Introduction
The Tooth-billed Pigeon Didunculus strigirostris, locally known as “Manumea” (MNRE 2006), is endemic to Samoa and is evolutionarily distinctive on a global level (Jetz et al. 2014). It has been assessed as ‘Critically Endangered’ (CR) on the IUCN Red List since 2014 (BirdLife International 2015). Ecological and behavioural knowledge about this bird is scarce and mostly anecdotal (Beichle Reference Beichle1982a,Reference Beichleb, Reference Beichle1987a,Reference Beichleb, 1989). All available information on Manumea was recently reviewed by Collar (Reference Collar2015).
The demise of Manumea was brought to the attention of the conservation community by Beichle (Reference Beichle2006) who reported that only a “few hundred” birds survived at the time, implying a 90% drop in numbers since the mid-1980s (Stattersfield and Capper Reference Stattersfield and Capper2000, Beichle and Baumann Reference Beichle and Baumann2016). The bird has been listed as ‘Endangered’ since the year 2000 (BirdLife International 2015) and a recovery plan was written and approved for implementation in 2006 (MNRE 2006).
The perilous status of Manumea was confirmed by a survey in 2012 of the bird’s presumed stronghold in the remote uplands of Samoa’s largest island, Savaii, in which no birds were observed (Butler Reference Butler, Atherton and Jefferies2012). This prompted the reclassification of its conservation status in 2014 to ‘Critically Endangered’. Despite the existence of the recovery plan, very few of its recommendations have been implemented since it was approved (Ulf Beichle pers. comm., Serra Reference Serra2017). The situation was summarised by Collar (Reference Collar2015) who stated “The great difficulty throughout this century has simply been to find even a single representative of the species […]” while Pratt and Mittermeier (Reference Pratt and Mittermeier2016) state that Manumea has turned into “an immediate conservation priority” for Samoa.
This paper reports on part of a biodiversity survey run between October 2015 and June 2016 funded by the Global Environment Facility Trust Fund (Serra Reference Serra2016). One of the objectives of the survey programme was to systematically and formally record traditional ecological knowledge (TEK) at village level (Sinclair et al. 2010, Berkes Reference Berkes2012, Wilder et al. 2016), especially information relating to rare and threatened fauna, including Manumea.
TEK often goes unrecorded and can be considered as only “anecdotal information” in scientific zoological literature. However, the “observational value” of TEK was highlighted by Sinclair et al. (2010) while its relevance in relation to detecting rare birds was described by Serra et al. (2004) and Blair (Reference Blair2005). Sourcing this type of information requires spending long periods of time establishing a trusting and viable working relationship with indigenous holders of TEK. By contrast, modern “rapid” survey methods such as the BIORAP (Conservation International 2016), based on a scientific ecological knowledge (SEK) approach, seem to be not ideal for the detection and hence assessment of the status of rare and elusive fauna (Powell Reference Powell2008).
This paper contributes to the forthcoming review and update of the Manumea Recovery Plan 2006–2016 (Serra Reference Serra2017), especially in terms of assessing the bird’s current conservation status and the hunting threat. It also identifies forest areas where it is still extant and provides information on its behavioural ecology, and on the hunting practice at village level.
Methods
Study area
The study areas included four of the eight designated terrestrial Key Biodiversity Areas (KBAs) of Samoa (Conservation International 2010): Uafato-Tiavea Coastal Forest (UTCF) and Apia Catchments (AC) on Upolu island (Figure 1); Central Savaii Rainforest (CSR) and Falealupo Peninsula (FP) on Savaii island (Figure 2).
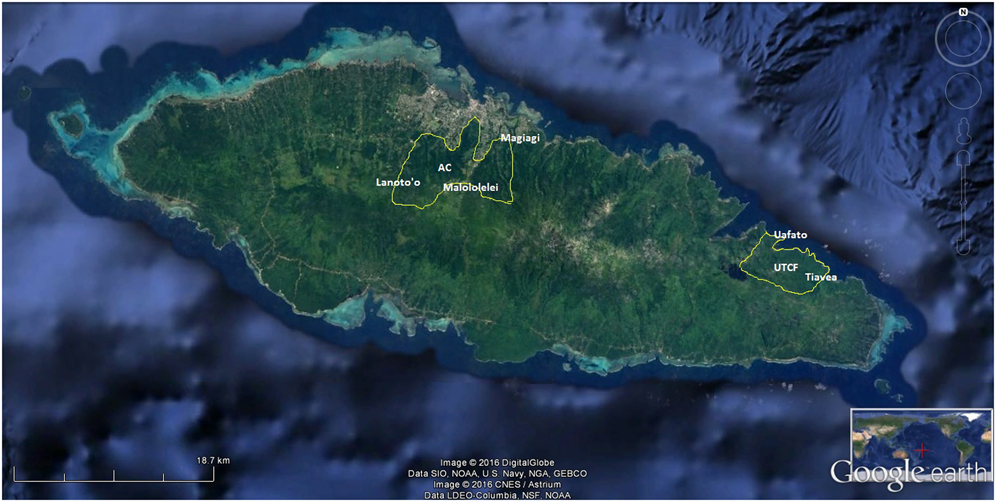
Figure 1. Villages sampled in the two terrestrial KBAs of Upolu island (borders in white): Apia Catchments (AC) and Uafato-Tiavea Coastal Forest (UTCF). Image courtesy of Google Earth.
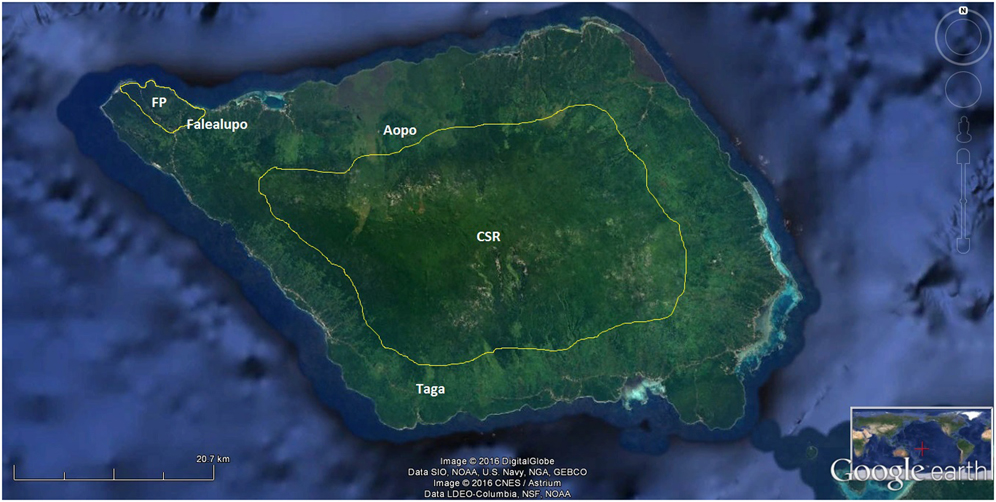
Figure 2. Villages sampled in the two terrestrial KBAs of Savaii island (borders in white): Central Savaii Rainforest (CSR) and Falealupo Peninsula (FP). Image courtesy of Google Earth.
Traditional Ecological Knowledge from standardised interviews
We discussed and designed a standard questionnaire aimed at interviewing villagers with experience of the natural environment to gather verifiable TEK relating to native biodiversity found in their ancestral forests (Annex S1). The respondents were not randomly selected and were considered by the village council of elders as “the most knowledgeable about native biodiversity and sincere”. We followed recommendations on how to best design the questionnaire in order to minimise response biases (White et al. 2005). We did not offer remuneration for participating, albeit past practices with earlier international projects may have raised expectations.
Special care was used to test the ability of interviewees to identify birds, the most biologically diversified group targeted by hunters in Samoa, in an attempt to verify the credibility of their replies to the questionnaire. This was done using five visual and four audio tests that were integrated within the questionnaire. First, visual and audio tests involved birds very different to each other to test the identification skills between different families. The questionnaire was designed to quickly identify within the first 2–3 questions those providing biased responses, or those with inadequate knowledge.
The second round of tests assessed identification skills between bird species within the same family, e.g. playing the recorded calls of the three sympatric pigeons which occur in rainforest habitats in Samoa – Manumea (recorded call provided by Ulf Beichle), Pacific Pigeon Ducula pacifica, known locally as “Lupe”, and White-throated Pigeon Columba vitiensis.
The questionnaire was in Samoan. The following approach was used to avoid interviewers influencing the responses by interviewees. Psychologically (Kalton and Schuman Reference Kalton and Schuman1982, Podsakoff et al. 2003) and culturally sound (Grattan Reference Grattan1985) questions were designed to be directed to hunters so that they were neutral (not “leading”) and did not signal what an answer “should be” (Annex S1). The aim was to obtain objective detailed anatomical, ecological and behavioural descriptions from interviewees while carefully avoiding any influence through presenting them with colour plates or referring to local names.
The reliability and skills of each interviewee were independently assessed by three interviewers by scoring replies on a scale of 1 (minimum) to 10. The focus of these assessments was to ensure interviewees could consistently and accurately distinguish between the different pigeon species and to gauge the quality of other information the interviewees provided.
Impressions about the general attitude of the interviewee (for instance the degree of self-confidence and assertiveness) were also considered when the scores were assigned. A short-list of experienced and skilled hunters was finalised. An interviewee scoring ≥ 6 was considered sufficiently experienced and skilled to become a reliable source for acquiring TEK of native biodiversity (hereafter referred to as “reliable hunter”).
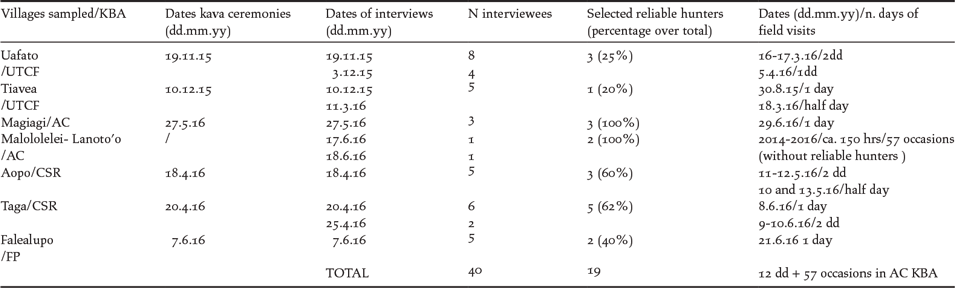
Our survey was designed to sample two representative forest areas for each KBA using the TEK approach. A GIS map with updated forest cover and village layers was used to select two villages adjacent to extensive primary and secondary forest areas in the four target KBAs. Customary protocols (Grattan Reference Grattan1985) were followed before requesting interviews with the most senior hunters of the village. In total 40 hunters from seven villages located within four KBAs were interviewed and their bird identification skills assessed (Table 1).
Table 1. Dates and figures relative to the villages sampled, the reliable hunters selected and the forest field visits. The kava ceremony in the presence of the village council is a ritual traditional protocol that is required in order to establish a viable working relationship. For the definition of “reliable hunters” see Methods.
TEK and SEK from field observations
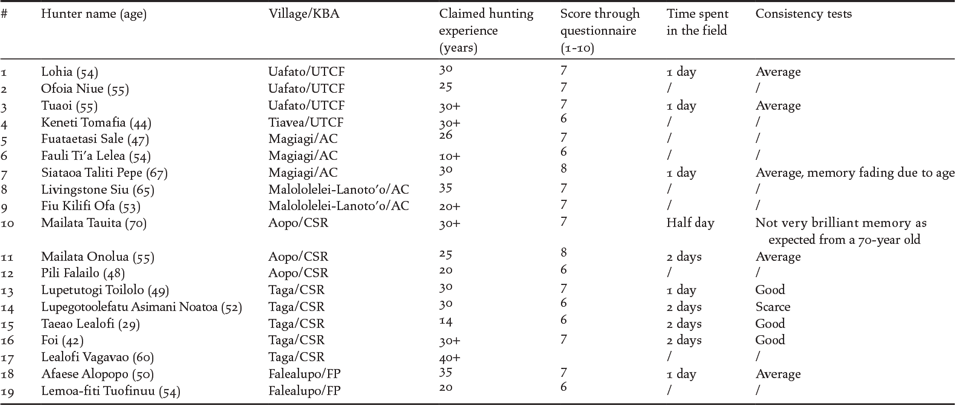
Based on performance with the identification tests we selected a subset of hunters who were regarded as the most reliable sources of information (Table 2). From this subset a further selection of one or two reliable hunters were identified from each village. These hunters were then asked to lead observations over one or two days in their ancestral forests (Table 1).
Table 2. Details of the 19 hunters assessed as reliable. For the definition of “reliable hunters” refer to Methods.
During these field visits, all TEK about rare native species was recorded based on informal unhurried discussions. Any key information gathered during previous interviews and from questionnaires was verified and “ground-truthed” as far as feasible (see “consistency”, Table 2). At the end of both the interviews and the field visits we thanked the hunters for sharing their knowledge and we reassured them it would be always credited to them and acknowledged in presentations, reports and publications.
The field visits were documented photographically and all observations included GPS grid references. Field identification of Manumea calls was attempted through a combination of different approaches within the Upolu and Savaii KBAs:
- Mixed TEK-SEK approach in Uafato forest (UTCF KBA). Two automatic audio recorders (Wildlife Acoustics SM3) were set up in locations in the Uafato forest which were recommended by reliable hunters (dates and criteria detailed in Table 3) and left running for 7–15 days with the aim of recording the “coo” calls of pigeons (sensu Beichle Reference Beichle1991). A total of 297 hours of recorded sounds were analysed using the program “Song Scope” (Wildlife Acoustics 2016). The coo calls detected from the recordings were then sent to Ulf Beichle and Sabine Baumann by the Ministry of Natural Resources and Environment (MNRE) for identification.
- TEK approach in Aopo and Taga forests (CSR KBA) and in Falealupo forest (FP KBA). During surveys in these forests, the selected hunters detected and identified “on the spot” coo calls of pigeons heard, in the presence of G. Serra (Table 3). A total of 910 hours of recordings from these three forests (same method described above) has been archived for future more in-depth and specialised sound analysis (Serra in prep.).
- SEK approach in Malololelei forest (AC KBA). A forest location was chosen to set up an automatic recorder based on hearing possible calls of Manumea on different occasions without the assistance of local experts (Table 3). A total of 83 hours of forest sounds were analysed using the program Song Scope. The coo calls detected from the recordings were then sent to Ulf Beichle and Sabine Baumann by MNRE for identification.
Table 3. Details about forest visits to four KBAs and deployment of automatic sound recorders.
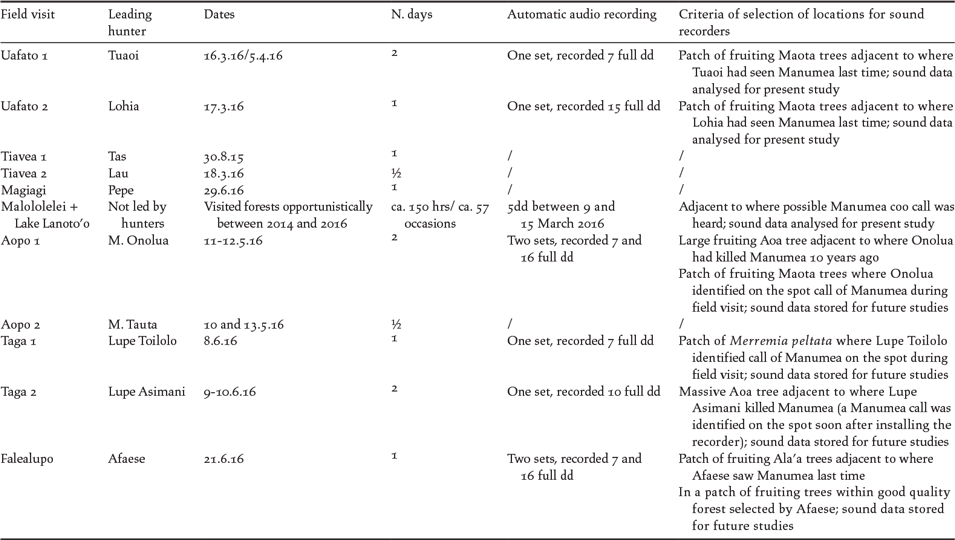
Within the AC KBA, only the Magiagi forest visit was led by a reliable hunter but no audio recorder was set up. The Malololelei and Lanoto’o forest (AC KBA) surveys only involved the authors. Despite not being led by local reliable hunters, these forests were observed the longest during this study – at least 150 hours and 57 occasions during 2014–2016 (G. Serra and G. Sherley pers. obs.).
In order to provide additional independent SEK information about Manumea’s visual and acoustic detectability, we recorded the number of claimed definite sightings and knowledge about the call from all government staff and international experts based long-term in Samoa, who were known to have expertise in local ornithology. On top of TEK about native biodiversity we aimed to gather as much information as possible about the hunting practice at village level through both the standardised questionnaire approach and informal conversations during forest surveys.
Results
Assessment of hunters’ reliability
The percentage of hunters we scored as “reliable” over the total interviewed for each village varied between 20 and 100% (Table 1). Overall, we selected 19 hunters as reliable out of a total of 40 interviewed (47.5%) (Table 2). The total amount of hunter-years of experience consulted is 510 which amounts to an average of 26.84 years of experience per hunter (s = ± 7.39; n = 19) (Table 2).
TEK from standard interviews
Of the 12 reliable hunters who were asked about trends in the numbers of Manumea, 10 (83%) stated that the species had certainly declined during their life time (Table 4). Most hunters mentioned major cyclones of the early 1990s as the main cause of decline while only four blamed hunting. On the other hand, out of 17 hunters who have seen Manumea at least once, seven (41%) admitted having killed at least one in their career (Table 4) and one said he had killed three. One of those who claimed never to have killed Manumea stated that it is “often confused with Lupe by hunters”.
Table 4. Key Traditional Ecological Knowledge on Manumea collected from 19 selected reliable hunters at seven villages in four KBAs in Samoa.
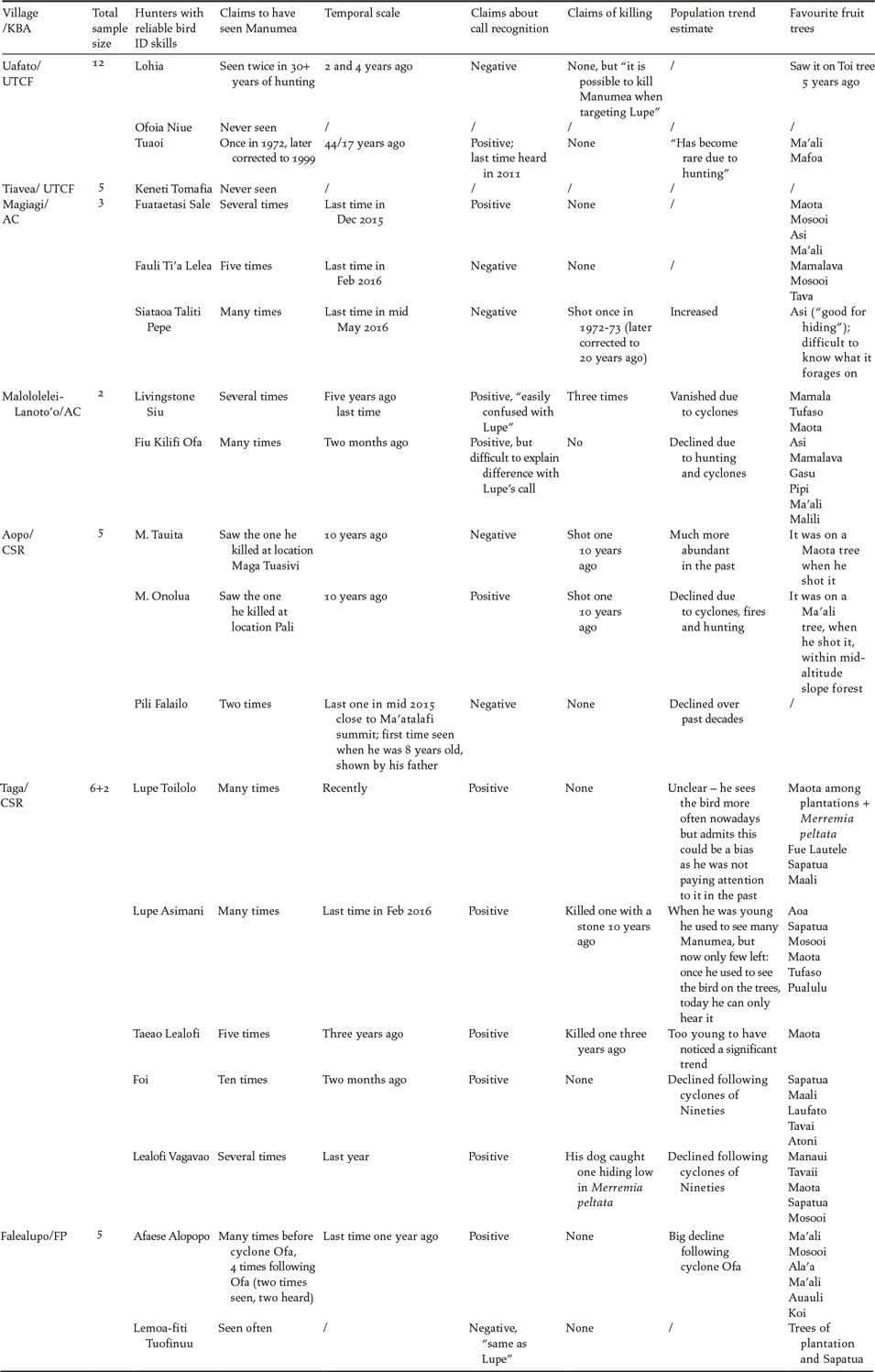
Opinions on the palatability and taste of the meat of Manumea varied considerably with two hunters describing it as equivalent to Lupe, while three others said it did not taste as good because it is “oily”. Another hunter claimed that the taste of Manumea largely depends on which kind of fruits it had been feeding on before being killed. The number of definite sightings of Manumea by the nineteen identified reliable hunters is reported in Table 4. Manumea was claimed to be seen 0–2 times over an average of 28 years by four hunters interviewed in Uafato and Tiavea villages. In Aopo three hunters sighted Manumea 1–2 times over an average of 25 years. In Magiagi, Manumea was claimed to be seen multiple times by three hunters over a period of 10–30 years. In Malololeilei, Taga and Faleolupo two, five and two hunters, respectively, saw Manumea repeatedly over an average of 27 years (Table 4).
The oldest last sightings of Manumea are from Uafato and Aopo villages (10–17 years ago, n = 3) although one hunter from Aopo said he saw one about one year ago. Thirteen hunters from the other five villages claimed to have seen the bird within the last year from the date of interviews with the exception of one hunter from Malololelei who last saw a Manumea about five years previously (Table 4).
Only 11 out of 19 (58%) reliable hunters claimed to be able to recognise the coo call of Manumea as evidenced by them correctly distinguishing its call from that of the Lupe during the audio tests (Table 4). Four of these 11 acknowledged that the calls of Manumea and Lupe are very similar and therefore can be easily confused.
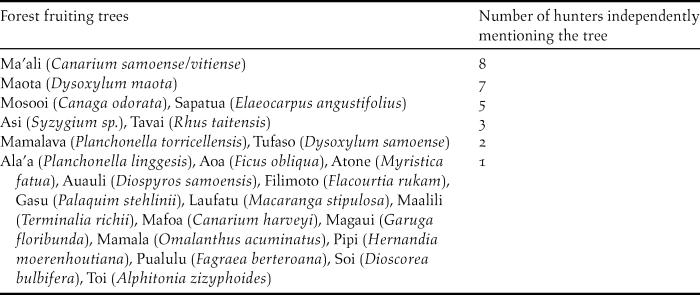
The native forest fruiting trees that are used by Manumea, according to the hunters, in order of importance, are listed in Table 5, a total of 23 species. Ma’ali Canarium samoense/vitiense was mentioned by the highest number of independent hunters (n = 8) of different villages. Maota Dysoxylum maota ranked second. Mosooi Canaga odorata and Sapatua Elaeocarpus angustifolius ranked third equal; interestingly Sapatua was introduced into Samoa (Whistler Reference Whistler2000). Finally, Asi (Syzygium sp.) and Tavai (Rhus taitensis) were noted by 3–5 hunters as important. Thirteen of nineteen (68%) hunters from both Upolu and Savaii used the name “Manuma” to refer to Manumea while two of them recognised the latter as a valid alternative name.
Table 5. Forest tree species whose fruits are eaten by Manumea according to 19 selected reliable hunters of 4 KBAs.
TEK and SEK from field observations
Details of field visits to ancestral forests led by the reliable hunters and of automatic audio recording activity are shown in Table 3 and described in Annex S2 (maps with tracks of forest surveys are included). While no Manumea calls were detected by two reliable hunters during two different forest surveys, west and east of Uafato village (UTCF KBA) in March 2016, several coo calls were automatically recorded in the following weeks at two locations recommended by the two same hunters (see Table 3 for details). One recorded call from the eastern and two from the western site were subsequently identified by Sabine Baumann and Ulf Beichle as Manumea.
A series of coo calls recorded at Malololelei forest (AC KBA) were identified as Manumea by Sabine Baumann and Ulf Beichle. Two residents living in Malololelei and Tiavi forests (AC KBA), claiming to have seen Manumea, were interviewed in 2015 and 2016 using the standard approach described in the Methods. Their descriptions of morphology and behaviour were sufficiently accurate to allow the authors to conclude that they probably had sighted Manumea. Information provided during one of these interviews led G. Serra to make a definite visual observation of Manumea in February 2016 in the vicinity of Malololelei forest.
Two series of coo calls were heard in two locations in upland forest adjacent to Aopo (CSR KBA) in May 2016. The local reliable hunter leading the survey confirmed these were made by Manumea. In June 2016, a coo call was heard in disturbed forest covered with the invasive vine Merremia peltata a few kilometres north-east of the village of Taga (CSR KBA). The call was confirmed as of Manumea by the selected reliable hunter.
During two days of forest observations, Manumea coo calls were confirmed several times by the selected reliable hunter in the Punaoa Community Cloud Forest Reserve, not far from Taga village. On the other hand, all coo calls detected during the forest survey in Falealupo Peninsula KBA were identified as Lupe calls by the selected reliable hunter.
Sightings reported by government employees and international experts based long-term in Samoa are described in Table 6. Defining characteristics of the Manumea’s call and its differences from Lupe’s call, based on both SEK and TEK, are listed in Table 7 (n = 15 observers).
Table 6. Definite visual identifications of Manumea claimed by foresters, ecologists, ornithologists and birdwatchers, based in Samoa, taken place during the past 20 years. Categories of “forest frequentation” used: scarce (at least a visit every two months), often (> once a month), frequent (at least once a week). Categories of “search effort” used in relation to Manumea: low (scarce knowledge of bird ID but interested in Manumea and involved in Manumea work / scarce forest frequentation), medium (good knowledge of bird ID / scarcely to often visiting forests), intensive (good knowledge of bird ID / visiting forests frequently).
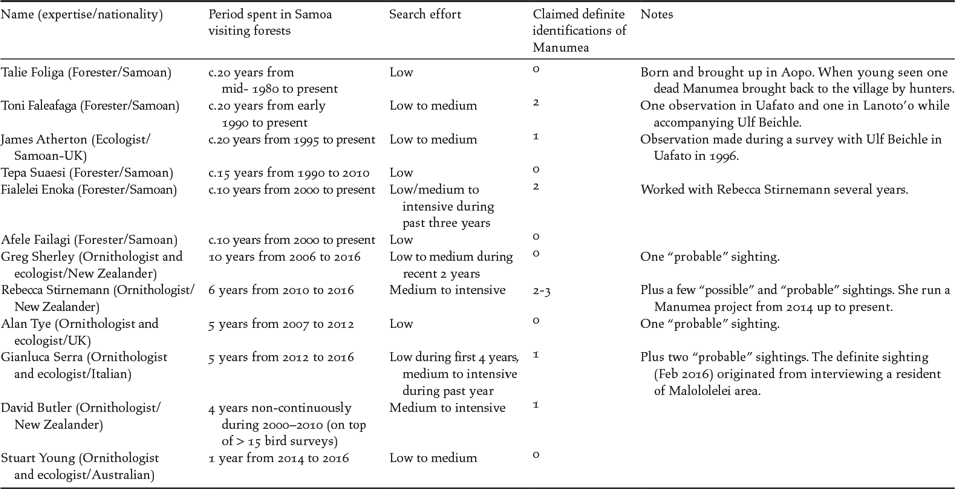
Table 7. Characteristics of Manumea’s call and differentiation in regard to Lupe’s call according to SEK and TEK experts.

Several of these reported characteristics are inconsistent or even contradictory. Only the following features of Manumea call, reported independently by different individuals, seem consistent:
- the call ascends and then descends steadily whereas the Lupe call is more undulating and modulated (n = 5 observers);
- lower frequency than Lupe’s call (n = 2);
- it does not start with a separated syllable as sometimes Lupe does (n = 2).
Hunter M. Onolua from Aopo village reported that his father told him that in the past Manumea was very abundant in the forest and he used to see it feeding and even nesting on the ground of the forest. Lupe Toilolo from Taga claimed to have seen Manumea on the ground several times in recent years. In one case, in Taga, a Manumea was reported to have been caught by a hunting dog while hiding amongst Merremia peltata close to the ground. Moreover, a definite sighting of a Manumea on the ground was reported in the recent past in Nu’utele island (D. Butler pers. comm.) and a probable identification of a Manumea walking on the ground within AC KBA was also reported in 2009 (A. Tye pers. comm.). Two hunters from Taga independently reported that Manumea is often associated with the invasive vine Merremia peltata where it hides.
Based on field observations made in June 2015 and February 2016 within AC KBA, G. Serra described the flight of Manumea as unusual for a pigeon because of its observed habit of flapping mostly the distal part of the wings. The whole appearance of the flight is clumsy and slow and very different from the flight patterns of the other two pigeons of similar size co-occurring in Samoa (Lupe and White-throated Pigeon). Consistently and independently, three reliable hunters described the flight of Manumea as “slow” and “weird”; while Fialelei Enoka described a similar kind of flight pattern based on his observation made in June 2015, especially the flapping of the tip of the wings. A very similar description was provided of a flying Manumea spotted in Uafato forest during the recent BIORAP survey in August 2016 (M. O’Brien pers. comm.).
Hunting assessment
Lupe, native fruit bats Pteropus tonganus and P. samoensis and feral pigs Sus scrofa are the key targets of forest hunters of the seven villages in the four KBAs sampled – with the addition of coconut crab Birgus latro in Falealupo. As regards to birds, hunters all claimed to be interested in Lupe only. White-throated Pigeon and Manumea, despite their similar body size to Lupe, do not have a good reputation for their taste, although this view is not unanimous. The two species of dove commonly occurring in Samoa forests (Samoa Fruit Dove Ptilinopus fasciatus and Many-coloured Dove Ptilinopus perousii) are regarded as too small to “be worth a bullet”, as are all the small birds (Passerines).
Of grave concern is the possibility that a good portion of the hunters (probably the least experienced) aim to kill indiscriminately all large birds they see through the forest canopy in the hope of taking a Lupe; and in so doing they put the Manumea at risk. Most of the hunters stressed the fact that wild meat is used only for particular occasions and celebrations and mostly for family (including extended) consumption. Some admitted that they have sold shot birds to affluent people from Apia (the country’s capital) and nearby resorts or even to the local pastors. The total number of hunters reported for each village was very variable from only a few to “almost all men in the village” - this information was often not consistent even between interviewees from the same village.
The consensus amongst hunters was that October–December is the “best” period for hunting pigeons in Samoa. There are apparently two reasons for this. First, two very important religious celebrations fall in this period (White Sunday and Christmas); and second, this is the time of the year when Lupe is apparently “fatter” and thus tastier (reportedly this is its main breeding season). According to some hunters, the best hunting period actually extends to January–February. Some hunters mentioned also May as a “good” period for hunting. In fact, according to others hunting in Samoa is good all year round, it all depends on the knowledge, strategic for the hunters, of which trees are fruiting and when. Several hunters stressed the fact that hunting is becoming increasingly less popular and the number of hunters is decreasing due to the cost of cartridges, licencing and constraints set by village councils. Some of them emphasised that hunting is sustainable at village level and is not negatively affecting the Lupe populations.
Discussion
Conservation status, hunting threat and current occurrence
In line with the suggestions based on SEK (Beichle Reference Beichle2006, MNRE 2006, Butler Reference Butler, Atherton and Jefferies2012, Pratt and Mittermeier Reference Pratt and Mittermeier2016), the present study provides further and complementary evidence that Manumea has sharply declined in recent decades. Most hunters claimed that major cyclones occurring during period 1990–1991 were the main cause for the decline, consistent with the findings of Tarburton (Reference Tarburton2001) and MNRE (2006).
The evidence from TEK about the relevance of hunting as a direct threat to Manumea survival supports what SEK approach has suggested in these regards so far (MNRE 2006, Beichle and Baumann Reference Beichle and Baumann2016). In fact, 41% of reliable hunters interviewed in the present study admitted having killed Manumea at least once in their life by mistake pursuing Lupe – consistent with what has also been reported by MNRE (2006), Beichle and Baumann (Reference Beichle and Baumann2016) and R. Stirnemann (pers. comm.).
However, this figure is probably an underestimate given the taboo around shooting a known protected species and publicly admitting this potentially shameful act. It should be noted that most men from villages of Samoa hunt or try to hunt during their life: only a few actually become experienced and regular forest hunters. Therefore, the percentage of those killing Manumea accidentally, drawn from a random sample of villagers including a good majority of inexperienced hunters, should be expected to be much higher than the figure we obtained by sampling a selected subset of experienced hunters.
As noted by Beichle and Baumann (Reference Beichle and Baumann2016), while the large-scale destruction of native forests that has taken place since the 1970s and major cyclones from the 1990s have most likely taken a critical toll on Manumea population, together with the spreading of predatory invasive alien species (MNRE 2006), killing as a by-product of Lupe hunting cannot be ruled out as a current key threat to the survival of this species, especially considering that Samoa’s forests are now much reduced in size and are quite fragmented. The remaining Manumea individuals are probably struggling to survive by flying between the last patches of their natural habitat in search of food, while hunter density has meanwhile increased and their killing efficiency greatly improved. This is important as a few observers may still believe that the 1993 national ban on Manumea hunting (MNRE 2006) succeeded in mitigating this threat (J. Atherton pers. comm.) while others are doubtful that hunting is a current key threat for the species (M. O’Brien pers. comm.).
Certainly Manumea is currently ‘Critically Endangered’ due to a deadly cocktail of threats while climate change does not improve its status: another cyclone of the same intensity as those from the 1990s, or higher, could quite possibly seal the fate of this unique piece of Samoa’s natural heritage. Various opinions about the palatability of Manumea were reported in this study which agree with MNRE (2006). The claim by some interviewees that pigeon hunting is a sustainable practice cannot be considered completely unbiased and independent. On the other hand it is a fact that Lupe, despite being the main target for all Samoan bird hunters, and despite being reportedly hunted primarily during its breeding season (information that would be worth verifying through SEK), is still quite a common forest bird in Samoa (Pratt and Mittermeier Reference Pratt and Mittermeier2016, G. Serra pers. obs.) It is possibly not as common as in the recent past and perhaps not able to fully fulfill the key ecological role of forest seed disperser it used to play.
Overall Manumea is rarely seen and known at village level, with the notable exceptions of Magiagi and Taga villages. Ignorance of Manumea seemed most noticeable in inexperienced, young hunters. This study provides evidence that Manumea is still extant at least within three of the four KBAs surveyed. However, four of the 19 reliable hunters (21%) stated either that they had never seen it (Uafato and Tiavea) or never saw it again (or rarely) following the two major cyclones which occurred between 1990 and 1991 (Malololelei-Lanoto’o and Falealupo) (Tarburton Reference Tarburton2001, MNRE 2006).
On the other hand, 84% of selected reliable hunters have seen the bird either recently or sometime in the past. In Uafato it has been observed between five and 17 years ago making it the forest area with the oldest last observations. In all other five villages, Manumea was claimed to have been seen more recently (from a few months to 4–5 years ago).
Manumea current occurrence was documented by the present study, through a TEK and a mixed TEK-SEK approach, in four forest areas of Samoa: Uafato (UTCF KBA) and Malololelei (AC KBA) on Upolu island; Aopo and Taga (CSR KBA) on Savaii island. Two recent BIORAP SEK-based surveys, targeting CSR KBA in 2012 and the same four KBAs targeted by the present study in July–August 2016 (Butler Reference Butler, Atherton and Jefferies2012 and M. O’Brien in prep., respectively), were less efficient in reliably detecting Manumea in the field, producing only one definite detection of Manumea in Uafato forest in August 2016.
Manumea is known to require good quality forest habitat (Collar Reference Collar2015). It is probably not a coincidence that Manumea was detected in the above-mentioned sites as these are the best quality forests sampled in this study as compared with Tiavea and Falealupo forests (observations on the quality of different forests surveyed are reported in Appendix S2).
The three acoustical detections of Manumea in Uafato forest produced by the present study are particularly important as the bird had not been detected in that area during the last Manumea SEK-based surveys (Beichle Reference Beichle2006, Butler and Stirnemann Reference Butler and Stirnemann2013). In fact the last definite sighting in Uafato forest is from 1996 (J. Atherton pers. comm.). On the other hand, the Malololelei forest is not even mentioned among the “key sites for the conservation of Manumea” listed in the Manumea Recovery Plan 2006–2016 (MNRE 2006); while Taga forest was not even reported among the historical sites where Manumea had been observed during the period 1978–2000 (MNRE 2006).
Occurrence of Manumea was photographically documented in 2013 at the edge of Tafua forest in Savaii island by MNRE staff (Uili Reference Uili2014); this record was confirmed in 2015 by S. Baumann and U. Beichle (pers. comm.). Currently highly threatened with development (S. Baumann and U. Beichle pers. comm.), Tafua is the last remaining lowland forest of significant size in Samoa: it is not included among the terrestrial KBAs of Samoa (Conservation International 2010) but it is mentioned among the “key sites for the conservation of Manumea” (MNRE 2006).
Detectability
How to detect Manumea in the field via visual cues or its vocalisations remains unanswered. The present study clearly highlights this issue for the first time and the implications for accurately assessing the conservation status of the species. Contrary to Beichle’s (1991) description of Manumea calling “from the top of trees”, observations from this study indicated a highly cryptic behaviour to the extent that we concluded that visual cues cannot be relied on to survey the species.
In particular, the behaviour of calling from top of trees has never been observed by the authors nor reported by the reliable hunters in this study. In fact, the opposite was recorded in the field: whenever a potential call of Manumea was heard it came from middle tiers and all attempts to spot the bird failed (at least 23 attempts recorded and described during 2014–2016; G. Serra and G. Sherley pers. obs.). As hunter Pepe from Magiagi village reflected: “this is a bird living in the deepest corners of forest, and perching close to trunks to hide better”. And yet at the time Ulf Beichle studied the bird in the late 1970s to early 1980s Manumea was apparently still relatively common and easy to see (U. Beichle pers. comm.).
The dramatic change in detectability of Manumea between the early days of its study to the present may be explained by a sharp drop in numbers and possibly by its cryptic behaviour in response to hunting pressure. Hunting of pigeons was traditionally carried out by use of bows and arrows or trapping (MNRE 2006) and the increase in cryptic behaviour may be linked to the increase of the human population in Samoa and the increase in the availability and use of firearms by villagers throughout the country which has occurred in recent decades (U. Beichle pers. comm.). Interviews with reliable hunters confirmed the highly cryptic behaviour of the species which is also consistent with the observation by U. Beichle of captive birds “slowly moving and hiding in the upper shady corners of cages on the approach of any person” (Collar Reference Collar2015). This is likely to be responsible for the extremely low rate of definite sightings claimed by a good portion of the reliable hunters interviewed in Uafato, Tiavea, Malololelei-Lanoto’o, Aopo and Falealupo and the 12 Government staff and other international experts based long-term in Samoa, during recent decades.
Further complicating the issue of assessing the conservation status of Manumea is the extreme difficulty of identifying its call in the field. The coo call of Manumea is extremely similar to one of the calls of the relatively common sympatric Lupe, while the call of the other sympatric pigeon, the White-throated Pigeon, is easily distinguishable (G. Serra pers. obs.).
Interestingly, this overlap in calls between the two species, so relevant in terms of conservation, was mentioned and described only very recently (Beichle and Baumann Reference Beichle and Baumann2016, Pratt and Mittermeier Reference Pratt and Mittermeier2016, Serra Reference Serra2016) and also suggested by Butler and Stirnemann (Reference Butler and Stirnemann2013) in relation to the lack of detections of Manumea during the recent BIORAP of upland Savaii (Butler Reference Butler, Atherton and Jefferies2012). This “underappreciated similarity” (Pratt and Mittermeier Reference Pratt and Mittermeier2016) is confirmed through TEK by the fact that even an appreciable portion of reliable hunters (42%), selected through present work, seemed to have problems in telling the two calls apart. The remaining hunters seemed to struggle in telling them apart which was apparent either through the audio tests included in the standard questionnaire and during the field surveys. Consistently, the reported characteristics of Manumea calls as described by individual reliable hunters and by Government and international experts differ from each other and are unclear and contradictory.
Notwithstanding the above, three distinguishing features of Manumea call seem apparent. Our observations tentatively agree with Pratt and Mittermeier (Reference Pratt and Mittermeier2016) in that the frequency of coo calls are variable, both intra- and inter-sequence, and thus it seems that it is not useful for differentiating between species. Recently, Beichle and Baumann (Reference Beichle and Baumann2016) suggested that a high repetition rate of coo calls within a series may reveal the identity of Manumea over Lupe, especially during the breeding season (presumed to be by these authors the dry season between April and August). Based on the present research we think that nowadays within Samoa only a few elderly indigenous experts remain who can reliably recognise the call of Manumea in the field; while it appears that other experts from the government and past and present Samoa-based foreign experts cannot reliably distinguish between Manumea and Lupe calls (with the notable exception of Ulf Beichle and Sabine Baumann, the world authorities for this species).
Ecology and behaviour
Hunters provided valuable information on the fruiting trees used by Manumea for foraging. The fact that maota was not reported as the favourite fruiting tree for this bird is at variance from most past reports and studies (Collar Reference Collar2015, Beichle and Baumann Reference Beichle and Baumann2016). Moreover, 15 fruiting tree species mentioned by local hunters as used by Manumea are also not mentioned in the literature on Manumea (Uili Reference Uili2014, Collar Reference Collar2015).
Two hunters from Taga confirmed independently the observation made by Fialelei Enoka about Manumea being associated with the invasive vine Merremia peltata found in disturbed forests and at the edge of plantations. This is a habitat not mentioned by Collar’s (2015) review of Manumea. Three independent hunters, one from Aopo and two from Taga, confirmed the behaviour of feeding on the ground suggested by several authors from the past (Collar Reference Collar2015). One hunter from Taga village claimed to have seen this behavior in recent times as did ornithologist David Butler (pers. comm.). The peculiar flying pattern of Manumea described in this study has not been reported elsewhere (e.g. Collar Reference Collar2015) and could materially assist in field identification of Manumea.
Rigorous acquisition of TEK
This study represents the first attempt to use the TEK approach in surveying rare and elusive fauna in Samoa – and is among the few carried out in the wider tropical Pacific region (Sinclair et al. 2010). In fact, past studies based on TEK carried out in Samoa focused on medicinal plants and ethnobotany (Cox Reference Cox1993, Whistler Reference Whistler2000, Castro and Tsuda Reference Castro and Tsuda2001). Collection and interpretation of TEK relating to biodiversity through a verifiable approach is appropriate and valuable – especially if the species concerned has had historical cultural significance (Sinclair et al. 2010). Native bats and pigeons are culturally highly significant as food species in Samoa (Grattan Reference Grattan1985). The rigorous assessment of identification skills was crucial in obtaining factual and objective information about rare and threatened fauna occurring in the forest.
There have been several awareness projects at national level on Manumea in the past and recent past (Butler Reference Butler1995, MNRE 2006, Uili Reference Uili2014). It was selected in the mid-1990s as the “national bird” of Samoa (Butler Reference Butler1995), even featuring on the 20-tala currency note and the 50-sene coin. Following these initiatives, national awareness of this bird has increased significantly during the past 20 years. Paradoxically the term Manumea has become popular in Samoa while very few people have ever seen it. But due to its current reputation and the consequent interest of international organizations, unfounded claims by people to know or have seen this bird have soared in recent years. It was determined that, in most cases, people are referring to the White-throated Pigeon which is a forest inhabitant of almost the same size, similar colour and partly showing secretive behaviour similar to Manumea (G. Serra pers. obs.).
Because of the Manumea’s popularity, we were extremely careful to ask questions without prejudice or “leading” the respondent. Hence, we made sure that any information gathered on the species from interviewing hunters with proven bird identification experience, originated from the interviewees themselves and was not in any way influenced or prejudiced by the interviewers. Certainly, TEK surveys targeting fauna should never be based on local names. It is in fact quite a common experience to find that the same species is called by different names in different villages in the same country (G. Serra pers. obs.). The present study confirmed the confusion over the local name even for this species (MNRE 2006, Collar Reference Collar2015). Some solid evidence was gathered that the local name for Didunculus strigirostris may not be restricted to Manumea. A majority of hunters from both islands indicated that the name they use is Manuma, whereas according to Watling (Reference Watling2001) this name (or Manuma’a) is the local name for Many-coloured Dove. Two hunters acknowledged that both Manumea and Manuma are used to indicate the Tooth-billed Pigeon. The term Manumea was adopted by the scientific literature as the most used local name through conservation awareness projects run during the 1990s and 2000s (Butler Reference Butler1995, Collar Reference Collar2015). However, Beichle and Baumann (Reference Beichle and Baumann2003) suggested that a shift of the local name used, from Manumea to Manuma, actually took place during the 1990s (Collar Reference Collar2015). This could be a reflection of a shift of the bird’s behaviour induced by increased hunting pressure during recent decades. In fact, Manuma means “shy bird” in the Samoan language which seems very appropriate for a highly cryptic species like this.
Overall the methodology employed by the present study seemed sufficiently rigorous and effective in gathering objective and unbiased information. The results collected were consistent with known SEK about Manumea; not only in relation to this particular species but also, independently, to other rare and threatened species surveyed such as the Samoan swallowtail Papilio godeffroyi, the Pacific sheath-tailed bat Emballonura semicaudata and the Samoan Wood-rail (Pareudiastes pacificus) (Serra Reference Serra2016). The present study confirms that the TEK “slow” survey approach is quite effective in detecting cryptic and rare bird species like Manumea.
Short-term recommendations
Conservation status
This study confirmed with evidence from TEK that Manumea in Samoa is declining and possibly nearing extinction which is in agreement with the most recent SEK assessments (MNRE 2006, BirdLife International 2015). Recommendation: retain the IUCN Red List categorisation of Manumea as ‘Critically Endangered’ based on the TEK collected in the present study and the lessons learnt from the recent loss of Hawaiian avifauna (e.g. Powell Reference Powell2008); and conforming to the precautionary principle (Cooney Reference Cooney2004). The new recovery plan in preparation (2017–2027) should reflect the conservation status upgraded in 2014 (BirdLife International 2015).
Current occurrence and threats
In addition to its occurrence in Tafua forest of Savaii (Uili Reference Uili2014, U. Beichle and S. Baumann pers. comm.), the present study provides solid evidence that Manumea is also extant at four other forest sites (Uafato, Malololelei, Aopo and Taga) which are within three KBAs on Upolu and Savaii islands. Further, evidence was collected that Manumea is killed by hunters as by-catch when targeting Pacific Pigeon (Lupe). The study supports the hypothesis that this is an arboreal-terrestrial bird and therefore it is most likely very vulnerable to invasive alien species such as feral cats (Felis silvestris) and rats (Rattus rattus and R. exulans) (Pratt and Mittermeier Reference Pratt and Mittermeier2016). According to Birdlife International (2015) “There is now an urgent need for intensive conservation actions to halt the species’ perilous slide towards extinction.” Recommendations: (a) implement ways in the short term to control and mitigate hunting and threats from invasive alien species in the five forests mentioned. (b) The 19 selected indigenous reliable hunters identified by the present study should be supported by an awareness and capacity building programme in order to raise their status within their villages as the guardians of their ancestral forests and local champions of Manumea conservation. (c) Upgrade the recently established Malololelei forest reserve of 12 ha into a larger and functional protected area according to international standards (IUCN 2016). Due to its proximity to Apia and being under no customary control, this forest area could become the first Manumea Conservation Area in Samoa with significant potential in terms of awareness and sustainable ecotourism development.
Detectability
The present study has uncovered the serious issue of reliably identifying Manumea in the field using visual and vocal cues. A total of 1,290 hours of forest sounds, recorded automatically at specific forest locations recommended by indigenous reliable experts, need to be analysed. Recommendations: (a) fully take into account the issue of detecting Manumea in the field in the new Manumea Recovery Plan (2017–2027). (b) Design a viable research plan to analyse recorded sound data already in storage in cooperation with relevant agencies and individuals (contribution to Objective #5 of Manumea Recovery Plan 2006-2016). A few reliable hunters selected through the present study should be able to assist in the process using their unique and valuable TEK.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000259
Acknowledgements
We acknowledge and thank the Ministry of Natural Resources and Environment of Samoa and the UNDP Samoa multi-country office for their assistance: special thanks go to the ACEO of MNRE Tauti Fuatino Leota, the staff of SMSMCL project and the United Nations resident coordinator Lizbeth Cullity. We are indebted to hunters Tuaoi, Lohia, M. Tuaita, M. Onolua, Pili, L. Toilolo, L. Asimani, Afalese and Pepe, and all the other hunters interviewed at seven villages, for the valuable knowledge they shared with us. We commit to always credit and acknowledge them. Many thanks also go to Sabine Baumann and Ulf Beichle for sharing their knowledge about the Manumea call.











