LEARNING OBJECTIVES
After reading this article you will be able to:
• understand the epidemiological and clinical features of nitrous oxide misuse
• understand the mechanisms of action of nitrous oxide
• describe potential treatment options in nitrous oxide misuse.
Case studyFootnote a
In September 2017 a 37-year-old non-vegetarian woman of Somalian origin attended an accident and emergency department, 2 weeks after inhaling 6 boxes of 24 ‘bulbs’ of nitrous oxide at a house party. She described immediately feeling euphoric but then unwell and nauseated. Within a day she started experiencing tactile hallucinations that woke her from sleep; auditory command hallucinations telling her to pull off her nails and fly; and visual hallucinations of unfamiliar faces. She had started to shower with clothes on owing to a belief that she was being watched, and had developed paranoid delusions that someone was interfering with her mobile telephone. At her initial interview she was laughing incongruously as a response to auditory hallucinations. She described her head feeling fuzzy and was noted to be unable to remember her children's dates of birth. She was seen by the local early intervention service and received a diagnosis of drug-induced psychosis and commenced on aripiprazole up to 10 mg daily.
In the months that followed she had polymorphous psychotic symptoms that appeared to wax and wane, and started developing limb weakness, sensory changes and tiredness, which were all put down to antipsychotic medication or dismissed. She did not disclose any further inhalations of nitrous oxide. She appeared to no longer be able to understand how to fill in forms, she accumulated debt and ignored letters from Court. In August 2018 she fell down the stairs, prompting admission to hospital.
Her neurological history was of recent-onset falls and being unable to get up. She complained of headaches, back pain, bilateral leg weakness and patchy sensory changes throughout her arms and legs. Neurological examination revealed that she was unable to sit unsupported. She had a flaccid paraparesis with areflexia, flexor plantar responses and absent vibration sensation in the legs.
She was initially diagnosed with Guillain–Barré syndrome and started on intravenous immunoglobulin. However, when her history of nitrous oxide misuse came to light during her neuropsychiatric assessment, relevant blood tests were requested and her diagnosis amended to nitrous oxide-induced functional vitamin B12 deficiency causing myeloneuropathy.
Blood tests identified a normocytic anaemia (Hb 97 g/L, MCV 82.3 fL, RDW 24.2%). Vitamin B12 was low-normal, at 193 ng/L (normal range: 180–1000 ng/L), but plasma methylmalonic acid (MMA) was grossly elevated, at >3800 nmol/L (normal range: 0–360), as was homocysteine, at 77.4 µmol/L (normal range: 5.0–15.0), consistent with a functional B12 deficiency. A variety of other blood tests and cerebrospinal fluid analysis were unremarkable. Electromyography confirmed a severe predominantly motor demyelinating neuropathy. Magnetic resonance imaging (MRI) of the cervical cord showed a high signal within the dorsal columns between C1 and C3 (Fig 1) and C6 and C7.
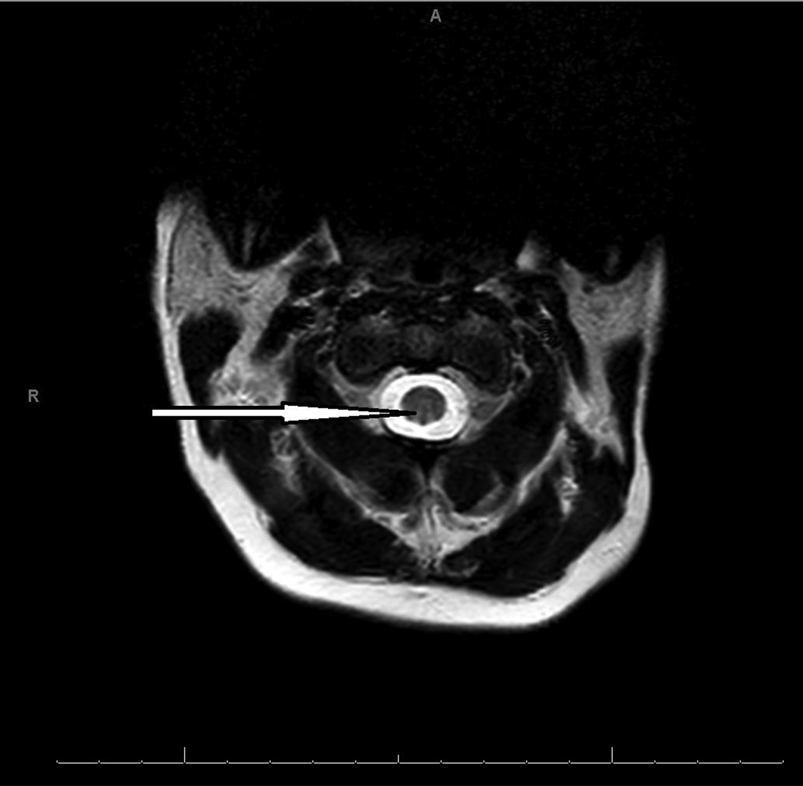
FIG 1 Magnetic resonance imaging scan of the cervical cord of a woman with myeloneuropathy caused by nitrous oxide-induced functional vitamin B12 deficiency. The scan shows a high signal in the dorsal column between C1 and C3.
Addenbrooke's Cognitive Examination version 3 (ACE-III) in September 2018 revealed a total score of 58/100, divided into: attention, 17/18; memory, 8/26; fluency, 5/14; language, 20/26; and visuospatial, 8/16. Unfortunately, the test was not subsequently repeated.
She was treated with hydroxocobalamin (vitamin B12) 1 mg intramuscular injections on alternate days for 7 weeks until her clinical progress improved and plateaued, at which point she was switched to 3-monthly injections. She was also treated with risperidone 1 mg twice daily. She remained in hospital for 4 months and made slow but steady progress with intensive rehabilitation. Fleeting visual hallucinations remained and worsened following a further inhalation of nitrous oxide while on the ward. She was discharged home walking with one stick, and thereafter lost to follow-up.
a Informed consent for the case described was obtained, witnessed, signed and formally recorded.
Introduction
‘The first time I inspired nitrous oxide, I felt a pleasurable sensation of warmth over my whole frame, resembling that which I remember once to have experienced after returning from a walk in the cold snow into a warm room. The only motion which I felt inclined to make was that of laughing at those who were looking at me’ (Samuel Taylor Coleridge, cited in Davy Reference Davy and Davy1839: p. 306).
Nitrous oxide (N2O) is a colourless gas, discovered by Joseph Priestley in 1775 and commonly known as laughing gas or, more recently, ‘hippy crack’. It has been used as an anaesthetic agent in medical, dental and obstetric procedures since the 19th century, and has also been used recreationally since Victorian times, when ‘laughing gas parties’ were held, as described above by Samuel Taylor Coleridge. It is primarily a euphoriant and as such produces a sense of joy and empathy. It also causes giggling and laughing, a deep ‘silly’ voice, and relief of pain and of anxiety (van Amsterdam Reference van Amsterdam, Nabben and van den Brink2015).
It has other uses as a propellant in the food industry (especially in whipped cream canisters, where it propels the cream out), in the manufacture of semiconductors, in improving the performance of car engines and to mimic decompression sickness in the training of divers. N2O for the car industry has sulphur dioxide added to deter inhalation. In medical practice, N2O is commonly mixed with oxygen before sale. Recreational users most commonly buy it over the internet as a whipped cream charger, also known as a bulb, ‘whippit’ or ‘nang’. However, in the UK it is covered by the Psychoactive Substances Act 2016, which makes it illegal to sell for recreational use, but not illegal to use, unless in prison or while driving. It is now becoming clear that it is a major drug of misuse, particularly in some parts of the world.
How is it taken, how much is taken and how long does it last?
The inhalation of nitrous oxide is sometimes referred to as ‘nagging’ or ‘nanging’ and is mostly from bulbs or party balloons. A bulb or ‘whippit’ (Fig. 2) is a non-reusable metal cartridge of 10 mL of N2O in a pressurised liquid form, which is comparable to 4 L of gas under normal pressure (van Amsterdam Reference van Amsterdam, Nabben and van den Brink2015). This is then released into a balloon by a metal ‘cracker’, with one bulb enough to fill a balloon. When intended for use in the food industry, nitrous oxide is thought to be contaminant free, however some people use ‘caplets’ from China, which have been found to contain particulate matter and an oily residue (Kaar Reference Kaar, Ferris and Waldron2016).

FIG 2 A nitrous oxide bulb or ‘whippit’.
Reported recreational intake of nitrous oxide varies. One study (Ehirim Reference Ehirim, Naughton and Petróczi2018) reported that 75% of young adults who had taken nitrous oxide took 1–5 inhalations in one sitting, with 45% using 1 or 2 inhalations per sitting, whereas another (Kaar Reference Kaar, Ferris and Waldron2016) reported that the median number of inhalations per session was between 10 and 15. Both studies are described in more detail below. Heavy use might be defined as ≥10 inhalations in one session, because adverse effects have been reported when users take on average 12–27 inhalations in any one session (Kaar Reference Kaar, Ferris and Waldron2016). The effect can last for up to 5 min, but in most cases (88%) lasts less than 1 min (Ehirim Reference Ehirim, Naughton and Petróczi2018).
Epidemiology of nitrous oxide misuse
In the 2014 Global Drug Survey (GDS), an international survey with 74 864 respondents, nitrous oxide was confirmed as being a very commonly used drug (Kaar Reference Kaar, Ferris and Waldron2016). Of all respondents, 19% had used it, rising to 39% of those in the UK. Similarly, respondents in the USA, New Zealand and Australia reported high lifetime use (29, 27 and 23% respectively). Importantly, the UK users differed significantly from the whole sample and the other high-income nations in that the reported use in the previous 12 months was 20.5%, compared with 6.5% globally. In addition, use within the previous 7 days was high, at 8%, compared with 2% globally. This survey found that nitrous oxide was the eighth most common substance used in the UK. Use was more common among males than females (7.2 v. 4.2% in the previous year) and most common in those in their early 20s. Importantly, this was a self-selecting sample, so is unlikely to be representative of the true prevalence of nitrous oxide use.
Ehirim et al (Reference Ehirim, Naughton and Petróczi2018) obtained similar results in a UK study. They assessed the use of nitrous oxide among 140 adults aged 18–25 in south-west London and found that 28% had taken it in the previous 12 months. In this small sample there was again a trend towards use being more common among males than females.
In the 2017/2018 crime survey of 34 400 households in England and Wales, the 1-year prevalence of nitrous oxide use was 2.3% for people aged 16–59, rising to 8.8% for people aged 16–24 (Home Office 2018). These numbers showed no significant change since the first survey in 2012/2013. Among 16- to 59-year-olds, men were again more likely to have used nitrous oxide than women (2.9 v. 1.8%).
The GDS specifically included clubbers and found that the 12-month prevalence of nitrous oxide use in that group was 9.4%, compared with 3.0% in non-clubbers (Kaar Reference Kaar, Ferris and Waldron2016). A survey of Dutch clubbers found that the previous-month prevalence of nitrous oxide use increased by a factor of ten between 2008 and 2013, to 33%, and the lifetime prevalence in 2013 was 71% (Nabben Reference Nabben, Benschop and Korf2014).
Summary
The 12-month prevalence of nitrous oxide use is unclear, but likely to be highest among young people, men and people attending clubs, in high-income countries and particularly in the UK, where its use may be increasing. Smaller studies tend to give higher estimates of prevalence, and the largest UK survey to date (Home Office Reference Ehirim, Naughton and Petróczi2018) gives lower prevalence estimates.
Mechanism of action in medical use
Nitrous oxide has multiple effects reflecting analgesia, anxiolysis and anaesthesia. In medical practice, N2O is commonly mixed with oxygen 50%/50%, often in the form of Entonox®.
Analgesia
Nitrous oxide is hypothesised to stimulate the release of endogenous opioids presynaptically, which in turn stimulates post-synaptic opioid receptors as a partial agonist. This is thought to be dependent on the actions of the enzyme nitric oxide synthase (NOS), which produces nitric oxide (NO). The release of the endogenous opioids is dependent on NO. This is supported by the fact that the opioid receptor antagonist naloxone can reverse the analgesic effects of nitrous oxide, and that competitive inhibition of NOS can also cause a reversal of analgesic effects (Emmanouil Reference Emmanouil and Quock2007).
Anxiolysis
Nitrous oxide is thought to act in a similar way to benzodiazepines, acting on the gamma-aminobutyric acid A (GABAA) receptor using the benzodiazepine binding site. This stimulation facilitates GABAergic activity. Evidence for its similarity to benzodiazepines is that anxiolytic use is reversed by flumazenil, there is cross-tolerance between benzodiazepines and N2O, it has similar effects in animal models, and its effects are also reversed by inhibition of NOS (Quock Reference Quock, Emmanouil and Vaughn1992; Emmanouil Reference Emmanouil and Quock2007).
Anaesthesia
Nitrous oxide is most famous for its anaesthetic effects, as it was the very first drug used in anaesthesia. The use of N2O for anaesthesia is thought to rely on it being an N-methyl-d-aspartate (NMDA) receptor antagonist. In animal studies, N2O was found to inhibit currents activated by NMDA but not by GABA (Jevtović-Todorović Reference Jevtović-Todorović, Todorović and Mennerick1998). Its effect in these studies is age dependent, and similar to the mechanism of action of ketamine. NMDA antagonism can cause cortical damage, and dual NMDA antagonism from ketamine and N2O may synergistically worsen cerebrocortical damage in rats (Jevtovic-Todorovic Reference Jevtovic-Todorovic, Benshoff and Olney2000).
Depression
There has been one published trial of N2O for the treatment of depression (Nagele Reference Nagele, Duma and Kopec2015). In this 20-patient placebo-controlled cross-over study comparing N2O+oxygen (in a 50/50 mix) with nitrogen+oxygen (50/50), 7 patients (35%) achieved either response or remission within 24 h after nitrous oxide treatment.
Inhalation of N2O was recently found to induce cortical excitation followed by depression (slowing) of electroencephalogram (EEG) activity within the delta and theta range, which is an effect shared with the onset of action of electroconvulsive therapy (Kohtala Reference Kohtala, Theilmann and Rosenholm2019). Similarly again to the novel antidepressant ketamine, it also affects the signalling of brain-derived neurotrophic factor (BDNF), activating the BDNF receptor glycogen synthase kinase 3 beta (GSK-3β) (Kohtala Reference Kohtala, Theilmann and Rosenholm2019). BDNF is involved in synapto- and neurogenesis, which is increasingly understood to be related to antidepressant response.
Other mechanisms of action
Imaging studies
In positron emission tomography (PET) imaging, inhalation of 20% N2O compared with room-air placebo resulted in deactivation of hippocampal, parahippocampal, posterior cingulate and visual association cortices (Gyulai Reference Gyulai, Firestone and Mintun1996). The first two areas are crucial for learning and memory, and deactivation in these areas could well account for the cognitive impairments described in nitrous oxide misusers. The anterior cingulate cortex is activated, which may be related to the previously mentioned analgesic effects of N2O, as this activation has been found in opioid release and is especially important in affective responses to pain, such as pain-induced aversion (LaGraize Reference LaGraize, Borzan and Peng2006). The anterior cingulate has connections to the nucleus accumbens and as such is part of the mesolimbic reward system (Navratilova Reference Navratilova, Xie and Meske2015), which may explain the misuse potential of N2O and its ability to cause psychotic symptoms in acute intoxication.
Biochemical actions
Nitrous oxide misuse is a well-established cause of functional vitamin B12 deficiency, i.e. although vitamin B12 (cobalamin) is present it cannot function. Vitamin B12 normally plays a pivotal role in converting homocysteine to methionine (essential for myelination) and in converting 5-methyltetrahydrofolate to tetrahydrofolate (crucial for DNA synthesis). It also converts L-methylmalonyl-Co-A (the coenzyme-A linked form of methylmalonic acid, MMA) to succinyl-Co-A (which is an important part of the citric acid cycle) (Wong Reference Wong, Harrison and Mattman2014). N2O inactivates B12 owing to the oxidation of the cobalt ion from 1+ to 3+, leading homocysteine and MMA to accumulate in serum (Fig. 3). This is especially important if a patient already has low or low-normal stores of B12. Vitamin B12 is poorly absorbed and is found only in animal products, putting people with gastrointestinal conditions and vegans at greatly increased risk of deficiency. Box 1 shows known causes of B12 deficiency. In the presence of these additional risk factors, nitrous oxide misuse would be particularly hazardous. In experimental conditions it has been found that 180 bulbs must be inhaled over 3 days to completely exhaust vitamin B12 levels; however, these were individuals with initially normal levels of B12 (van Amsterdam Reference van Amsterdam, Nabben and van den Brink2015).

FIG 3 The biochemical pathways of vitamin B12.
BOX 1 Causes of B12 deficiency apart from nitrous oxide misuse
Dietary: vegan or vegetarian diet (lacto-vegetarians are thought to be safe); excessive folate supplementation
Substance misuse: alcohol misuse
Demographic: increasing age
Infections: fish tapeworm, HIV infection, altered bacterial flora in terminal ileum
Surgical: ileal or gastric bowel resection
Medications: achlorhydria from proton pump inhibitors or histamine-1 blockers; colchicine, neomycin; metformin
Inflammatory and autoimmune: Crohn's disease, pancreatitis, pernicious anaemia
Preneoplastic: atrophic gastritis
Genetic: transcobalamin II deficiency
Miscellaneous: tropical sprue
(Adapted from Garakani Reference Garakani, Welch and Jaffe2014).
Megaloblastic madness
Vitamin B12 deficiency has been known for over 100 years to cause psychiatric symptoms (e.g. Langdon Reference Langdon1905). Sometimes termed ‘megaloblastic madness’, it is characterised by cognitive impairment up to frank dementia, depression, psychosis, and a ‘great variety of neurotic and psychotic presentations’ (Smith Reference Smith1960). However, the exact mechanism for cognitive, affective and psychotic symptoms in B12 deficiency is unclear, as is the contribution of other factors.
As mentioned above, B12 helps convert 5-methyltetrahydrofolate to tetrahydrofolate and is therefore involved in the folate cycle. A B12 deficiency will lead to a functional folate deficiency. This is significant from a psychiatric point of view, as a functioning folate cycle is necessary for the synthesis of the monoamine neurotransmitters (serotonin, (nor)adrenaline, dopamine, melatonin) and nitric oxide. The reduction in neurotransmitter levels with B12 deficiency has been found in animal models (Deana Reference Deana, Vincenti and Deana1977), but not clearly in humans.
Despite a clear theoretical relationship between lowered neurotransmitter levels and depression, the evidence for B12 deficiency being associated with depression is considered to be debatable, with both positive (Tiemeier Reference Tiemeier, van Tuijl and Hofman2002) and negative studies (Bjelland Reference Bjelland, Tell and Vollset2003). Depression itself may lead to B12 deficiency via a reduction in appetite and malnutrition, or vice versa (Harrison Reference Harrison, Kopelman, David, Fleminger and Kopelman2009).
Psychosis has been clearly described in B12 deficiency from its very first characterisation (Addison Reference Addison1849). Zucker et al (Reference Zucker, Livingston and Nakra1981), in their careful review of 15 cases of B12 deficiency and psychiatric symptoms, found that 10 of the 15 individuals had paranoid delusions and 7 had hallucinations. Conversely, Shulman's prospective case series of 27 individuals with pernicious anaemia found that none developed psychotic symptoms (Shulman Reference Shulman1967). More recently, B12 levels have been found to be lower in never-medicated patients with first-episode psychosis compared with dietary-, age- and other variable-matched controls (Kale Reference Kale, Naphade and Sapkale2010).
Vitamin B12 deficiency has been implicated in cognitive impairment and dementia. Vitamin B12 replacement is more likely to improve cognition in deficient patients with mild cognitive impairment than in those with dementia (Eastley Reference Eastley, Wilcock and Bucks2000). However, B12 deficiency is thought to be the cause of only 1% of reversible dementias (Harrison Reference Harrison, Kopelman, David, Fleminger and Kopelman2009).
Summary
Nitrous oxide has diverse biochemical, metabolic and physiological effects, with actions involving the anterior cingulate, hippocampus and parahippocampus. Some of these effects may be medically beneficial, whereas others maybe unhelpful and damaging. It is highly unlikely that vitamin B12 inactivation is the sole mechanism by which psychiatric symptoms emerge in the setting of nitrous oxide misuse.
Clinical features of nitrous oxide misuse
Garakani et al (Reference Garakani, Jaffe and Savla2016) conducted a systematic review of the published case literature regarding nitrous oxide misuse up until 21st January 2016. Cases were classified according to neurological, psychiatric or medical manifestations, and included cases of nitrous oxide misuse resulting in death. A total of 91 cases were included, from 77 publications. Males made up 71%, and the median age was 26.
Neurological manifestations were the most frequently reported sequelae, including myelopathy, myeloneuropathy and subacute combined degeneration of the spinal cord. Out of the 91 cases, 72 had a primarily neurological manifestation. Most patients were treated with cyanocobalamin (vitamin B12) injections and, where outcomes were given, symptoms improved in 46, persisted in 3 and fully resolved in 10 individuals. Eight patients had non-neurological medical manifestations, mostly affecting the respiratory system. In addition, 29 of the case reports described death from nitrous oxide misuse, most of them involving asphyxiation.
Eleven reports described patients with predominantly psychiatric manifestations of nitrous oxide misuse.
We have updated the review by repeating the search for the period January 2016 to December 2018 using the same strategy and databases as Garakani et al. This yielded three further cases, two of which we have placed in the psychiatric category (Chen Reference Chen, Zhong and Jiang2018; Hew Reference Hew, Lai and Radford2018), while the third was primarily a neurological presentation (Mancke Reference Mancke, Kaklauskaitė and Kollmer2016).
Psychiatric manifestations
Table 1 provides clinical information about the cases with psychiatric manifestations reported in the literature. The 13 individuals ranged in age from 23 to 64 years old and 12 were males. The duration of nitrous oxide intake ranged from 20 years of regular use to a few days before being seen, but was not reported clearly in two cases. The amount inhaled was generally unreported, but ranged from 20–100 bulbs a day to a 20 lb canister over 4 days. The last inhalation or use of nitrous oxide was also not clearly reported in most cases. Interestingly, in one case the patient had reportedly been abstinent for a year, in another for a ‘few weeks’ and in a third for a few days before presentation. In the last two cases, symptoms resolved quickly, whereas in the first, cognitive deficits persisted.
TABLE 1 Summary of case literature on psychiatric manifestations of nitrous oxide misuse (adapted from Garakani Reference Garakani, Jaffe and Savla2016)

Npsychol, neuropsychological testing; MCV, mean corpuscular volume blood test; Utox, urinary toxicology; CT, computed tomography scan; IFB, intrinsic factor blocking antibody test; HCY, homocysteine; MMA, methylmalonic acid; IM, intramuscular; THC, tetrahydrocannabinol (cannabis); +, positive; −, negative; LN, low-normal; →, normal; ↓, low; ↑, high; x/7, days; x/52, weeks; x/12, months.
The most common presenting psychiatric symptoms in the 13 cases were delusions (reported by 8 individuals), cognitive impairment (6), visual hallucinations (4), bizarre or inappropriate behaviour (4), affective lability (3), anxiety symptoms (3) and auditory hallucinations (2). Depression was a feature in two reports, and grandiosity or manic behaviour in two.
There was no standardised set of investigations and the abnormalities reported varied greatly, but homocysteine was elevated in five individuals and MMA was elevated in four. Vitamin B12 was tested in nine individuals. In one of these it was normal, in two low-normal and in six it was low.
Neuropsychological testing was reported in four cases. Brodsky & Zuniga (Reference Brodsky and Zuniga1975) found a mild bilateral temporal lobe dysfunction, with impaired immediate memory, poor motor speed and perseveration. Testing was not repeated at follow-up. In the case described by Sterman & Coyle (Reference Sterman and Coyle1983), deficits on the Digit Symbol subtest (from the Wechsler Adult Intelligence Scale) and the Symbol Digit Modalities Test were noted; both tests would have required speed and attention. They repeated this testing 2 and 7 days later, demonstrating substantial improvements. Of particular interest is a case reported by Van Atta (Reference Van Atta2004), of a dentist who claimed abstinence from nitrous oxide for 12 months before testing. He showed deficits on tests of verbal memory, visual memory and construction, and his processing speed was slow. He scored overall in the 8th percentile for general cognitive functioning for someone of his age and education. In Schwartz & Calihan (Reference Schwartz and Calihan1984), the patient was reported to have defects in recent memory, but details of the cognitive testing undertaken were not provided. Furthermore, he had been discovered with his head still in the plastic bag used to inhale the nitrous oxide, so he may have suffered a degree of hypoxic brain injury. This would likely have influenced the results and potentially the clinical presentation. Overall, it appears that in nitrous oxide misuse where cognitive impairment occurs it is primarily found in processing speed and recent or immediate recall.
Treatment and prognosis
In nine of the ten case reports where outcome was reported, the outcome was favourable. Resolution of symptoms occurred mostly after around 1–3 weeks, but in one case was ‘immediate’ and in another in 2 days. In eight cases antipsychotic drugs were given and in one case lithium. In three cases antipsychotics alone were given and the patient recovered, but in one case an antipsychotic was given first, was ineffective, and only intramuscular vitamin B12 resulted in a full recovery. Seven patients were given intramuscular B12, of whom five were also given an antipsychotic. In one of these cases where the patient did not improve significantly, his presentation was complicated by alcohol dependency. First-generation antipsychotics (zuclopenthixol acetate, haloperidol, trifluoperazine) were given in three cases, an unknown antipsychotic in one and second-generation antipsychotics (risperidone, olanzapine, quetiapine) in four. It is unclear whether abstinence alone was sufficient for recovery, and therefore whether the B12 and antipsychotics these patients received actually caused the improvements in patients' conditions. In addition, one patient was positive for cannabis on urinary drug screening. Urinary drug screens can be useful, but are not diagnostic and can lead to adverse physical and psychological outcomes if they give a false-positive result (Sheldon Reference Sheldon2019). However, if the result is a true positive this might have affected the patient's presentation and duration of symptoms.
Overall, and accepting their limitations, what appears to emerge from these cases is that psychosis accompanied by cognitive disturbance appears to be the most common psychiatric presentation of nitrous oxide misuse. Vitamin B12 levels are likely to be low or low-normal, while homocysteine and MMA are likely to be raised. Resolution of psychiatric symptoms is to be expected within a few weeks of abstinence, and most cases of recovery are associated with intramuscular B12, in combination with antipsychotics.
As with all case reports, these are limited by recall bias, confounders (such as hypoxic brain injury, cannabis use or alcohol dependency) and other potential biases (such as regression to the mean). There is uncertainty regarding the volume of nitrous oxide inhaled, whether other pollutants may have complicated the picture, and other factors, such as patients’ risk factors for psychosis. In the absence of a direct measure of N2O concentration in all these patients, most studies used indirect measures of B12 functioning – MMA and homocysteine. Some patients may have had pernicious anaemia, but this was measured (by intrinsic factor antibodies, IFB) in only three cases (and in one was positive). This would itself lead to B12 deficiency.
Treatment uncertainties
How should nitrous oxide misuse be treated?
There are no published treatment trials for nitrous oxide misuse, whether psychological therapies or medications. However, the multiple case reports described above lend credence to the importance of treating complications, i.e. functional vitamin B12 deficiency, which itself can cause devastating long-term effects, and which we outline in Fig. 4 and in the treatment recommendations Box 2.

FIG 4 Diagnosis and immediate intervention for suspected nitrous oxide misuse, using the diagnostic pentad of weakness, numbness, paraesthesia, psychosis and cognitive impairment.
BOX 2 Treatment recommendations for nitrous oxide misuse
• All psychiatric patients should be asked about nitrous oxide misuse. If they report use, they should be asked what form they take it (most likely whipped cream charger bulbs), how much they use, what effect it has on them, how they obtain it and whether they drive while intoxicated.
• Fully inform patients of the neuropsychiatric risks and their prevention.
• If nitrous oxide misuse suspected: do a full neurological symptom screen and examination, and take blood for B12 levels, plasma homocysteine and, ideally, methylmalonic acid.
• If neurological symptoms or signs are present, liaise directly with neurology.
• If neurological or psychiatric symptoms are present, initiate 1 mg hydroxocobalamin intramuscularly on alternate days (before blood results are back) until no further improvement and then once every 2 months.
• For empirical prevention of B12 deficiency, oral cyanocobalamin 250 µg daily may be used, although this is not thought to be as well absorbed as intramuscular hydroxocobalamin.
Atypical antipsychotics appear also to be helpful. A psychoeducational approach may be beneficial in terms of educating patients about the risk of harm from nitrous oxide, but has not been tested.
Does nitrous oxide merely unmask psychosis in vulnerable individuals?
Although this is possible, there have been many cases reported where patients appeared to have a low baseline risk of psychosis (e.g. Grigg Reference Grigg1988; Sethi Reference Sethi, Mullin and Torgovnick2006; Wong Reference Wong, Harrison and Mattman2014).
Does nitrous oxide misuse cause permanent cognitive impairment?
From the data overall this is not clear, although in the case that we describe at the beginning of this article and one other (Van Atta Reference Van Atta2004), this appeared to be so. Where B12 deficiency itself has caused mild cognitive impairment and dementia, there is evidence that individuals (not nitrous oxide users) with mild cognitive impairment improve with B12 treatment and those with dementia do not (Eastley Reference Eastley, Wilcock and Bucks2000).
Who is at risk of complications (e.g. psychosis or functional B12 deficiency) from nitrous oxide misuse?
Although higher frequency and heavy use of nitrous oxide inhalation is likely to be associated with greater risk of psychosis and B12 deficiency, individuals who are already vitamin B12 depleted, and those who have low-normal stores or other risk factors for B12 deficiency (e.g. are vegans) are at especially high risk of functional B12 deficiency and its complications. Other than individuals with generic risk factors for psychosis, individuals who also use ketamine along with nitrous oxide may be at particular risk of psychosis and this combined use has been shown to increase cortical damage in rodents (Jevtovic-Todorovic Reference Jevtovic-Todorovic, Benshoff and Olney2000).
Does nitrous oxide use cause dependence?
This is a controversial question and has been a topic of debate for a long time, with anecdotal evidence of psychological dependence (van Amsterdam Reference van Amsterdam, Nabben and van den Brink2015). There is also evidence of tolerance in animals and humans (Emmanouil Reference Emmanouil and Quock2007). However, there is no evidence of withdrawal effects.
Conclusions
Although there is likely to be a large number of occasional users of nitrous oxide who do not develop significant neuropsychiatric symptoms, it is clear from the case literature that repeated heavy use of nitrous oxide is very hazardous to health.
There is an urgent need for longitudinal prospective data and research interest in nitrous oxide misuse and its long-term psychiatric effects, as well as its treatment. We believe that psychiatrists, as the only medically trained members of the psychiatric multidisciplinary team, should take the lead in treating patients with psychiatric symptoms and nitrous oxide misuse, as its use may be becoming more widespread and adverse outcomes can be devastating.
Vitamin B12 replacement should be initiated with a low index of suspicion in patients where nitrous oxide misuse is suspected and especially in the presence of any symptom within the pentad of weakness, numbness, paraesthesia, psychosis and cognitive impairment, just as vitamin B1 (thiamine) is when Wernicke's encephalopathy is suspected. And will missing a case become just as medico-legally unacceptable?
MCQs
Select the single best option for each question stem
1 The mechanisms of action of nitrous oxide are best described as:
a endogenous opioid release; GABAA receptor activation; NMDA receptor antagonism; inactivation of B12
b endogenous opioid release; glutamate agonism; NMDA receptor agonism; inactivation of B12
c endogenous opioid antagonism; GABAA receptor activation; NMDA receptor antagonism; inactivation of B12
d inhibition of nitric oxide synthase; GABAA receptor antagonism; NMDA receptor agonism; inactivation of B12
e antagonism of BDNF receptor GSK-3β; GABAA receptor activation; NMDA receptor antagonism; inactivation of B12.
2 The most common psychiatric manifestations of nitrous oxide misuse are:
a depression and anxiety
b psychosis and cognitive impairment
c anxiety and personality change
d bipolar disorder
e cognitive impairment and depression.
3 A blood test battery used to demonstrate functional B12 deficiency is:
a Hb, MCV, B12
b MCV, B12, IFB
c MCV, clotting, B12
d FBC, serum NfL, immunoglobulins
e B12, HCY, MMA.
4 Patients with nitrous oxide misuse presenting with psychosis should initially receive:
a CBT for psychosis and antipsychotics
b antipsychotics and neurorehabilitation
c ketamine and neurorehabilitation
d electroconvulsive therapy ± antipsychotics
e intramuscular B12 replacement ± antipsychotics.
5 Nitrous oxide misuse is most likely among:
a males aged 16–24, attending nightclubs
b females aged 24–50, attending nightclubs
c males aged 60+, attending social clubs
d males and females aged 13–18, non-nightclubbers
e transgender people aged 24–50, attending nightclubs.
MCQ answers
1 a 2 b 3 e 4 e 5 a








eLetters
No eLetters have been published for this article.