or if there is a supporting sexual history. Endocrine and metabolic possibilities should be borne in mind, such as hypo- or hyperglycaemia. Thyroid function should be checked in such a presentation as a routine measure.
In a patient of this age and with psychiatric disturbance, Wilson's disease should not be missed and warrants consideration because it is a potentially reversible cause of psychosis and cognitive decline. Although rare, around one-third of cases of Wilson's disease present with psychiatric symptoms (European Association for the Study of the Liver 2012). When indicated, testing includes serum ceruloplasmin and copper levels, liver function tests and slit lamp examination for Kayser–Fleischer rings. If there is concern about the possibility of Wilson's disease a 24 h urinary copper excretion test should be undertaken (Ferenci Reference Ferenci, Czlonkowska and Schilsky2017).
Psychiatric differentials
A primary psychiatric disorder such as bipolar affective disorder or schizophrenia is quite plausible. Phenomenologically, there is a fair amount in the history that suggests an affective component. Chiefly, the psychomotor agitation and potential egocentrism implied by being ‘haughty’ and ‘dismissive’. There is also a sense that the patient has come to understand a great truth, that she is the sole possessor of this knowledge and must uncover it, thus giving the suggestion of the ‘special mission’. However, there is a persecutory feel to the content, which does not rule out a bipolar illness, but which is less common in the delusional semiology of that disorder (Picardi Reference Picardi, Fonzi and Pallagrosi2018). The timing of onset is more in keeping with a manic episode in a bipolar illness. In schizophrenia one would typically expect a more insidious onset with an identifiable prodromal phase. A schizoaffective illness is also within the differential, as are entities such as the brief or transient psychotic disorder. A collateral history would be instructive in outlining any major psychosocial stressors.
As the case progresses we have a convincing description of a catatonic patient. There has been progression from excitement to stupor and the movements mentioned may represent catatonic posturing and psychomotor agitation. It is important to remember the excited catatonia phenotype, so as to avoid considering only akinetic and mute patients as exhibiting catatonia. Indeed, the alternation of excitement and stupor is characteristic of catatonia (Bush Reference Bush, Fink and Petrides1996).
It might seem reasonable to attribute the catatonia to a primary psychotic disorder. But this may be premature. Classically catatonia has been coupled with the major psychiatric diagnoses, principally schizophrenia, but there is now greater recognition that there are a number of aetiologies (Fink Reference Fink and Taylor2001).
Abnormal movements in the psychiatric patient
Characterisation of the abnormal movements is an important task and could have implications in progressing our understanding of what is happening in this case. The abnormalities may represent a primary movement disorder and this raises an important diagnosis not to miss in this case (some important abnormal movements are described in Box 3). Antipsychotic treatments can of course precipitate movement disorders. Although in our case the antipsychotic seems to have only been started since the patient was admitted, it is not stated when exactly it was initiated compared with the onset of the movements. Akathisia can occur within 12 h of antipsychotic initiation, acute dystonias within 24 h and acute Parkinsonism within days (Mathews Reference Mathews, Gratz and Adetunji2005). Despite this, the timeline seems unlikely here and our patient's writhing movements sound more like chorea. Chorea and dystonias are common movement disorders in the context of autoimmune encephalitis, principally N-methyl-d-aspartate receptor (NMDAR) antibody-mediated encephalitis. Importantly, catatonia is also described in the disorder (Varley Reference Varley, Webb and Balint2019).
BOX 3 Some important dyskinesias (abnormal involuntary movements)
Hyperkinetic dyskinesias (excessive movement)
Akathitic movements: arising from a subjective sense of restlessness and urge to move.
Dystonia: sustained muscle contractions, may be associated with repeated twisting of a body part, sustained postures or both.
Tremor: rhythmic oscillations around a body part.
Chorea: unpredictable, non-repetitive movement fragments that can appear jerky or writhing in nature.
Athetosis: similar to chorea but unbroken, continuous and flowing movements.
Ballism: severe form of chorea with sudden, large-amplitude movements of an entire limb.
Myoclonus: sudden brief jerks or relaxation of muscles or muscle groups.
Hypokinetic dyskinesias (reduced movement)
Bradykinesia: slowness of movement.
Rigidity: significant resistance to passive joint movement even at low speed unrelated to musculoskeletal factors such as joint pain. The classical presentation is ‘lead pipe’ rigidity in Parkinsonism, which may be felt as ‘cog-wheel’ rigidity if there is also a tremor.
(Sanger Reference Sanger, Chen and Fehlings2010; Fahn Reference Fahn2011)NMDAR-antibody encephalitis
The most likely diagnosis in this case is NMDAR-antibody encephalitis, one of a number of causes of autoimmune encephalitis (Box 4). A characteristic and classic picture has emerged in this case in keeping with what is now recognised as the most common type of autoimmune encephalitis. This is often a multistage disorder which often starts with a prodromal flu-like illness and progresses to psychosis, movement disorder, catatonia and seizures. With further progression and no treatment, dysautonomia and coma can result. The mortality has been estimated at 7% (Titulaer Reference Titulaer, McCracken and Gabilondo2013), the disorder having been first described in 2007 (Dalmau Reference Dalmau, Tuzun and Wu2007).
BOX 4 Some autoimmune causes of encephalitis
Antibodies against neuronal surface antigens
NMDAR-antibody encephalitis (linked with ovarian teratoma)
Leucine-rich glioma-inactivated 1 (LGI1) antibody encephalitis (linked with thymoma)
Antibodies against intracellular antigens
Anti-Hu (linked with small cell lung tumours)
Anti-Ma (linked with testicular tumours)
Anti-GAD (linked with type 1 diabetes, coeliac disease and small cell carcinoma)
Syndromes
Acute disseminated encephalomyelitis (ADEM)
Bickerstaff's encephalitis
(Gagnon Reference Gagnon and Savard2016; Ellul Reference Ellul and Solomon2018)Importantly, in our case a CNS infection has not yet been clearly excluded, and a lumbar puncture, if not contraindicated, would be essential. A lumbar puncture poses a challenge in a psychiatric setting, where a significant proportion of these patients can present, on the grounds of available expertise, not to mention the logistics of trying to undertake this investigation in agitated and disturbed patients. Nevertheless, practical difficulties alone should not exclude a lumbar puncture when it is required and safe to do so. In vignette 1 we highlighted the dangers of missing a CNS infection.
In this case, CSF analysis is very important and would confirm the diagnosis, therein complementing blood testing for the associated antibodies (Graus Reference Graus, Titulaer and Balu2016). In addition, an EEG may show characteristic abnormalities (Graus Reference Graus, Titulaer and Balu2016). Early collaboration with the neurology team is recommended to help guide management, which may include steroids, immunoglobulins or plasmapheresis. Autoimmune encephalitis is often a paraneoplastic process, so patients should be investigated for the possibility of a malignancy. As well as appropriate history and examination, investigations such as a CT of the chest, abdomen and pelvis or a PET scan may be useful. Although it is increasingly recognised that the majority of cases are not related to a cancer, the original description of NMDAR-antibody encephalitis was in young women and girls with ovarian teratoma (Dalmau Reference Dalmau, Tuzun and Wu2007). Appropriate management and treatment of the malignancy should be curative of the disorder.
Subacute cognitive decline
Case vignette 3
A 70-year-old man attends your out-patient clinic. His wife is concerned about 18 months of cognitive decline. He struggles to recall new information and has lost interest in activities he has previously enjoyed, such as reading the newspaper and going for walks. His wife has had to take over household management as he has made various mistakes with this responsibility, for example failing to pay bills on time. He struggles with planning tasks and is more easily frustrated. He has had a number of falls during this period and was admitted to hospital after one such occasion.
On physical examination the patient has a fixed facial expression with infrequent blinking. He has marked rigidity in his limbs. There is a bent-over posture with a small-stepping gait. There is slowness of movement bilaterally and arm swing is also reduced, more on the right-hand side. Cognitive screening has been undertaken and shows fronto-executive dysfunction as well as visuospatial deficits, impaired attention and poor delayed recall. Blood tests are normal.
It later emerged that our patient had been diagnosed with a neurological condition when he was aged 60.
Approaching a case of cognitive impairment
In coming to a diagnosis we need to think first about the timing here. This does not appear to be an acute process and so we may be considering some different potential aetiologies from those discussed already. The chief complaint appears to be cognitive decline leading to functional impairment. There are also features to suggest difficulties in movement. We are going to be thinking about causes of cognitive decline with added problems with movement.
Before thinking about the category of degenerative processes it is worth considering ‘low-hanging fruit’, that is, factors that are potentially reversible. For example, protracted or recurrent infections might be present. See Box 5 for recommended blood tests in working up a case of possible cognitive impairment.
BOX 5 Screening investigations for suspected dementia
In most cases:
Full blood count
Erythrocyte sedimentation rate (ESR)
Urea and electrolytes
Calcium
Glycated haemoglobin (HbA1c)
Liver function tests
Thyroid function tests
Serum B12 and folate levels.
If clinically indicated:
Midstream urine
Chest X-ray
Electrocardiogram (ECG)
Syphilis serology
HIV
(Arvanitakis Reference Arvanitakis, Shah and Bennett2019)This could be a depressive episode, the so-called pseudodementia phenotype. The loss of interest in previously enjoyed activities may represent anhedonia, and the apparent cognitive impairment might relate more to depressive bradyphrenia.
It is important to search in the history and on examination for any systemic features that might point towards a malignancy. A primary or secondary CNS malignancy here seems much less likely given the fairly protracted timeline (Batash Reference Batash, Asna and Schaffer2017).
Another differential related to malignancy would be a paraneoplastic syndrome. Such conditions arise when antibodies produced against cancer cells cross-react with antigens which are expressed in the central or peripheral nervous system. See Box 6 for examples of paraneoplastic syndromes that affect the CNS. Several of these syndromes can cause confusion and memory problems.
BOX 6 Some paraneoplastic neurological syndromes
Paraneoplastic encephalomyelitis: Confusion, amnesia, seizures, brainstem abnormalities, sensory ataxia
Paraneoplastic limbic encephalitis: Amnesia, personality change, irritability, depression, seizures
Paraneoplastic cerebellar degeneration: Cerebellar signs, e.g. ataxia, nystagmus, dysarthria
Lambert–Eaton myaesthenic syndrome: Proximal weakness, constipation, dry mouth
Paraneoplastic opsoclonus–myoclonus: Eye movement abnormalities and myoclonic jerk. Associated cancers: small cell lung, breast, gynaecological
(Voltz Reference Voltz2002)Normal pressure hydrocephalus is a condition associated with a classic triad of symptoms, namely: cognitive decline, gait disturbance and incontinence. The investigation of choice would be MRI of the brain. If imaging supported this, a trial lumbar puncture might be used as a way of determining likely response to, and therein possible benefit from, CSF diversion strategies (Shprecher Reference Shprecher, Schwalb and Kurlan2008).
We must of course consider a dementia syndrome as the diagnosis in this patient, with all the varying aetiologies that this encompasses. Common entities being common, Alzheimer's and vascular dementia should be in our minds as differentials at this stage. Frontotemporal dementia is also a possibility and further detail in the history will hopefully guide us. The history is not strongly suggestive of a Lewy body dementia at this point, but it would remain in the differential and we will return to it below.
A general cognitive screening test (e.g. the Mini-Addenbrooke's Cognitive Examination, Mini-ACE) and a frontal assessment battery may help both to support a diagnosis and provide a baseline for future monitoring. Neuroimaging should also be considered, although this will be informed by the physical examination and, as a general rule, if you are ordering imaging you should have physically examined the patient to aid both the appropriate choice of imaging and clinical interpretation of radiology reporting.
How to think about dementia-plus syndromes
In broad terms, we have a patient who is presenting with a possible dementia syndrome and movement disorder. It is useful to have a heuristic about possible differentials when these features co-exist. See Table 2 for a suggested approach to thinking about these symptoms.
TABLE 2 Dementia and movement disorder – a simple differential
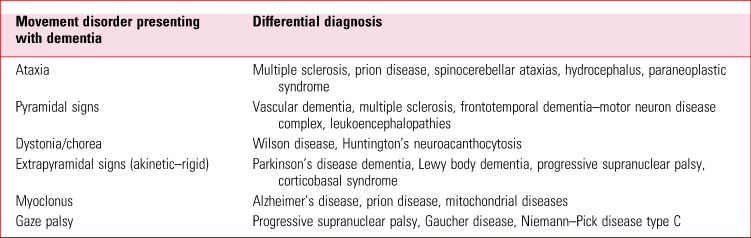
Source: Clarke (Reference Clarke, Howard and Rossor2016).
The core features of Parkinson's disease are tremor, rigidity and bradykinesia. These typically show at least some asymmetry in the early stages. From the details of the physical examination there do appear to be Parkinsonian features in our patient. The reduced blink rate and asymmetrical reduction of arm swing are certainly features often seen in Parkinson's disease, and there also appears to be bradykinesia generally. There is a reduced range of facial expression, termed hypomimia. He is noted to be stiff, has a stooped posture and the description of his gait also fits with a diagnosis of Parkinson's disease (Kalia Reference Kalia and Lang2015).
The neuropsychiatry of Parkinson's disease
Although the motor symptoms of Parkinson's disease are often the symptoms that bring patients to the clinic there are a range of non-motor symptoms. These include sleep disturbance, fatigue, sexual dysfunction, cardiovascular symptoms, and urinary and gastrointestinal symptoms such as constipation. This group of symptoms also includes mood disturbance, principally anhedonia and apathy, as well as difficulties with attention and memory. It is reported that such symptoms can appear years in advance of a diagnosis of Parkinson's disease, and rapid eye movement (REM) sleep behaviour disorder may precede a neurodegenerative diagnosis such as Parkinson's disease by several decades (Pont-Sunyer Reference Pont-Sunyer, Hotter and Gaig2015).
Psychiatric symptoms are therefore very common in Parkinson's disease and it is important to consider the presence of depression and anxiety in assessing patients with the disease (Martinez-Martin Reference Martinez-Martin, Schapira and Stocchi2007). Cognitive impairment may be the chief complaint in the clinic, yet the main issue may be an affective disorder. These symptoms are amenable to treatment and are associated with significant impairment in quality of life (Aarsland Reference Aarsland, Creese and Politis2017). Cognitive impairment is very common in Parkinson's disease and it is a consequence of the disease process (Aarsland Reference Aarsland, Creese and Politis2017). It has been reported that cognitive impairment may be found in approximately 20% of people with early and untreated Parkinson's disease (Aarsland Reference Aarsland, Bronnick and Larsen2009). When compared with age-matched controls, patients with Parkinson's disease have a four times greater relative risk of developing dementia (Agrawal Reference Agrawal, Faruqui and Bodani2020). Around a quarter of all people with Parkinson's disease have dementia, with over 80% developing it by 20 years after disease diagnosis (Aarsland Reference Aarsland, Creese and Politis2017; Reid Reference Reid, Hely and Morris2011). Patients who are 10 years post-diagnosis, such as our case, will most likely have developed some degree of cognitive impairment, if not frank dementia.
Our patient has most likely developed Parkinson's disease dementia (PDD). A typical profile of cognitive deficits in PDD involves four main cognitive domains: impaired attention and executive functions, impaired visuospatial function and impaired recall (Clarke Reference Clarke, Howard and Rossor2016). The impairment has to span more than one of these domains and a 1-year rule is applied. This is to say that PDD is diagnosed when the dementia syndrome evolves more than 1 year after a diagnosis of idiopathic Parkinson's disease (Emre Reference Emre, Aarsland and Brown2007). In the circumstance where dementia develops before or concurrently with Parkinsonism this is termed dementia with Lewy bodies (DLB) or Lewy body dementia. The core features here are fluctuating cognition, which can include pronounced variations in attention and alertness, as well as visual hallucinations and Parkinsonism (Emre Reference Emre, Aarsland and Brown2007). PDD and DLB exist along a clinical continuum and there can be difficulty defining the specific clinical entity in some patients.
An example of this is that we often think of the visual hallucinations in DLB as being characteristic and a useful point of discernment clinically. Yet this is not the case, as there is a relatively high prevalence of psychosis in Parkinson's disease, ranging from visual and auditory hallucinations to delusions (Ffytche Reference Ffytche and Aarsland2017a). There are three contexts in which this can arise. The psychosis can be associated with dementia and it can also be related to the pharmacological treatment of Parkinson's disease. It is also possible for the psychosis to occur independently (Ffytche Reference Ffytche, Creese and Politis2017b).
A final aspect which is important when thinking about cognitive symptoms in Parkinson's disease are the effects of treatment for the disease. Treatment with levodopa can contribute to confusion, and this response is considered a risk factor for developing PDD (Clarke Reference Clarke, Howard and Rossor2016). Dopamine agonists, which are used in early treatment to lessen the need for levodopa, can give rise to impulse control disorders. Common behaviours that can occur with impulse control disorders include hypersexuality, pathological gambling, compulsive eating and compulsive spending (Husain Reference Husain and Schott2016). Hobbyism describes an enraptured focus in a new activity. Punding is the compulsive performance of a previously goal-directed behaviour such as packing or sorting out items which has now become purposeless, repeated and time consuming (Clarke Reference Clarke, Howard and Rossor2016; Weintraub Reference Weintraub and Claassen2017). These features may need to be proactively sought from the history as they may not be volunteered by the patient.
Rarer Parkinson's-plus disorders to consider
Corticobasal syndrome is a clinical phenotype of a neurodegenerative process termed corticobasal degeneration. In corticobasal syndrome a typical presentation will involve asymmetric Parkinsonism and a characteristic limb clumsiness, which occurs in approximately 50% of cases. This speaks to the pattern of neurodegeneration which evolves, as the name suggests, to involve the basal ganglia and the cortex (Mahapatra Reference Mahapatra, Edwards and Schott2004). Higher cortical dysfunction is often seen and typically manifests clinically as ideomotor and limb apraxia. Apraxia means an inability to plan and undertake motor tasks that were previously possible for a patient, despite preservation of strength, sensation and simple coordination. In trying to draw out apraxia in the clinic it is useful to ask the patient to mime the performance of a motor activity such as using a hammer and nail, brushing their teeth or combing their hair (Armstrong Reference Armstrong, Litvan and Lang2013).
There are two further Parkinsonian conditions worth brief thought in our clinical scenario. Multiple system atrophy is a condition with varying degrees of Parkinsonism, cerebellar ataxia and autonomic dysfunction. The autonomic nervous system dysfunction can result in symptoms such as significant orthostatic hypotension and difficulty in micturition (Kollensperger Reference Kollensperger, Geser and Ndayisaba2010). These problems are not present in our patient.
Progressive supranuclear palsy is a disorder involving neurodegeneration which presents with a pattern of symmetrical bradykinesia and rigidity that is more proximal than distal. The Parkinsonism, which is not typically responsive to levodopa, is accompanied by early dysphagia, dysarthria and early cognitive impairment (Litvan Reference Litvan, Agid and Calne1996). The axial rigidity often leads to a characteristic hyperextended neck posture termed retrocollis. This rigidity also leads to the more upright posture as opposed to the stooped gait in Parkinson's disease. Overactivity of the frontalis muscle and eyelid retraction give a characteristic ‘surprised’ facies called the procerus sign (Warren Reference Warren and Burn2007).
Case vignette 4
A 60-year-old right-handed female is referred to the cognitive clinic for assessment. She has noticed over the past 9 months that her writing has deteriorated. Anything she writes is unintelligible to herself and others. She has also noticed some difficulty when dressing, despite having no clear pain, loss of sensation or weakness in her limbs.
A year before this presentation she had been seen by her general practitioner (GP). At that point the chief complaint had been a decline in her reading as well as a history of having had some minor car accidents, such as reversing into her garage door and into several plants that line her driveway. She had been seen by the optician and there was no evidence of any problems with her visual acuity or ocular media. Visual fields seemed broadly intact to a moving target (finger) when her GP saw her in clinic.
Her GP thought that she was very anxious at that time and that some social stressors may have been contributing to her reported difficulties, hence referral to psychiatry. She was diagnosed with an anxiety disorder and prescribed an SSRI.
You complete cognitive screening. There are no marked deficits in attention, memory, fluency and language. The patient is unable to write although can spell words verbally when asked. She struggles to read text. She is also unable to perform simple calculations. Curiously, when you are undertaking the finger–nose test, she is unable to identify her index finger. When you ask her to show you her right little finger she is also unable to do this. Indeed, she generally seems unable to discern left from right.
Additionally, when shown a picture and asked to describe it the patient is only able to name specific objects and is unable to comment on what is happening in the picture scene. The patient appears to have some difficulty in fixing her eyes on objects and has an associated problem in that she struggles to reach for objects when asked to do so.
Making sense of cognitive complaints
Trying to localise a problem is often the first step in neurology. In this case, there is a suggestion of both dysgraphia and acquired dyslexia. It is possible that this reflects a dominant hemisphere disorder (probably left hemisphere in a right-handed patient such as this). More specifically, a lesion in the left parietal lobe could explain both dysgraphia and acquired dyslexia. The history of bumping into objects without a clear physical cause raises the possibility of a visuospatial disorder. Visual neglect or a small visual field defect are still possible, as quick visual field testing with a large moving target such as a finger can miss more subtle deficits. Parietal and occipital lobe lesions might be involved in this. Finally, we have the difficulty in dressing that has no obvious cause. A dressing apraxia seems likely here, given the other features, and could additionally suggest non-dominant parietal lobe involvement.
To help us with possible aetiologies of parietal lobe dysfunction we can next consider the timeline in this case. For the most recent problems there is a 9-month history. The patient was seen in the psychiatry clinic a year ago and thus her symptoms may well have been going on for longer than a year. This does not appear to be an acute process, even at onset. There is no suggestion that the difficulties are episodic, although careful history taking will assure us of this. Therefore this is unlikely to be a vascular or infective event. There is a suggestion of steady progression. As ever, there are unlikely diagnoses to be considered which add a caveat to this assertion. Some infective processes do evolve over time, such as neuroborreliosis, syphilis and HIV, although these would be likely to have additional supporting evidence from the history or examination. Screening blood tests in the investigation of cognitive impairment have been mentioned in vignette 3 (Box 5) and are relevant here.
If a series of vascular events, such as ischaemic strokes, were giving rise to the patient's cognitive problems an episodic or paroxysmal chronology superimposed on progressive decline might be seen. This is absent in our case. A seizure, or series of seizures, would not necessarily show progression. Neither of these possibilities appears to be the case with our patient. Sometimes this episodic nature in the history is obscured as the patient may editorialise somewhat in telling the story in the service of brevity, succinctness or embarrassment. Close questioning on the detail will bring this to light.
The main categories of disorder we must next consider encompass neurodegenerative processes and primary psychiatric conditions. Hierarchically, ruling out a degenerative disease will take precedence. There does appear to be progressive decline in more than one cognitive domain here and of paramount importance is an attempt to delineate just what cognitive impairments the patient is suffering from. A cognitive screening test must be undertaken at assessment and augmented with some lobar specific screening such as the Frontal Assessment Battery to gain an overview of cognitive performance.
Anxiety is very common and does have an impact on cognitive performance (Eysenck Reference Eysenck, Derakshan and Santos2007) but it is not a specific finding. Very often the chief complaint in an anxious patient is of forgetting and overall memory performance. This is likely based on deficits in both attention and concentration (Langarita-Llorente Reference Langarita-Llorente and Gracia-Garcia2019). Our patient does not fit that phenotype and instead primarily has a range of complaints that seem to encompass motor skills and visuospatial function.
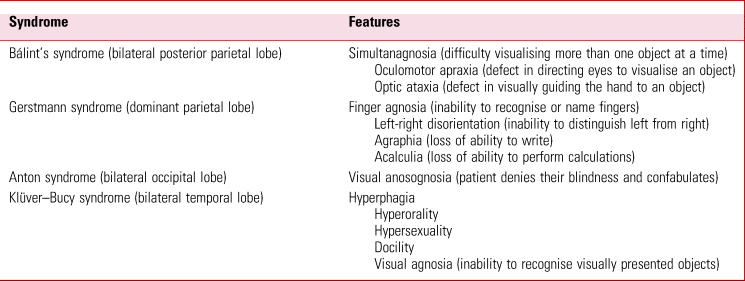
The neurocognitive syndromes
The difficulties this patient is experiencing conform to the described abnormalities in both Gerstmann syndrome and Bálint syndrome. The former arises due to pathology of the dominant parietal lobe, specifically the inferior parietal lobule. The latter is often a consequence of bilateral lesions affecting the posterior parietal cortex and the parieto–occipital junction (Agrawal Reference Agrawal, Faruqui and Bodani2020). The symptoms and signs in this case therefore localise to the posterior regions of the cerebral cortex, probably bilaterally.
Table 3 outlines the clinical features of various eponymous neurocognitive disorders, including Gerstmann and Bálint syndromes.
Posterior cortical atrophy
From the pattern of cognitive difficulties in our case we were considering a lesion involving the posterior aspects of the cerebral hemispheres. On the MRI brain scan there was severe atrophy of the parietal lobes, which was marked when compared with the surrounding parenchymal volume. This fits with our pattern of neurocognitive defects (Fig. 1 shows an illustrative MRI).
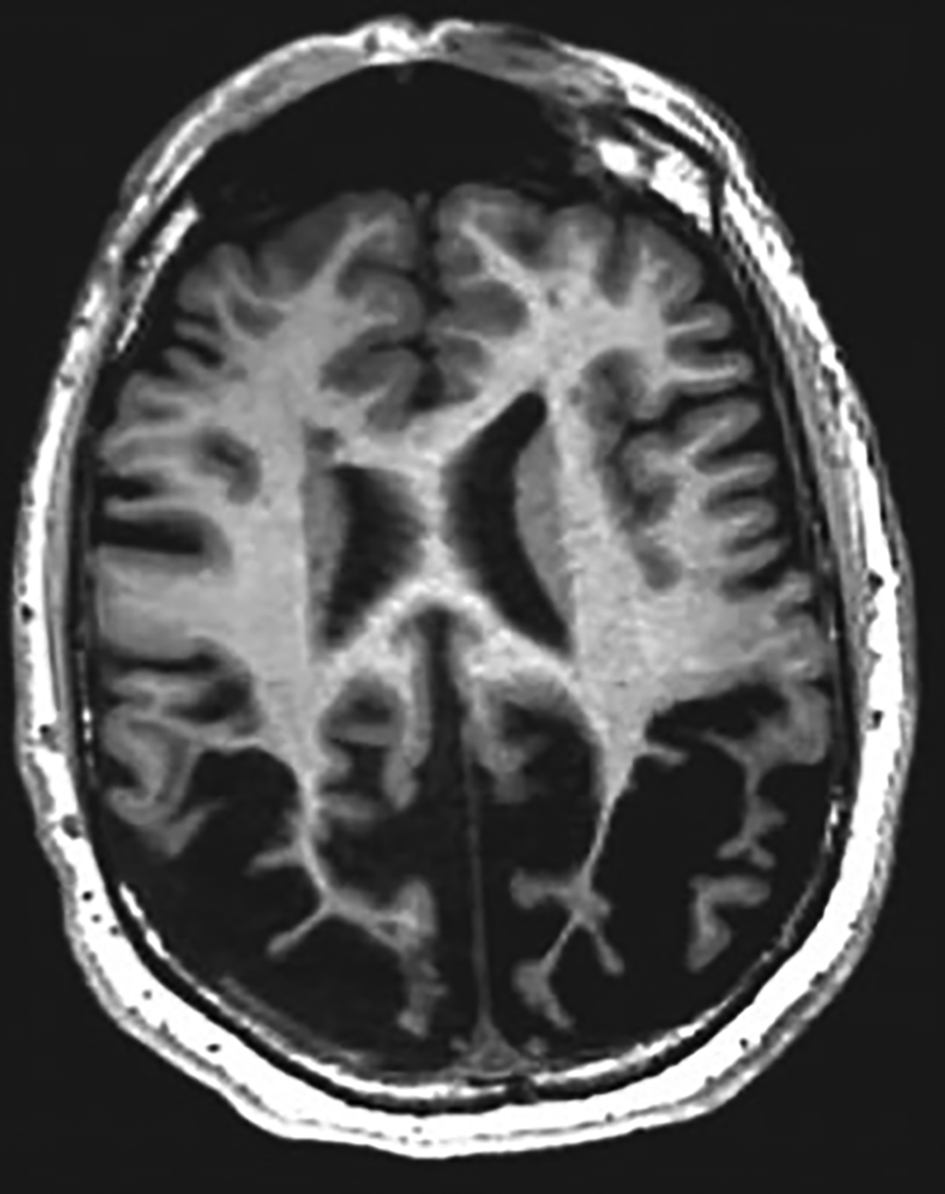
FIG 1 A magnetic resonance imaging scan of the brain showing posterior cortical atrophy resulting in visual impairment. © 2019 Elsevier. Reprinted with permission from Keuss et al (2019).
The most likely cause of this pattern of atrophy and the clinical presentation in our patient would be posterior cortical atrophy. This condition, also known as Benson syndrome, is often related to an Alzheimer disease phenotypic variant, although Lewy body dementia or even prion disease can also cause it (Crutch Reference Crutch, Lehmann and Schott2012; Holden Reference Holden, Bettcher and Pelak2020). The posterior lobar predilection leads to the classic symptoms of parieto-occipital dysfunction.
Posterior cortical atrophy is insidious and gradually progressive. Although not common, it serves as a reminder that neurodegenerative conditions can present in a number of very different ways. Some of these patients may be diagnosed with a functional cognitive disorder owing to the seemingly idiosyncratic problems they present with. It is therefore important to keep in mind that functional cognitive disorders almost exclusively present with concerns about memory. Our patient's concerns are very atypical in this sense and should instil caution about making such a diagnosis until organic causes have been carefully considered (McWhirter Reference McWhirter, Ritchie and Stone2020).
Final thoughts
We have shown how considering key neurological differential diagnoses is important in psychiatry to help prevent delays in the appropriate management of patients. A number of the differentials we have discussed could have adverse consequences if missed. Among psychiatry patients will be some with intercurrent or predominant neurological illness. Detecting such cases, where appropriate with assistance from neurology or general medical services, will help optimise patient care.
As psychiatrists we may believe that we are more inclined, predisposed even, to holistic care in thinking about our patients. This is a great strength and is a principle that we are not only taught from very early in our training but one that we quickly realise through working with patients with complex problems. Sometimes though, the biological part of our biopsychosocial approach must take a more central role. It is therefore important to keep updated on this front, and this has been the aim of our article. In doing so we will maintain secure and renewed links with our once inseparable and still ever-present cousin, Neurology.
Author contributions
Both authors made substantial contributions to the conception, writing, revision and final approval of the manuscript.
Funding
This article received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at https://doi.org/10.1192/bja.2021.67.
MCQs
Select the single best option for each question stem
1 Which of the following causes of encephalitis is most important to consider when assessing an acutely confused patient in the UK?
a Varicella zoster virus
b Enteroviruses
c Measles virus
d Herpes simplex virus
e Adenovirus.
2 Which of the following neurological conditions is most likely to present with symptoms of déjà vu and jamais vu?
a Posterior cortical atrophy
b Viral encephalitis
c Temporal lobe epilepsy
d Normal pressure hydrocephalus
e Generalised tonic–clonic seizures.
3 Which of the following clinical signs is a classic feature of Parkinson's disease?
a Asymmetrically reduced arm swing
b Brisk knee reflexes and upgoing plantars
c Increased rate of blinking
d Eyelid retraction
e Abnormal cerebellar signs.
4 A 22-year-old women with no medical history of note presents with a 1-day history of rapidly worsening confusion and headaches. A standard set of blood tests and an emergency MRI brain scan were reported as ‘normal’. Which is the most important next step?
a Lumbar puncture
b EEG
c Appropriate antimicrobials and lumbar puncture
d Repeat the MRI with contrast
e Repeat the MRI after 24 h to look for any change.
5 A 70-year-old man with Parkinson's disease reports much better control since he saw a specialist 3 months before. His wife is concerned, however, that he has suddenly started gambling in the past 2 months. Which of the following is most likely to reveal the cause?
a An MRI scan of the brain
b Auto-antibody testing
c A lumbar puncture
d An EEG
e Medicine review.
MCQ answers
1 d 2 c 3 a 4 c 5 e







eLetters
No eLetters have been published for this article.