Introduction
Land-use changes are driving biodiversity loss at unprecedented rates (Newbold et al. Reference Newbold2015). At coastal wetlands within tropical and subtropical latitudes, large areas occupied by mangroves and saltmarshes have been transformed to shrimp farming (Valiela et al. Reference Valiela, Kinney, Culbertson, Peacock, Smith and Duarte2009). These significant land-use changes have reduced habitats for migratory shorebird populations during the non-breeding season (e.g. Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014), both intertidal areas for foraging during low-tide and supratidal areas for roosting during high tide (Rogers et al. Reference Rogers, Piersma and Hassell2006). Migratory shorebirds are essential components of the biodiversity at coastal wetlands (Butchart et al. Reference Butchart2010), coupling ecosystem functioning across entire hemispheres (Bauer and Hoye Reference Bauer and Hoye2014). Despite this pivotal role, many shorebird populations are declining worldwide (Wetlands International 2012). As an example, shorebird populations have decreased on average by 70% across North America since 1973, with underlying causes still unclear (e.g. Munro Reference Munro2017). One reason for these declines may be the loss or alteration of habitats in non-breeding areas. Therefore, understanding how alteration of coastal wetlands associated with shrimp farms affects migratory shorebirds could help to mitigate population declines, and ultimately reduce biodiversity loss.
Wetlands modified by human activities for productive systems (such as salt works and aquaculture) can function as alternative foraging sites and thus may help buffer the adverse effect of the natural habitat loss for the conservation of waterbird populations (e.g. Sebastián-González and Green Reference Sebastián-González and Green2016). Recent studies in a tropical coastal lagoon showed that a single shrimp farm is used as an alternative foraging site by significant numbers of shorebirds during the non-breeding season (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a, Reference Navedo, Fernández, Valdivia, Drever and Masero2017). Hence, shrimp farms can have a similar function of other productive systems such as rice fields (Elphick Reference Elphick2000), salt ponds (Masero Reference Masero2003), coastal pastures (Navedo et al. Reference Navedo, Arranz, Herrera, Salmón, Juanes and Masero2013), or even other aquaculture systems (Walton et al. Reference Walton, Vilas, Coccia, Green, Cañavate, Prieto, van Bergeijk, Medialdea, Kennedy, King and Le Vay2015, Rocha et al. Reference Rocha, Ramos, Paredes and Masero2017). These systems can serve an essential role in the conservation of migratory shorebirds (Sánchez-Guzmán et al. Reference Sánchez-Guzmán, Morán, Masero, Corbacho, Costillo, Villegas and Santiago-Quesada2007, Sripanomyom et al. Reference Sripanomyom, Round, Savini, Trisurat and Gale2011, Marquez-Ferrando et al. Reference Marquez-Ferrando, Figuerola, Hooijmeijer and Piersma2015). However, the use of shrimp-farm ponds by shorebirds as foraging grounds can be limited to specific time-windows, i.e., 40 days during the harvesting season (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a, Reference Navedo, Fernández, Valdivia, Drever and Masero2017). Also, some studies suggest that waterbirds, especially shorebirds, may use artificial wetlands only when natural wetlands are unavailable or of poor quality (Ma et al. Reference Ma, Li, Zhao, Jing, Tang and Chen2004). Therefore, although shorebirds consistently forage at a shrimp-farm associated to a small coastal wetland (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a), the link between natural intertidal wetland availability and the use of shrimp farms as foraging or roosting grounds by non-breeding shorebirds is unclear (but see Basso et al. Reference Basso, Fonseca, Drever and Navedo2018). Evaluating the use of shrimp farms associated with critical natural coastal wetlands during the non-breeding season could be useful to integrate these modified areas within conservation planning and management of wetlands for shorebirds, particularly at tropical non-breeding sites where the information about their ecology is scarce (e.g. Fernández and Lank Reference Fernández and Lank2008).
The coast of Sinaloa (northwestern Mexico) is a crucial region for shorebirds during the non-breeding season (Engilis et al. Reference Engilis, Oring, Carrera, Nelson and López1998, Morrison and Ross Reference Morrison and Ross2009). Numerous coastal wetlands in the region have been classified as important for shorebirds within the Western Hemisphere Shorebird Reserve Network (WHSRN), a non-regulatory network of public and private partners established in 1985 to protect the most important breeding, stopover, and non-breeding sites for shorebirds throughout the Americas. Among them, Bahía Santa María (BSM) stands out as both a WHSRN Site of Hemispheric Importance and a Ramsar site. Despite its crucial role for conservation, several landscape changes on the coast of Sinaloa have occurred during the last decades, with the development of agriculture and aquaculture being the most important (de la Fuente and Carrera Reference de la Fuente and Carrera2005, Berlanga-Robles et al. Reference Berlanga-Robles, Ruiz-Luna, Bocco and Vekerdy2011). There are 7,117 hectares dedicated to semi-intensive shrimp-farming within BSM (CESASIN 2016).
Here we assessed the shorebird assemblage, habitat use, and abundances of Nearctic migratory shorebirds using shrimp farms in Angostura Municipality within BSM. We also estimated shorebird densities at intertidal areas within BSM to assess whether shorebird habitat use differed during and after the shrimp-harvesting season. Our objectives were to (1) estimate the minimum population size of each shorebird species using the studied shrimp farms; (2) determine whether shorebirds use shrimp-farms situated within vast coastal wetlands as foraging and/or roosting habitat; and (3) compare the shorebird assemblages on intertidal units during and after the harvesting season at shrimp farms. We conclude by evaluating the potential importance of these human-modified habitats to support Nearctic shorebird populations. These results form a critical part of the ecological understanding necessary to inform useful inclusion of shrimp-farms into management plans for the conservation of Nearctic shorebirds (e.g. Senner et al. Reference Senner, Andres and Gates2016), which is necessitated by their extensive use of these human-created wetlands.
Methods
Study area
BSM (25°02’N, 108°18’W) is about 90 km northwest of Culiacán City in northwestern Mexico. BSM is the largest wetland on the Sinaloa coast. The bay has two main channels to the ocean and is composed of 1,350 km2 of a diverse mosaic of habitats, including an outer bay, intertidal mudflats, mangroves, brackish flats, emergent brackish marshes, and freshwater marshes (Serrano et al. Reference Serrano, Ramírez-Félix and Valle-Levison2013). Over 380,000 individuals of 24 shorebird species were estimated during the winter at BSM, and they are widely distributed among the mosaic of habitats (Engilis et al. Reference Engilis, Oring, Carrera, Nelson and López1998). The most abundant species were Western Sandpiper Calidris mauri, dowitchers Limnodromus spp., Willet Tringa semipalmata, and American Avocet Recurvirostra americana, common shorebird species on the Pacific Coast (Page and Gill Reference Page and Gill1994). Shrimp aquaculture at BSM, similar to many other sites in northwestern Mexico, is semi-intensive, with rustic shrimp ponds that are sequentially harvested by emptying the water, usually one pond at a time. Following harvesting, ponds become available for shorebirds to forage (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a). However, ponds are only available for use by foraging shorebirds for a few days because the substrate quickly dries as harvest gates are sealed to prevent the ponds being flooded during high tide (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a, Reference Navedo, Fernández, Valdivia, Drever and Masero2017). Most of the shrimp farms were built on saltmarshes or brackish flats, while a few were constructed on mangrove areas (Berlanga-Robles et al. Reference Berlanga-Robles, Ruiz-Luna, Bocco and Vekerdy2011). The timing of harvest varies widely among shrimp farms, and factors such as size and price of shrimp, as well as sanitary conditions, influence the timing and the speed at which the harvest season progresses within and among shrimp farms.
Shorebird surveys
We conducted shorebird surveys at selected shrimp farms to determine species composition, shorebird abundance, and habitat use (foraging or roosting) on these human-modified habitats. We also conducted shorebird surveys at sample units within intertidal mudflats of the BSM to explore the connectivity of natural habitats and shrimp-farms for shorebirds during the non-breeding season.
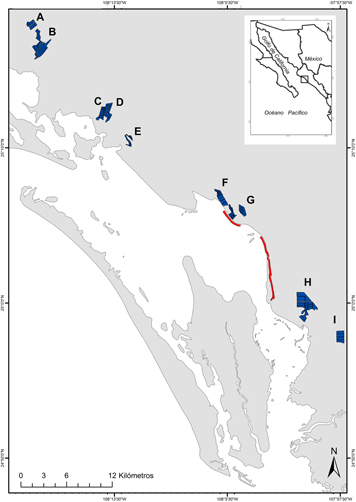
We conducted fieldwork during two shrimp-harvesting seasons in 2014–2015 (September–February) and 2015–2016 (end of August–January) in the Municipality of Angostura, the northern portion of BSM, which has an area of 3,101 ha of shrimp ponds (CESASIN 2016). During the 2014–2015 season, we visited 10 shrimp farms with 103 ponds, covering 994 ha. During the 2015–2016 season, we visited eight shrimp farms, six of the previously-surveyed farms and two others, with 86 ponds covering 924 ha (Figure 1). Although focal shrimp farms were not randomly selected, we assumed that they were a representative sample because they were independently owned, of different sizes (see below), and were scattered across the municipality. Focal shrimp farms were visited only when each owner allowed us to conduct the shorebird surveys. This agreement did not imply any commitment to change management practices. The harvesting cycle was overall completed by the end of November in the first season but was extended up to the end of December in the second season, when some farms extended shrimp growth following market decisions. By 1 January all ponds have to be harvested based on a sanitary regulation (CESASIN 2016). The surveyed shrimp farms were located throughout the study area, very close to the adjacent intertidal flats (0.11 ± 0.08 km; range: 0.03–0.31 km; Figure 1). The total area, size and number of ponds were variable among focal shrimp farms (total area: 145.0 ± 104.5 ha, range: 43–371.8 ha; pond size: 13.5 ± 14.4 ha, range: 3.1–49.9 ha; number of ponds: 14 ± 6 ponds, range: 4–23 ponds). As mentioned above, each shrimp farm independently decided when to start harvesting the shrimp ponds.

Figure 1. Location of selected shrimp farms (polygons A-I) at the Angostura Municipality, Bahía Santa María, Sinaloa (Northwestern Mexico), during the harvest season of 2014–2015 and 2015–2016, and surveyed intertidal units (lines). Focal shrimp farms were: A) Maricultura SA de CV; B) Baturi Acuícola SA de CV, Agropecuaria Osli SA de CV, and Palmitas de Angostura SPR de RI; C) Acuícola MV SA de CV; D) SCPPA El Playón del Esterón SCL de CV; E) SCPPA La Ensenada SCL de CV; F) Acuícola Rosarito SA de CV; G) SCPA Acuícola El Botetero SC de CV; H) Acuícola Visión SC de RL de CV, and Acuícola Camarones del Pacífico SC de RL de CV; and I) Granja Las Bocas SC de RL de CV.
When each shrimp farm harvested the first shrimp pond, we started the shorebird surveys and then we systematically visited the shrimp farm every 1–2 weeks, thus accounting for the variable availability of intertidal habitats associated with moon phases (Calle et al. Reference Calle, Gawlik, Xie, Green, Lapointe and Strong2016; Basso et al. Reference Basso, Fonseca, Drever and Navedo2018). Each farm was visited at least five times throughout the season before all harvested ponds were dried. All focal shrimp farms were visited within a maximum of four consecutive days because there were 1–3 two-people teams with a field vehicle to conduct shorebird surveys. Each two-people team visited a different shrimp farm per day, and they covered all ponds and surveyed all shorebirds with binoculars (10x) or spotting scopes (20–60x). Each pond on focal shrimp-farms was surveyed at both low and high tide to explore the ecological function of the ponds for shorebirds (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a). We conducted shorebird surveys within the three hours of each tidal peak, i.e. from 1.5 hours before to 1.5 hours after either low or high tide peak. All shorebirds were identified to species, except for the Short-billed Dowitcher Limnodromus griseus and Long-billed Dowitcher L. scolopaceus, which could not be reliably distinguished in the field; all counts were combined into one dowitcher group. We conducted focal observations on individual birds and quantified the proportion of all birds counted that were actively feeding. If the activity of an individual could not be determined instantaneously (e.g. a bird with its back to the spotting scope), the individual was observed for 1–5 s to determine its foraging activity (Navedo and Masero Reference Navedo and Masero2007).
We conducted concurrent shorebird surveys at five sample units at intertidal mudflats in the BSM (Figure 1) in November (during the shrimp harvesting cycle) and January (after the shrimp harvesting cycle) in both 2014–2015 and 2015–2016. In all cases, we surveyed the same sampling units. The sample units in the intertidal zone were established as discrete units with similar habitat (mudflats) of a relatively small size (total: 230.0 ha; average: 46.0 ± 16.4 ha; range: 26.1–68.1 ha) that can be covered in less than 20 minutes with an airboat. These sample units were not randomly selected due to logistical constraints, but we assumed they represented shorebird abundances within the most critical habitats for migratory shorebirds at BSM (Engilis et al. Reference Engilis, Oring, Carrera, Nelson and López1998). Using a standardized protocol, we searched the area with the help of an airboat to estimate all shorebirds in each sample unit during a falling spring tide (from 4 hrs after high tide). To minimize potential differences in overall intertidal habitat availability, we covered all sample units in two days during spring tide periods (three sample units one day and the other two sample units the next day). The survey team was comprised of a crew member to operate the boat and four trained observers using 10x binoculars. Observers were the same as those surveying shorebirds at the shrimp farms. The airboat travelled at a constant speed (∼ 15 km/hr) parallel to the mangroves. The survey area was limited to a transect 200–300 m wide, the mudflat available between the mangrove and the airboat. Because it was not possible to count each shorebird, we identified to species level (except Limnodromus; see above), and numbers of shorebirds were determined by direct counts or by flock estimations when larger concentrations were encountered. Although we did not account for detectability of birds on shrimp farms or intertidal areas, we assume full detectability due to the openness of the habitat.
Analyses
To determine shorebird species composition and abundance across the harvesting season, we summed the counts for all ponds from each 2–4 consecutive day count at each shrimp farm at low tide and high tide for each of the 15 survey periods. We used these figures to obtain an overall estimate of the use of the shrimp farms from September to January. Furthermore, we considered the maximum count of each species during a single 2–4 consecutive day count as the minimum population abundance using the shrimp farms at each season. To provide a biogeographic context of population size of birds using the shrimp farms, we averaged maximum counts over both seasons to obtain an estimate of the population of each species using the studied shrimp farms at BSM and then divided by the total population size estimates provided in Andres et al. (Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012).
We tested for differences in overall abundance of each species at shrimp farms between years by using Mann-Whitney tests. To investigate differences among shorebirds in abundance and activity at the shrimp farms during high and low tides, we used Wilcoxon matched-pair tests for each species. We conducted these analyses for the most common shorebird species at the shrimp farms: American Avocet, Black-necked Stilt Himantopus mexicanus, Marbled Godwit Limosa fedoa, dowitchers, Western Sandpiper, and Willet. These species represented over 85% of the total shorebird abundance. A General Linear Mixed Effects Model with period (during and after the harvesting cycle) and season (2014–2015 and 2015–2016) as fixed factors and intertidal unit (n = 5) as the random factor was used to test for differences in shorebird density among intertidal areas. All values are presented as means ± SE.
Results
A total of 25 shorebird species were observed at the surveyed shrimp farms (Table 1). Although there were some differences in species abundances, the shorebird assemblage was similar among shrimp farms across both seasons but dropped off earlier in 2014–2015 than in 2015–2016 (late November vs. early January) (Figure 2). Shorebird abundance was relatively higher at the beginning of the harvesting season (September–October) and then decreased as the season progressed (Figure 2). However, Western Sandpiper, which was the most abundant species, showed the maximum peak in late December 2015, accounting also for the maximum shorebird abundance during the study period, which was 20,773 shorebirds (93% Western Sandpiper) (Figure 2). Overall, the number of shorebirds using the surveyed shrimp farms during the 2015–2016 season was higher than in the 2014–2015 season. Specifically, Western Sandpiper (Z = -2.21; P < 0.027), dowitchers (Z = -2.31; P < 0.020), and American Avocet (Z = -2.11; P < 0.035) had higher numbers in 2015–2016 than in 2014–2015. For Marbled Godwit, Willet and Black-necked Stilt, abundance was similar between two seasons (P > 0.18 in all cases).
Table 1. Categories of conservation concern (USSCPP 2016) and maximum number of the most frequent migratory shorebird species counted at focal shrimp farms (c.1,000 ha) of Angostura Municipality, Bahía Santa María, Sinaloa, during the shrimp harvest seasons of 2014–2015 and 2015–2016, and estimated percentage of the biogeographic populations (based on an average maximum count between the two harvest seasons) based on Andres et al. (Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012). In bold shorebird species for which surveyed shrimp farms support more than 0.35% of their biogeographic populations.

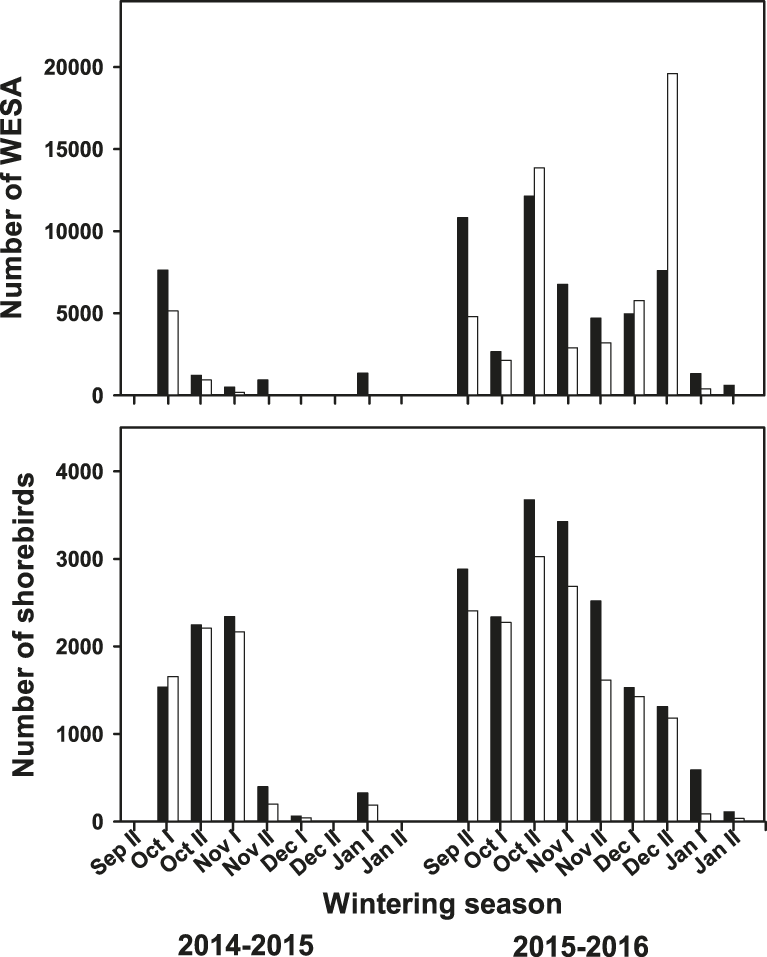
Figure 2. Total number of Western Sandpipers (WESA, upper panel) and other shorebird species (lower panel) counted during low- (white bars) and high- (black bars) tide at the focal shrimp farms in Angostura Municipality, Bahía Santa María, Sinaloa, within the same week throughout the study period during the 2014–2015 and 2015–2016 harvest seasons.
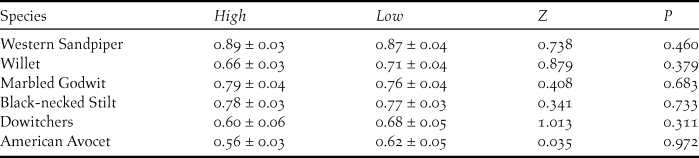
During both shrimp harvesting seasons, the average maximum abundance of shorebirds at the surveyed shrimp farms resulted in important fractions of the biogeographic populations for Willet (0.61%), Marbled Godwit (0.51%), Western Sandpiper (0.39%), and Red Knot Calidris canutus (0.41%) (Table 1). There was a slight increase in shorebird abundance at shrimp farms during high tide with respect to low-tide period, due to significant differences for Western Sandpiper (Z = 1.96; P < 0.05), Willet (Z = 2.67; P < 0.01), and nearly significant for Marbled Godwit (Z = 1.70; P = 0.08) (Fig. 3). Finally, a large and similar proportion of shorebirds were observed foraging during both high tide (71.9 ± 2.0%; n = 94) and low tide (74.0 ± 1.9%; n = 91) (Table 2), with no significant differences in abundances for any species (P > 0.31 in all cases; Figure 3).

Figure 3. Compared number of individuals (means ± SE) of the most abundant shorebird species during high (black bars) and low tide (white-bars) periods at the focal shrimp farms in Angostura Municipality, Bahía Santa María, Sinaloa, within the same week during the harvesting season. Species: Western Sandpiper (WESA), Willet (WILL), Marbled Godwit (MAGO), Black-necked Silt (BNST), dowitchers (UNDO), and American Avocet (AMAV). Note that WESA abundance was divided by 10 for representation purposes. * = P < 0.05; ** = P < 0.01.
Table 2. Proportion of foraging birds (means ± SE) of most abundant shorebirds counted during low and high tide at the focal shrimp farms of Angostura Municipality, Bahía Santa María, Sinaloa, during the harvest seasons of 2014–2015 and 2015–2016.
At the intertidal sample units, we detected 16 shorebird species, all of which were present at shrimp farms, with the same six species showing the highest densities in both natural and anthropogenic habitats. There were high variability and species-specific differences among the five surveyed units for Marbled Godwit and dowitcher densities (Table 3) but did not differ between the two periods (during and after the harvesting period of shrimp farms) (Figure 4) nor between study seasons (Table 3).
Table 3. Results of GLMs showing effects of (fixed factors) period (during and after harvesting season), season (2014–2015 and 2015–2016) and its interaction (P x S), at five different (random factor) intertidal units, on densities of the most abundant shorebird species at Bahía Santa María, Sinaloa.
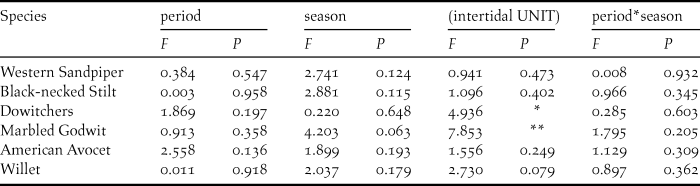

Figure 4. Average density (ind·ha-1) of the most abundant shorebird species recorded during surveys at intertidal units at Bahía Santa María, Sinaloa, in November (during the harvesting season: black bars), and January (after the harvesting season: white bars) of 2014–2015 and 2015–2016 non-breeding seasons. Species: Western Sandpiper (WESA), Willet (WILL), Marbled Godwit (MAGO), Black-necked Stilt (BNST), dowitchers (UNDO), and American Avocet (AMAV). Note that WESA abundance was divided by 10 for representation purposes.
Discussion
On survey days during the harvesting season, shrimp farms at BSM were regularly used by the entire shorebird assemblage found in northwestern Mexico during the non-breeding season (Page et al. Reference Page, Palacios, Alfaro, Gonzalez, Stenzel and Jungers1997, Morrison and Ross Reference Morrison and Ross2009). Because significant landscape changes have occurred since 1993–1994 (Berlanga-Robles et al. Reference Berlanga-Robles, Ruiz-Luna, Bocco and Vekerdy2011), when the last published information about shorebird populations at this area was recorded (Engilis et al. Reference Engilis, Oring, Carrera, Nelson and López1998), we were unable to make comparisons between these time periods. Nonetheless, on the assumption that abundances at BSM will be not higher nowadays due to overall declining trends of Nearctic migratory shorebird populations (Andres et al. Reference Andres, Smith, Morrison, Gratto-Trevor, Brown and Friis2012), a significant proportion of non-breeding shorebirds at BSM might use shrimp aquaculture ponds during the harvesting season. Abundance at recently harvested ponds was overall similar during low and high tide periods, but increased during high tide for the most abundant species, presumably suggesting that at least some individuals selected shrimp farms when available. However, shorebird abundance decreased significantly after the harvesting season within the shrimp farms, most probably because foraging areas became unavailable due to pond drying (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a, Reference Navedo, Fernández, Valdivia, Drever and Masero2017). In support of this, the year in which some farms delayed pond harvesting, overall shorebird abundance within farms dropped off one month later. Moreover, the primary activity of shorebirds using the ponds was foraging, irrespective of the daily tidal cycle (i.e. average foraging activity 73.0 ± 2.4%). Therefore, our results provide the first evidence for the ephemeral but consistent use of shrimp-farm supratidal habitats by foraging shorebirds through the harvesting season at the landscape level, a similar pattern of use during the non-breeding periods described for shorebirds at other anthropogenic wetlands (Masero Reference Masero2002, Kloskowski et al. Reference Kloskowski, Green, Polak, Bustamante and Krogulec2009).
We did not observe differences in shorebird densities at the intertidal mudflats between surveys conducted during and after the shrimp-harvesting season. This similar shorebird density contrasts with the pattern observed in the Estero de Urías, a smaller coastal wetland located south of BSM, where shorebird numbers over the entire wetland decreased sharply after the shrimp farm was harvested (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a). A possible explanation is that anthropogenic habitats can offer significant additional trophic resources for non-breeding shorebird populations where intertidal foraging areas are restricted (Basso et al. Reference Basso, Fonseca, Drever and Navedo2018), thus temporally increasing current carrying capacity at small coastal wetlands such as Estero de Urías (Fonseca et al. Reference Fonseca, Basso, Serrano and Navedo2017). By contrast, at large bays such as BSM, most individuals use the natural intertidal areas as foraging grounds, but some fraction of the population of Nearctic shorebirds may shift to a different habitat/resource when it becomes available during the non-breeding season. For example, the relative abundance of Willets at intertidal sample units was much lower than at shrimp farms at BSM and Estero de Urías (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a), where it is one of the most frequent and abundant species. Willets show high behavioural plasticity, being able to exploit very different food resources (Lowther et al. Reference Lowther, Douglas, Gratto-Trevor, Poole and Gill2001) and may prefer to forage within recently harvested ponds at shrimp farms when they are available. In addition, low-competitive individuals of different shorebird species not able to thrive within the best intertidal foraging areas in the wetland (e.g. Navedo et al. Reference Navedo, Sauma-Castillo and Fernández2012a) might be displaced to use shrimp-farms when available. Once the harvesting finishes, these birds may explore other (not surveyed) intertidal areas, or other natural habitats, such as saltmarshes that are available at BSM (Berlanga-Robles et al. Reference Berlanga-Robles, Ruiz-Luna, Bocco and Vekerdy2011).
During two consecutive seasons, we surveyed 994 ha and 923 ha of shrimp ponds, respectively. These areas represent 30–32% of the total area of shrimp aquaculture ponds available within the Angostura Municipality, and 13% of the total semi-intensive aquaculture shrimp-ponds at BSM. If the results presented in Table 1 are extrapolated to the whole BSM region, during the harvesting season shrimp farms would support an internationally significant (over 1% of the biogeographic population) fraction of Willet, Marbled Godwit, Western Sandpiper, and Red Knot, but probably also for Black-necked Stilt, Semipalmated Plover Charadrius semipalmatus, and Snowy Plover Charadrius nivosus. Four of these shorebird species (see Table 1) have been recently listed (at least) as of high conservation concern in USA (USSCPP 2016). Our results are similar to those of previous studies developed at a single shrimp farm (Navedo et al. Reference Navedo, Fernández, Fonseca and Drever2015a), as well as those from other fish-farm aquaculture (Walton et al. Reference Walton, Vilas, Coccia, Green, Cañavate, Prieto, van Bergeijk, Medialdea, Kennedy, King and Le Vay2015). This highlights the potential role of currently modified habitats dedicated to semi-intensive shrimp farming as alternative foraging grounds for shorebird populations during the non-breeding season.
Conservation implications
Shrimp-farming has been one of the primary sources of wetland habitat loss and degradation at coastal tropical areas during recent decades (Páez-Osuna et al. Reference Páez-Osuna, Gracia, Flores-Verdugo, Lyle-Fritch, Alonso-Rodriguez, Roque and Ruiz-Fernández2003, Valiela et al. Reference Valiela, Kinney, Culbertson, Peacock, Smith and Duarte2009), reducing ecosystem processes and ecological resilience (Cumming et al. Reference Cumming, Barnes, Perz, Schmink, Sieving, Southworth, Binford, Holt, Stickler and van Holt2005). Indeed, land-use changes have significantly contributed to the overall decline in migratory shorebird populations (Wetlands International 2012), especially within the East Asian-Australasian Flyway (Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014). Artificial wetlands cannot adequately compensate natural habitat loss for the conservation of waterbird populations (Sebastián-González and Green Reference Sebastián-González and Green2016). Therefore, we do not advocate transforming natural habitats such as brackish flats or saltmarshes, the primary habitat where shrimp farms were established in northwestern Mexico (Berlanga-Robles et al. Reference Berlanga-Robles, Ruiz-Luna, Bocco and Vekerdy2011), into new shrimp farms. This landscape change will result in the fragmentation of wetlands and reduce ecosystem resilience (Cumming et al. Reference Cumming, Barnes, Perz, Schmink, Sieving, Southworth, Binford, Holt, Stickler and van Holt2005). Similar to studies at other anthropogenic wetlands (e.g. Navedo et al. Reference Navedo, Masero, Sánchez-Guzmán, Abad-Gómez, Gutiérrez, Sansón, Villegas, Costillo, Corbacho and Morán2012b, Reference Navedo, Arranz, Herrera, Salmón, Juanes and Masero2013), the following recommendations are intended to apply only to existing shrimp farms.
BSM is classified as Site of a Hemispheric Importance for the conservation of shorebirds within the WHSRN, and another nearby critical wetland, Ensenada Pabellones, is classified as a Site of International Importance. Similar to other anthropogenic wetlands (Navedo et al. Reference Navedo, Hahn, Parejo, Abad-Gómez, Gutiérrez, Villegas, Sánchez-Guzmán and Masero2015b, Walton et al. Reference Walton, Vilas, Coccia, Green, Cañavate, Prieto, van Bergeijk, Medialdea, Kennedy, King and Le Vay2015), during harvest season shrimp farms can provide an ephemeral but crucial trophic resource to different shorebird species and other waterbirds such as egrets and herons (Cheek Reference Cheek2009, J.G. Navedo and G. Fernández pers. obs.), mainly because available foraging habitats within shrimp farms are not restricted by tidal periods. Also, though not yet studied, these supratidal ponds could also be used by shorebirds as roosting areas during periods outside of the harvesting season. Therefore, we recommend including current areas dedicated to semi-intensive shrimp farming in Sinaloa (over 25,000 ha; CESASIN 2016) within management plans of the WHSRN Sites (see Morrison and Ross Reference Morrison and Ross2009), since they could play an essential role in the conservation of Nearctic migratory shorebirds.
Shrimp farm owners involved are aware of the migratory shorebirds spending the non-breeding season at BSM and the importance of improving shrimp farm practices to favour alternative foraging habitat for this group of birds. Further work is needed to identify opportunities (e.g. by increasing net value of the product; Athearn et al. Reference Athearn, Takekawa, Bluso-Demers, Shinn, Brand, Robinson-Nilsen and Strong2012, Green et al. Reference Green, Sripanomyom, Giam and Wilcove2015) and to develop essential guidelines to improve ‘nature-kind’ shrimp farm practices that favour shorebirds at coastal wetlands during the non-breeding season. These practices can include measures to retain moisture of the substrate by providing some water supply to ponds after harvest (Navedo et al. Reference Navedo, Fernández, Valdivia, Drever and Masero2017). As an additional recommendation, external entities (government, NGOs) could ‘rent’ habitat from farmers on a seasonal basis and pay them to provide habitat during critical periods, similar to other landscape-scale conservation initiatives, such as the ‘Bird Returns’ programme (Reynolds et al. Reference Reynolds2017). These actions will integrate shorebird conservation into sustainable shrimp-farm management (Jones et al. Reference Jones2015). Last but not least, applying the precautionary approach (Cooney Reference Cooney2004) when managing habitats for the conservation of threatened resources, it should be first mandatory (i) to evaluate land-use changes resulting from shrimp-farm aquaculture at BSM against the spatial complexity and connectivity of the wetland landscape; and (ii) to determine the fitness consequences for migratory individuals using these anthropogenic wetlands as alternative foraging grounds (e.g. the amount of heavy metals and other pollutants potentially incorporated into bird tissues).
Acknowledgements
This study has been developed thanks to funding from David and Lucille Packard Foundation (Grant 2014-40252) to the Fondo Mexicano para la Conservación de la Naturaleza AC and administrated by FONNOR AC (M-1409-003). Other support for shorebird surveys at the intertidal mudflats was provided by the US Forest Service International Programs and the Mexican National Council for Science and Technology (CB2010-155353). The authors are sincerely indebted to the shrimp farm owners of Maricultura SA de CV, Baturi Acuícola SA de CV, Agropecuaria Osli SA de CV, Palmitas de Angostura SPR de RI, Acuícola MV SA de CV, SCPPA El Playón del Esterón SCL de CV, SCPPA La Ensenada SCL de CV, SCPA Acuícola El Botetero SC de CV, Acuícola Visión SC de RL de CV, Acuícola Camarones del Pacífico SC de RL de CV, Granjas Las Bocas SC de RL de CV, and Acuícola Rosarito SA de CV for granting access to their farms to study shorebirds. In addition, we thank Felipe Ruiz, Adrián Castro, Jesús Castro, David Sánchez, and all shrimp-farm owners of the Acuicultores de Angostura AC for their willingness to collaborate with this study. We also thank Rafael Valdéz and several field assistants that help to conduct the shorebird surveys, and Verónica Palacios who helped with the study area map. Mark Drever helped with final language edition. JGN was supported during writing by FONDECYT grant #1161224 (Gobierno de Chile).