Introduction
The Cyprus Warbler Sylvia melanothorax is a distinctive restricted-range species, and one of 31 bird species endemic to Europe (BirdLife International 2004). Prior to about 1990, the species was common and widespread over much of Cyprus, apart from the central plain, occurring in a wide variety of habitats, but primarily in low, dense scrub (Flint and Stewart Reference Flint and Stewart1992), which covers large parts of the island. It is an endemic breeder with an unknown, but apparently large proportion of the population wintering as far south as southern Egypt and northern Sudan (Shirihai et al. Reference Shirihai, Gargallo and Helbig2001).
Surveys in the west of Cyprus from 1997 to 2000 suggested a decline in Cyprus Warbler numbers within its breeding range, coinciding with an increase in the population of Sardinian Warbler S. melanocephala, which had first colonised Cyprus as a breeding species in the early 1990s (Frost Reference Frost1995, Cozens et al. Reference Cozens, Stewart and Pomeroy2000, Pomeroy and Walsh Reference Pomeroy and Walsh2000, Reference Pomeroy and Walsh2002, Flint and McArthur Reference Flint and McArthur2014). Meanwhile, breeding Sardinian Warblers were also recorded from a second and apparently quite separate area, in the eastern part of the Kyrenia range, on the north coast of Cyprus (Flint and McArthur Reference Flint and McArthur2014). The Cyprus Warbler’s decline suggests a recent change may have occurred in environmental conditions on its wintering grounds, or in Western Cyprus, the latter detrimental to Cyprus Warbler but benefiting Sardinian Warbler. In addition, concern has been raised that the Cyprus Warbler could be suffering from competitive displacement by its congener (Cozens and Stagg Reference Cozens and Stagg1998, Pomeroy and Walsh Reference Pomeroy and Walsh2000, Reference Pomeroy and Walsh2002), a process that can be amplified by climate change (Begon et al. Reference Begon, Townsend and Harper2006). However Cyprus Warblers were thought to prefer areas with more trees than Sardinian Warblers, and to require larger habitat patches (Tucker and Evans Reference Tucker and Evans1997). Furthermore, Ieronymidou et al. (Reference Ieronymidou, Collar and Dolman2012) suggested that the two species select subtly different scrub habitats, and although none of the habitat types they examined was used exclusively by one species, they suggested that the two were unlikely to compete for resources. In contrast, Jones (Reference Jones2006) found no significant differences in the vegetation structure or habitat composition of breeding territories of the two species in a range of scrub habitats. She also showed that the two warbler species nested in the same bush species, in bushes of a similar size, and that areas suitable for one species were suitable for the other.
Although the Sardinian Warbler was not confirmed as breeding on Cyprus until 1993, it may have begun breeding in the 1980s (Flint and McArthur Reference Flint and McArthur2014). This species, which is widely distributed around the Mediterranean, had previously been only a passage migrant and winter visitor to the island. In winter it was usually widespread and common, frequenting ‘scrub similar to and denser than that preferred by Cyprus Warbler’ (Flint and Stewart Reference Flint and Stewart1992). Although the exact place and year of first breeding are not known, records suggest that it was near to the Baths of Aphrodite, c.8 km west of Polis, on the north-west coast of the island, and 7 km from the tip of the Akamas peninsula (Figure 1) (Flint and McArthur Reference Flint and McArthur2014).
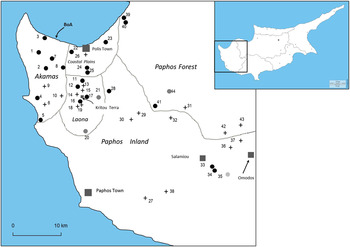
Figure 1. Map of Cyprus showing Paphos District and the locations of 44 survey sites, listed with corresponding site numbers, in Appendix 1. Filled squares indicate towns and villages (large and small squares, respectively). The Baths of Aphrodite, near which Sardinian Warblers were first thought to have bred, are also indicated (BoA). The five geographical regions surveyed (Figure 2) are defined by dotted lines. Circles: sites first surveyed in 1998.
![]() : Sardinian Warbler present in 1998–2000;
: Sardinian Warbler present in 1998–2000;
![]() : first recorded 2001–2005;
: first recorded 2001–2005;
![]() : first recorded post-2005. +: Sites first surveyed post-1998.
: first recorded post-2005. +: Sites first surveyed post-1998.
Here we describe a rapid decline in the numbers of Cyprus Warbler detected at survey sites in Paphos District during 1997–2011, closely coinciding with the colonisation of this area by the Sardinian Warbler. We also compare the rate of change in the Cyprus Warbler’s abundance at each site with measures of land-cover, vegetation density, altitude, rainfall and the duration of site occupancy by Sardinian Warbler. Finally, we compare changes in the Cyprus Warbler population in western Cyprus during 2006–2014 with those in central and eastern Cyprus, where Sardinian Warblers were sparse or absent.
Methods
Field methods
Annually from 1997 to 2011, DP and FW made counts of all species of breeding birds at varying numbers of sites between mid-April and mid-June (maximum range 19 April to 21 June). Of 72 sites surveyed in western Cyprus, 67 were in Paphos District, the remainder being in adjacent parts of Lefkosia and Lemesos Districts. For brevity, we refer to the full set of sites as ‘western Cyprus’. During the early years, a number of sites were only surveyed in one year, as they were primarily intended to provide data for the Cyprus Bird Atlas (Whaley and Dawes Reference Whaley and Dawes2003). In this paper, we analyse count data from the 44 sites in Paphos District that were counted for at least five out of the 15 years, in almost all cases consecutively, and at which one or both Sylvia species had been recorded in at least one year. Ten sites were surveyed in at least 12 years. Geographically, sites covered the whole District, but with fewer sites in the south (Figure 1).
The majority of sites were counted twice in each year, and the two counts were made in the first and second half of the field period, respectively. Overall, a half of the counts were started before 10h00, a quarter between 10h00 and 16h00, and the remainder after that, the allocation of time to sites being made randomly, except that sites at lower altitudes were first counted earlier in the season, to allow more time for territories to be established at higher altitudes (up to 980 m; Appendix S1 in the online supplementary material). No count was made in rain, or if rain appeared to be imminent, or if it was windy (when bird sounds are less detectable).
Between 1997 and 2006, we used Timed Species Counts (Bibby et al. Reference Bibby, Burgess, Hill and Mustoe2000, Freeman et al. Reference Freeman, Pomeroy and Tushabe2003), which do not have fixed routes, so far as possible within a particular habitat, but changed to line transects from 2003 to 2011; these are described below. Only a few sites had transects in the change-over period, mostly at the same sites as the TSCs, and these counts were made concurrently, this being possible since bird densities were generally fairly low.
Six land-cover types have been recognised for Paphos District (Government of Cyprus 1994). We divided one of these (arable) into two, as agriculture at medium-to-low elevations (i.e. below about 400 m) is quite different from that in upland areas (Table 1). Each study site consisted primarily of a single land-cover type. Details of these types are given by Pomeroy and Walsh (Reference Pomeroy and Walsh2006). In brief, they were as follows: forest, predominantly pines Pinus spp., often with species of oaks as shrubs or small trees; uncultivated land, covering a variety of maquis types of scrub, and some areas of long-abandoned agriculture; grass/phrygana; phrygana refers to low forms of maquis, intermixed with grass, and with few if any taller shrubs or trees; it is the ‘most widespread dwarf scrub vegetation of dry slopes, hills and islands in the Mediterranean climatic zone’ (Polunin Reference Polunin1980: 36). The remaining forms of land-cover are much more anthropogenic: arable land, mainly in the lowlands but with some small fields in upland areas (generally above 400 m), usually in formerly grass/phrygana areas; permanent crops, mainly citrus, olives and vines; and built-up land, as in towns and villages. For the analyses, the few upland agricultural sites were combined with grass/phrygana. For most practical purposes, these forms of land-cover were equated to habitats but, as emphasised by Fuller (Reference Fuller, Irvine, Davies, Armsworth, Gaston, Lepczyk and Warren2012: 7), the two are not always the same (e.g., ‘permanent crops’ included both citrus groves and vineyards, but the respective areas of these are not known, so they could not be separated).
Table 1. Estimates of the densities and population sizes of the two Sylvia species in seven land-cover categories (birds km-2 and estimated populations; 95% CLs). Counts were made in April–May of 2007–2011, and may include some juveniles. The area of each land-cover type in Paphos District is also given, as an indication of its relative importance. Land-cover data from Pomeroy and Walsh (Reference Pomeroy and Walsh2006).

In 2006 BirdLife Cyprus began a common birds monitoring scheme, covering sites in various parts of Cyprus, using line transects, which are now the standard method for land-bird monitoring in Cyprus, and contribute to the Europe-wide programme (see, for example, PECBMS 2012). Accordingly, we converted to monitoring transects on many of our sites, and added new sites where necessary to maintain coverage of the set of seven land-cover types. Transect counts consisted of walks along fixed routes, mainly on small roads and tracks, for distances of 800–2400 m. Because much of western Cyprus is hilly, land-cover often changes over short distances, and consequently some transects were relatively short. Despite these organisational changes, the method used to count Sylvia species was constant through all years.
Individual birds are not normally recorded in TSCs, but an exception was made for the two Sylvia species, because of their special interest. Hence, the numbers of these two species, both seen and heard, were recorded as part of both types of count, from the outset in 1997. TSCs were of a fixed time, 60 min, whereas on average, transect counts took only 55 min, which could have resulted in a small under-estimation of bird numbers after the change of method. Fewer sites were counted in the first few years. We therefore pooled the count data from each site surveyed during 1997–1999, by calculating the mean value from the 1, 2 or 3 years in which it was surveyed, and assigned this mean to 1998. We pooled the data from 2000 and 2001 in a similar way: assigning the mean value for these two years to 2001. Since some of their calls are similar (and we did not use play-back), the two species may have been under-estimated in the first 2–3 years, until we became more familiar with them. In most years, each site was surveyed 2–3 times, and the counts were averaged, though by 2011 only 24 of the 44 sites were surveyed, due to a lack of time.
During transect counts the perpendicular distance of each individual bird from the transect line was recorded, and used to estimate population densities of the two Sylvia species, for the whole District (Pomeroy and Walsh in press), using the program Distance (Thomas et al. Reference Thomas, Buckland, Rexstad, Laake, Strindberg, Hedley, Bishop, Matques and Burnham2010). Distances were estimated by eye, but frequently checked against a tape measure. For these analyses, we used the transect count data from 2007 to 2010, inclusive; the years for which the data were most complete. Estimates of density were converted to population estimates by multiplying estimated density by area, using published data on the extent of each land-cover category (Pomeroy and Walsh Reference Pomeroy and Walsh2006).
At each of our sites we estimated the per cent cover of woody vegetation, separately in four horizontal layers, namely 0–1, 1–3, 3–8 and >8 m. These assessments were made at ten points, at 100 m intervals (or slightly less for short transects), which was considered as being effectively unbiased. At each point, cover was estimated by eye for each of four quadrants, then averaged across the ten points (Pomeroy Reference Pomeroy1992). It is thus theoretically possible for the total vegetation of all four layers to reach 400%, although in practice it rarely exceeded 200%, and was usually much less. Native and planted vegetation types were recorded separately. Since both Sylvia species are most closely associated with maquis, we have used only data for native woody vegetation in the lower two layers (see Appendix S1). For nine sites, the cover in these two layers was estimated retrospectively, based upon detailed knowledge of the areas concerned. Altitude was obtained from standard GPS readings, and rainfall from a map for the period 1951–1980 (Cyprus Meteorological Service 1983), the most recent 30-year period covering the whole island. Population changes at these sites were compared with mean annual rainfall for the hydrological years 2004/05 to 2012/13 (Cyprus Meteorological Service 2014).
Data analysis
We used Linear Mixed Effects models to determine the rate of change in warbler abundance during 1997–2011 in each of five areas of Paphos District (Figure 1), and at all Paphos sites combined (n = 44 sites). Models were fitted using the lme command in R (3.0.1; R Development Core Team 2009). In each case, an index of warbler abundance on each survey site was entered as the dependent variable, study year as a fixed effect and site identity as a random effect. Warbler abundance indices were first normalised using a natural log+1 transformation. As noted above, data for 1997–1999 were pooled, and assigned to 1998. Model coefficients were used to estimate warbler abundance indices in study year 1 (1998) and 14 (2011), controlling for the effects of repeated measures from each site. These values were used to calculate the annualised rate of change throughout the study period, and per decade. In addition, using the lmer command in R, we compared Cyprus Warbler abundance indices with those of Sardinian Warbler throughout the survey period, entering both site identity and study year as random effects.
To identify variables associated with changes in Cyprus Warbler abundance in western Cyprus we first fitted a linear regression to an index of its abundance at each site over the study period. We used General Linear Models to compare changes in Cyprus Warbler abundance (the regression slope coefficient at each site) with potential predictor variables, using the lm command in R (3.0.1; R Development Core Team 2009). The full model included the following predictor variables: altitude (covariate), annual rainfall (covariate), land-cover type (six-way factor), and percentage woody vegetation cover at 0–1 m and at 1–3 m (covariates). We also included a measure of the change in mean rainfall within Paphos District between two periods: 1916/17 to 1969/70 and 1970/1971 to 1999/2000, from Rossel (Reference Rossel2001).
To test whether changes in the status of Cyprus Warbler at each site were correlated with those of Sardinian Warbler, we included in the model three measures of the latter’s presence throughout the survey period: its annual rate of change (covariate; slope coefficient from a linear regression of abundance over survey year); mean abundance throughout the survey (covariate); and duration of occupancy (covariate: last year surveyed minus first year on which detected at the site). Since the number of years surveyed at each site varied, we also included two interaction variables (Sardinian Warbler mean abundance*duration of occupancy, and rate of change*duration of occupancy), on the assumption that any effects of the Sardinian Warbler’s presence on Cyprus Warbler abundance would increase with the duration of its occupancy.
All environmental predictors, measures of Sardinian Warbler presence and both two-way interactions were included in the full model. We used the R step command to sequentially eliminate non-significant terms whose removal from the model reduced the AIC value by < 2, leaving a final, minimal model in which all variables selected were significant at P < 0.05. We used the R plot command to check that warbler abundance values, and residuals of the final model, reasonably met with model assumptions (Crawley Reference Crawley2013). All probabilities are quoted as two-tailed.
Using data collected by BirdLife Cyprus during 2006–2014, we compared the rate of change in Cyprus Warbler abundance on sites surveyed in western Cyprus with that evident in central and eastern Cyprus. Many of the latter sites were located on or near to the central plain, where mean annual rainfall is less than 400 mm, and Cyprus Warblers have always been largely absent. Of the 127 sites monitored, 36% were first surveyed during 2006–2009, and in most cases for six or more years. The remaining sites were first surveyed during 2013–2014, and hence contributed data only for the last 1–2 years of the survey period. For continuity we restricted the analysis to those sites first surveyed early in the survey period. We used a Linear Mixed Effects model to determine the rate of change in Cyprus and Sardinian Warbler abundance in each region, entering site identity and habitat as random effects in the model.
Results
Population estimates
During the 2007–2010 breeding seasons, Paphos District held an estimated annual average of 28,340 Cyprus Warblers (95% CLs: 22,126–34,564) and 131,114 Sardinian Warblers (95% CLs: 109,403–152,824) (Pomeroy and Walsh Reference Pomeroy and Walsh2015). Thus, by 2008–2009 (the median years) the Sardinian Warbler was already c.3–7 times more numerous than its endemic congener (Table 1) (numbers of both species may have been under-estimated – see below – but we consider that the overall picture will not have been seriously affected). The apparently high densities of Cyprus Warbler present in permanent crops (Table 1) derive from three highland vineyard sites, in an area into which Sardinian Warbler had only recently spread. The total population of Sardinian Warbler in uncultivated land, estimated at c.57,000–72,000, is much larger than that for permanent crops (c.7,900–12,000), because the former is far more extensive. More importantly, much as uncultivated areas (scrub) are important to Cyprus Warbler, even there they were outnumbered by their congener by a factor of c.3.5–6.0.
Rate of spread of Sardinian Warbler
In the west of the island, Sardinian Warbler had already reached Salamiou, some 45 km from the Baths of Aphrodite (Figure 1), by the late 2000s, implying either that they had spread at about 5 km year-1, or that they had been at the Baths of Aphrodite area since earlier than 1990. Had they begun breeding as early as 1980, the rate would still be more than 2 km year-1. Our furthest site, some 15 km beyond Salamiou, had been reached by 2011, a continuing spread rate of at least 1.5 km year-1.
Rainfall
Cyprus receives most of its rain in winter, and this allows rapid growth of the vegetation in the following spring. Good rains during the winter months might therefore be expected to lead to higher arthropod populations in the following spring, higher fruit production, and therefore more food for breeding warblers, which in turn could lead to higher productivity of young and more adults in the following breeding season. The converse should also hold true. Note, however, that exceptionally heavy winter rainfall might reduce arthropod numbers (Speight et al. Reference Speight, Hunter and Watt1999). The winter rains of 2007–2008 were so low as to be classified by the Cyprus Meteorological Service (2013) as a severe drought, defined as less than 70% of the average from 1961–1990. The wettest winters during the survey period were in 2001–2002 and 2002–2003, when rainfall was 111–120% of the long-term average (Cyprus Meteorological Service 2013).
Population trends
We divided Paphos District into five geographical areas (Figure 1), broadly reflecting the eastward spread of Sardinian Warbler. The latter showed a significant increase in all five areas, whilst the Cyprus Warbler declined significantly in three areas (Figure 2). By 2011, the Sardinian Warbler was by far the commoner species in Paphos District. A mean of 1.3 Cyprus Warblers were recorded per site in 1998, dropping to 0.4 in 2011, whilst the mean number of Sardinian Warblers recorded in these years increased from 0.6 to 4.0, respectively (Figure 3). Moreover, by 2010–2011, Cyprus Warblers were detected on only 12 out of 29 sites on which they had been present earlier in the survey.
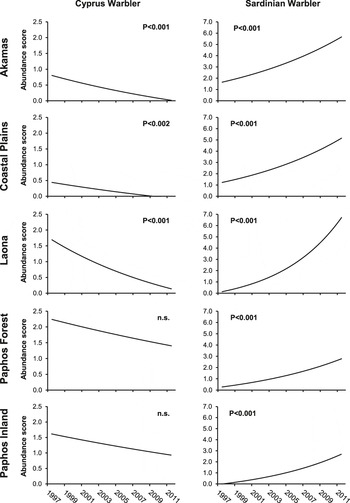
Figure 2. Changes in the abundance of Cyprus and Sardinian Warblers in five areas of Paphos District during 1998–2011. Fitted lines show the slopes derived from LME models of the relationship between each species’ abundance (log+1 transformed) on each site, and study year (1–14). Site identity was entered as a random term. Slope coefficient probabilities are indicated.
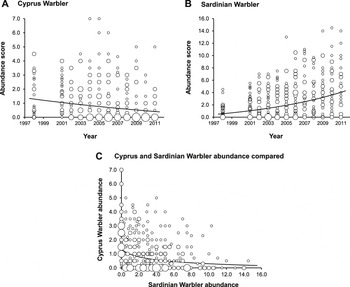
Figure 3. Cyprus and Sardinian Warbler abundance scores at all sites surveyed during 1998–2011. Symbol size indicates the number of sites at which a given abundance value was recorded:
![]() = 1 site;
= 1 site;
![]() >15 sites. Fitted lines show the slopes derived from LME models in which: A. Cyprus Warbler abundance = -0.037 (±0.005 SE)year + 0.867; F1,336 = 51.99, P < 0.0001; B. Sardinian Warbler abundance = 0.090 (±0.005 SE)year + 0.355; F1,336 = 260.62, P < 0.0001. Site identity was entered as a random term and ‘year’ = survey year. C. Abundance values of the two species compared, on sites and years in which at least one species was present. Cyprus Warbler abundance = -0.247 (±0.041 SE)Sardinian Warbler abundance + 0.850; F1,362 = 35.42, P < 0.0001. Site identity and survey year were entered as random terms. Each point represents one site in one survey year. Warbler abundance in each case was log+1 transformed.
>15 sites. Fitted lines show the slopes derived from LME models in which: A. Cyprus Warbler abundance = -0.037 (±0.005 SE)year + 0.867; F1,336 = 51.99, P < 0.0001; B. Sardinian Warbler abundance = 0.090 (±0.005 SE)year + 0.355; F1,336 = 260.62, P < 0.0001. Site identity was entered as a random term and ‘year’ = survey year. C. Abundance values of the two species compared, on sites and years in which at least one species was present. Cyprus Warbler abundance = -0.247 (±0.041 SE)Sardinian Warbler abundance + 0.850; F1,362 = 35.42, P < 0.0001. Site identity and survey year were entered as random terms. Each point represents one site in one survey year. Warbler abundance in each case was log+1 transformed.
Over the study period Sardinian Warbler showed a significant increase in abundance in Paphos District (log(Abundance+1) = 0.090 (± 0.005 SE)Study Year + 0.355, F1,336 = 260.6, P < 0.0001) while Cyprus Warbler showed a significant decrease (log(Abundance+1) = -0.037 (± 0.005 SE)Study Year + 0.867, F1,336 = 52.0, P < 0.0001) (Figure 3a,b). The Cyprus Warbler population in Paphos District thus showed an annualised decline of 8.5%; equivalent to a decline of 59% decade-1. In contrast the Sardinian Warbler’s population in Paphos District showed an annualised increase of 16.4%, or 356% decade-1; a rate of spread not unusual for newly-colonising species. Thus, over the course of the survey the abundance of Cyprus Warbler on a given site and year was inversely correlated with that of Sardinian Warbler (Figure 3c).
To eliminate any effects of misidentifying calls during the first 2–3 years of the study, or of pooling data for the years 1997–1999 and 2000–2001, we calculated the rate of change in Cyprus Warbler abundance for the period 2002–2011 only. During this shorter period the change evident in Cyprus Warbler abundance was very similar to that estimated for the full study period: log(Abundance+1) = -0.034 (± 0.007 SE)Study Year + 0.828, F1,279 = 22.1, P < 0.0001; equivalent to an annualised decline of 8.2%, or 58% decade-1. In the case of Sardinian Warbler the model parameters (log(Abundance+1) = 0.085 (± 0.008 SE)Study Year + 0.404, F1,279 = 119.7, P < 0.0001) indicated an annualised increase of 13.1%, or 244% decade-1 during 2002–2011; a slower rate of increase than was estimated for the full survey period.
To identify variables associated with changes in Cyprus Warbler abundance we compared the rate of change in abundance at each site with a range of potential predictor variables, including three measures of the presence of Sardinian Warbler. In the full GLM, change in Cyprus Warbler abundance was inversely related to the length of site occupancy by Sardinian Warbler; no other variables were selected. When non-significant explanatory variables were removed sequentially, four variables were retained in a minimal model: length of occupancy by Sardinian Warbler, its mean abundance over the period, occupancy*abundance and land-cover. The latter variable comprised six categories (forest, orchards/vines, arable, uncultivated, grassland, built), of which only grassland had a significant effect. We therefore reclassified land-cover into two categories, grassland and non-grassland, and examined its effect on change in Cyprus Warbler abundance in two models, in which we entered length of site occupancy by Sardinian Warbler, and the mean abundance of Sardinian Warbler, respectively. Individually, each measure of the presence of Sardinian Warbler on survey sites was significantly negatively correlated with change in Cyprus Warbler abundance. Based on their respective AIC values we selected the first of these as the final model, which compared change in Cyprus Warbler abundance with the duration of site occupancy by Sardinian Warbler and with land-cover (grassland, non-grassland) (Table 2).
Table 2. Results of a GLM investigating the relationship between annual rate of change in the abundance of Cyprus Warbler, duration of site occupancy by Sardinian Warbler and land-cover category (grassland/non-grassland).

* Non-grassland sites
The final model shows that declines in Cyprus Warbler numbers were steeper on sites occupied by Sardinian Warbler earlier than later in the survey period (and hence for longer), and on sites dominated by forms of land cover other than grassland (F2,41 = 14.69, P < 0.0001). On sites colonised by Sardinian Warbler, Cyprus Warblers would thus be expected to begin declining sooner on non-grassland than on grassland sites (Figure 4). Together, these two variables explained 39% of variation in the rate of change in Cyprus Warbler abundance over the survey period. The high proportion of unexplained variance may be linked to the low numbers of Cyprus Warbler present, since the chance sighting of one more, or one less bird during a survey visit may have had a marked effect on the slope of the regression between Cyprus Warbler abundance and study year, on a given site. Furthermore, we cannot exclude the possibility that the high proportion of unexplained variance may have been caused by unknown environmental factors, or linked to the poor spatial and temporal resolution of the environmental data available.
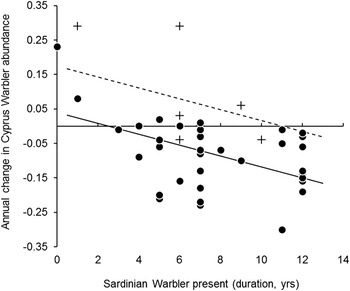
Figure 4. Annual change in the abundance of Cyprus Warblers on grassland (+) and non-grassland sites (
![]() ), in relation to the length of site occupancy by Sardinian Warbler. Change in abundance is expressed as the slope coefficient from a linear regression model for each survey site. Fitted lines show the slope of a GLM in which change in Cyprus Warbler abundance = -0.016 (±0.0045 SE)years of occupancy by Sardinian Warbler -0.135 (± 0.038 SE)land use + 0.175; F2,41 = 14.69, P < 0.0001, R2 = 0.39. Solid line: non-grassland sites; dashed line: grassland sites.
), in relation to the length of site occupancy by Sardinian Warbler. Change in abundance is expressed as the slope coefficient from a linear regression model for each survey site. Fitted lines show the slope of a GLM in which change in Cyprus Warbler abundance = -0.016 (±0.0045 SE)years of occupancy by Sardinian Warbler -0.135 (± 0.038 SE)land use + 0.175; F2,41 = 14.69, P < 0.0001, R2 = 0.39. Solid line: non-grassland sites; dashed line: grassland sites.
To further investigate changes in Cyprus Warbler abundance we examined the numbers of birds recorded on sites surveyed by BirdLife Cyprus during 2006–2014, restricting the analysis to those sites first surveyed during 2006–2009, most of which were surveyed for six years or more. These comprised 17 sites in western Cyprus and 29 in central and eastern Cyprus. Sardinian Warblers were recorded on nine sites (53%) in western Cyprus, but on only four sites (14%) in central and eastern Cyprus. In western Cyprus, the mean number of Cyprus Warblers recorded each year on each site declined overall during 2006–2014: (log(Abundance+1) = -0.036 (± 0.015 SE)Study Year + 0.716, t = 2.37, P = 0.018). This is equivalent to a decline of -8.6% p.a., or -59% decade-1. In contrast, over the same time period, Cyprus Warblers showed no significant change in abundance in central and eastern Cyprus. While Cyprus Warblers declined in western Cyprus, Sardinian Warblers continued to increase (log(Abundance+1) = 0.104 (± 0.023 SE)Study Year + 0.216, t = 4.58, P < 0.001), at a rate equivalent to +24.4% p.a., or +785% decade-1. We were unable to model the pattern of change shown by Sardinian Warblers in central and eastern Cyprus, due to the very low number of occupied sites.
Discussion
Our findings show that, during 1998–2011, the population of Cyprus Warbler declined by c.59% decade-1, in an area of Cyprus accounting for approximately a quarter of the species’ global breeding range. The decline in Cyprus Warbler abundance in the west of the island coincided with the rapid colonisation of western Cyprus by the Sardinian Warbler, which increased at a rate of about 356% decade-1. Although the rate of change in Cyprus Warbler abundance at individual sites was negatively correlated with the duration of site occupancy by Sardinian Warbler, the proportion of the variance explained was low, suggesting that trends in each species’ population might have been influenced by unmeasured environmental factors, beneficial to one species but detrimental to the other. Furthermore, during 2006–2014, when surveys were conducted by BirdLife Cyprus in both western Cyprus and in central and eastern Cyprus, Cyprus Warblers had continued to decline in the former, also at a rate of c.59% decade-1, while Sardinian Warbler numbers had continued to increase. In contrast, Cyprus Warbler numbers in central and eastern Cyprus, where Sardinian Warbler was largely absent, showed no significant change during this period.
Possible explanations for the Cyprus Warbler’s decline in western Cyprus include: a deterioration in the conditions on its wintering grounds; a recent change in its habitats in western Cyprus, detrimental to Cyprus Warbler but beneficial to Sardinian Warbler; and competition between the two congeners for similar resources. Regarding the first of these, a decline in numbers of wintering Cyprus Warblers has been noted over the past 20 years in Israel (H. Shirihai pers. comm. 19 May 2014), through which many migrating Cyprus Warblers also pass (Shirihai et al. Reference Shirihai, Gargallo and Helbig2001). Furthermore, there is some evidence of a drying and warming of the Cyprus Warbler’s wintering areas, including Israel, Egypt and the Red Sea (Meteorological Office 2011). However these changes would be expected to affect the whole population; not just birds from the west.
On the second point, Ieronymidou (Reference Ieronymidou2012) made detailed studies of habitats and habitat change in Cyprus, and particularly noted changes due to the keeping of livestock penned, rather than free-ranging. However, in western Cyprus, this affected only those villages where the keeping of livestock (mainly goats) was traditional; and in those, the change to penning is recent, mainly from 2010 onwards, with some increase in the vegetation in areas where they formerly grazed and browsed. In addition, a total of five new houses appeared along two of the transect routes. The reduction in browsing will only have had a small effect during our study period, but may have slightly benefited the warblers by providing more cover. The new houses affected less than 1% of the count area in the upland arable and vineyard sites where they appeared. In any case, such changes are not confined to western Cyprus, making the second reason unlikely to be a major factor. Nonetheless, we are unable to exclude the possibility that trends in either Sylvia species are linked to regional variation in the volume or pattern of rainfall in Cyprus, due to the poor spatial and temporal resolution of the meteorological data available.
With regard to the possibility of competition between the two congeners, while our results demonstrate a strong, inverse correlation between their population trends, they do not establish causation, and consequently we are unable to determine whether competition was implicated in the Cyprus Warbler’s decline. Similarly, in her detailed study over three breeding seasons, during which she used spatial mapping, playback, and measured productivity and nestling condition, Jones (Reference Jones2006) was unable to find evidence of competition between the two species. Where they occur together in the breeding season, records of interspecific aggression are relatively few, conspecific aggression being more frequent, although still not common, whilst the main expressions of territorial behaviour are song and display (Jones Reference Jones2006, Flint and McArthur Reference Flint and McArthur2014). In addition, Jones noted that home ranges of the two species often overlapped, and their centres were significantly closer than those of conspecifics. However, we should also note that whilst Jones (Reference Jones2006) found no direct evidence of competition, she could not rule out the possibility that breeding Sardinian Warblers might have introduced new pathogens to which Cyprus Warblers had not previously been exposed, but noted that this seems unlikely, given that the two species have been present together in winter for a long time (Jones Reference Jones2006), and that nine other Sylvia species occur in Cyprus on passage (Flint and Stewart Reference Flint and Stewart1992).
In contrast, and based upon their reinterpretation of results presented in Jones (Reference Jones2006), Flint and McArthur (Reference Flint and McArthur2014) suggested that some degree of interspecific territoriality or competitive displacement may exist during the breeding season, and further suggested that this may have contributed to the Cyprus Warbler’s decline. Although different observers used rather different methods for recording aggression, Flint and McArthur (Reference Flint and McArthur2014) also found that during the winter months, interspecific aggression and competition for territories is much more apparent, and this may have negatively affected the Cyprus Warbler’s over-winter survival rate, and hence its ability to establish territories in the following breeding season. Indeed, chases by these warblers, both intra- and interspecific, were more than a hundred times as frequent in winter as in spring, in itself a surprising result.
In our study, Cyprus and Sardinian Warbler differed to some extent in their land-cover preferences (Table 1), notably in the former’s virtual absence from built-up areas, and its being uncommon in arable areas. In contrast, Sardinian Warbler was found in all habitats (Ieronymidou et al. Reference Ieronymidou, Collar and Dolman2012, describe it as more of a generalist), although in varying densities. For those forms of land-cover where both species were relatively common there were differing preferences (Table 1), a finding also reported by Ieronymidou et al. (Reference Ieronymidou, Collar and Dolman2012), who found no evidence of competitive displacement, although that has been queried by Flint and McArthur (Reference Flint and McArthur2014). Ieronymidou et al. (Reference Ieronymidou, Collar and Dolman2012) also found, as we did, that in 2009 the Sardinian Warbler was more abundant inside its breeding range than its endemic congener, recording five times as many registrations per transect. One potentially important difference between the species is that resighting rates of female Cyprus Warblers in subsequent years were much lower than for female Sardinian Warblers (Jones Reference Jones2006), suggesting lower survival (or lower site fidelity) in the former. The fact that resighting rates were lower for females than males could further contribute to the declining population (e.g. Donald Reference Donald2011). In addition, the Cyprus Warbler had a higher incidence of blood parasites and also appears to have a slightly lower reproductive output than Sardinian Warbler (Jones Reference Jones2006).
The presence of migrants, particularly if they are closely-related or have similar diets, can affect the behaviour of resident species (Randler Reference Randler2013). Nine other species of Sylvia occur regularly on Cyprus, but they are mainly passage migrants, of which at least four are common (Flint and Stewart Reference Flint and Stewart1992). Before it became a resident breeder on Cyprus the Sardinian Warbler differed from other Sylvia migrants (apart from Blackcap S. atricapilla) in two respects: it was a winter visitor and it showed site fidelity in successive winters (Flint and Stewart Reference Flint and Stewart1992). A proportion of Sardinian Warblers now resident on Cyprus are also territorial during the winter (Jones Reference Jones2006, Flint and McArthur Reference Flint and McArthur2014). It seems likely that some external factor, such as climate change, was involved in the first Sardinian Warblers staying to breed as winter ended, because this happened at roughly the same time in two, perhaps three different parts of the island (Flint and McArthur Reference Flint and McArthur2014). The subsequent rate of spread of the Sardinian Warbler suggests that a large surplus of young from each breeding season survives to the following year, to become established on new territories beyond the existing breeding range, such that by 2010–2011 Sardinian Warbler had been recorded at least once on all 44 of the survey sites in western Cyprus.
If the decline of Cyprus warbler continues at its present rate, then its future prospects in western Cyprus look poor. However, a few observations offer some hope. Even in the Akamas peninsula, the first area known to be occupied by breeding Sardinian Warblers, the Cyprus Warbler is still to be found, albeit rarely. And even at a density of one pair km-2 (and it is still above that; pers. obs.) Paphos District alone would support a total population of several hundred, if their preferred scrubby habitats are adequately conserved. This may now be the case, with the recent increase in Special Protection Areas in Cyprus (Hellicar et al. Reference Hellicar, Anasatasi, Beton and Snape2014). The drying of the climate of Cyprus (Cyprus Meteorological Service 2013) seems to be less unfavourable to Sardinian than to Cyprus Warbler (see above; also Flint and McArthur Reference Flint and McArthur2014), suggesting that areas that may remain moister, such as higher ground in the west of the island, would be more favourable to the survival of the latter species. A totally different picture is given by Doswald et al. (Reference Doswald, Willis, Collingham, Pain, Green and Huntley2009), who predict that by the end of this century, the Cyprus Warbler, in response to projected climate change, may shift its breeding range 150–300 km north, into Turkey, where it already occurs occasionally in spring, presumably having ‘over-shot’ Cyprus (Kirwan et al. Reference Kirwan, Boyle, Castell, Demirci, Özen, Welch and Marlow2008). A spread into Turkey could mean expanding the species’ range by a factor of around 10, whilst perhaps making shorter migrations in winter. Meanwhile, the range of the Sardinian Warbler is not predicted to grow much, if at all (Doswald et al. Reference Doswald, Willis, Collingham, Pain, Green and Huntley2009).
It is clearly important to continue to monitor the numbers of Cyprus Warblers across the whole island. As noted above, Cyprus Warbler may be harder to detect than Sardinian Warbler, leading to an under-estimation of numbers, and Newson et al. (Reference Newson, Massimino, Johnston, Baillie and Pearce-Higgins2013) have pointed out that detectability of a species may change with time. For example, as Cyprus Warblers become increasingly outnumbered by Sardinian Warblers they may respond by becoming more cryptic. Further, Flint and McArthur (Reference Flint and McArthur2014) have also shown that (up to 2013) encounter rates and abundance of Cyprus Warblers were much higher where Sardinian Warblers were scarce or absent than where they were long-established and at high population levels. For these reasons, and in order to obtain more accurate population estimates, the methods of censusing need careful consideration; while transect counts are generally excellent for monitoring, they may be less suitable for estimating population densities, despite their wide use for this purpose.
In conclusion, the Cyprus Warbler, which is currently listed as ‘Least Concern’ (BirdLife International 2014), showed a steep (-59% decade-1) decline in western Cyprus both during 1998–2011 and, in a separate survey, during 2006–2014. The causes of this sustained decline have not been established. In central and eastern Cyprus, during 2006–2014, the species showed no significant change in abundance. If the decline observed in western Cyprus was linked to the region’s colonisation by Sardinian Warbler, and if the latter continues to spread eastwards, the Cyprus Warbler is likely to show a global population decline at or above the threshold for species qualifying as ‘Vulnerable’ under IUCN criterion A1, or as Endangered, under criterion A2 (IUCN 2014). Consequently, we strongly recommend the development of an Action Plan (IUCN 2015) for the species. This could be undertaken under the African-Eurasian Migrant Land Bird Action Plan (a programme of the Convention on Migratory Species), where Cyprus Warbler is one of the listed species.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270915000337
Acknowledgements
Vicky Jones and anonymous referees offered many pertinent comments on earlier versions of the paper. The Environment Study Centre at Kritou Terra, and especially its then Director, Nick Symons, provided an excellent base for much of the field work. PF also wishes to thank Alison McArthur and Colin Richardson for their comments and discussion on this topic. We also thank Will Cresswell, for providing invaluable assistance with parts of the analyses.








