Introduction
For many birds, reproductive success is dependent on the selection of a suitable nest site. Common factors that define a suitable nest site may include: shelter from adverse climatic conditions, protection from predation, presence of conspecifics, minimised disturbance, and/or proximity to food (Partridge Reference Partridge, Krebs and Davies1978, Cody Reference Cody and Cody1985, Walsberg Reference Walsberg and Cody1985, Kim and Monaghan Reference Kim and Monaghan2005). In a given habitat, cues to the locations where suitable nesting conditions could be met are often provided by certain features, such as vegetation composition and/or structure (Partridge Reference Partridge, Krebs and Davies1978, Cody Reference Cody and Cody1985). Identifying and understanding the factors that are most important, and the habitat features that provide them, is essential to the success of species-targeted habitat restoration initiatives.
The endangered Yellow-eyed Penguin Megadyptes antipodes, or “hoiho”, inhabits a restricted range in New Zealand (BirdLife International 2012, Seddon et al. Reference Seddon, Ellenberg, van Heezik, Borboroglu and Boersma2013). Throughout the South Island part of the hoiho's range, most of the coastal forest habitat that existed before European settlement has been cleared (Seddon and Davis Reference Seddon and Davis1989, Darby and Seddon Reference Darby, Seddon, Davis and Darby1990). As a consequence, hoiho that breed in this area nest primarily in alternative habitats that may reduce reproductive success (Darby and Seddon Reference Darby, Seddon, Davis and Darby1990). This issue has been addressed by the New Zealand Department of Conservation and the Yellow-eyed Penguin Trust, both of which identify the re-vegetation of nesting habitats as a primary management activity, and one of nine objectives in the “Hoiho recovery plan” (McKinlay Reference McKinlay2001, Yellow-eyed Penguin Trust 2012).
Similar to other penguin species at temperate latitudes, hoiho nest primarily in locations that are sheltered from direct exposure to sunlight, which is considered to reflect a strategy for avoiding negative effects that can result from insolation (Stonehouse Reference Stonehouse and Holdgate1970, Seddon and Davis Reference Seddon and Davis1989, Darby and Seddon Reference Darby, Seddon, Davis and Darby1990, Williams Reference Williams1995). However, unlike other penguins, hoiho nests are typically well concealed and widely dispersed, with an average inter-nest distance that can exceed 20 m (Seddon and Davis Reference Seddon and Davis1989, Darby and Seddon Reference Darby, Seddon, Davis and Darby1990, Marchant and Higgins Reference Marchant and Higgins1990, Moore Reference Moore1992). This results in the common visual isolation of each nest, which has been consistently documented (e.g. Richdale Reference Richdale1957, Seddon and Davis Reference Seddon and Davis1989, Marchant and Higgins Reference Marchant and Higgins1990, Moore Reference Moore1992) and has been considered an essential requirement for hoiho (Darby Reference Darby1985, McKinlay Reference McKinlay2001, Birdlife International 2012). Darby (Reference Darby1985) and Lalas (Reference Lalas1985) reported observations of nest failures that appeared to result from a lack of visual isolation from conspecifics. However, Seddon and Davis (Reference Seddon and Davis1989) considered that the visual isolation of nests from conspecifics may be only a consequence of hoiho selecting sites with substantial cover that provides ample protection from insolation.
Visual isolation from conspecifics has been observed to positively affect the breeding performance of Larus gulls. For example, Burger (Reference Burger1977) and Kim and Monaghan (Reference Kim and Monaghan2005) observed shorter inter-nest distances and greater reproductive success for gulls that nested in vegetation as opposed to bare ground. This correlation was partially attributed to the lower visibility between nests in vegetation, which reduced the frequency of aggressive interactions and other disturbance between neighbours, and therefore allowed incubating birds to spend more time resting and attending offspring (Burger Reference Burger1977, Bukacińska and Bukaciński Reference Bukacińska and Bukaciński1993, Kim and Monaghan Reference Kim and Monaghan2005).
Determination of whether visual isolation from conspecifics or protection from insolation is the primary driver of hoiho nest site selection has important implications for ongoing habitat restoration that seeks to maximise nesting densities. If visual isolation from conspecifics is an essential nest site requirement for hoiho, then, similar to Larus gulls, the availability and distribution of suitable sites within a nesting habitat could be influenced by the limits of visibility. However, if visual isolation is a result of selection for adequate shelter from insolation, then nest site selection, and hence distribution, may be influenced by the density or distribution of habitat features that provide a suitable amount of protective cover from sunlight. Previous studies recorded whether hoiho nest sites were visually isolated from each other (e.g. Seddon and Davis Reference Seddon and Davis1989, Moore Reference Moore1992), or derived an index of visual isolation based on the density and cover of vegetation at nest sites (Smith Reference Smith1987). We assessed the apparent importance of visual isolation from neighbouring conspecific nests by comparing the distance of visibility of active nests with unused sites, and whether inter-nest distance was correlated with the distance of visibility. To assess whether visual isolation may be a consequence of selection for adequate protection from insolation, we compared the amount of cover from insolation at nests, with that at unused sites, and tested for correlations of this variable with the distance of visibility and inter-nest distance. Our aim was to advance the understanding of hoiho nest site requirements, and thereby contribute to improving the effectiveness of habitat restoration and re-vegetation activities.
Methods
Study areas
We examined hoiho nest site selection and distribution in three habitat types at two study areas on the south-east coast of the South Island of New Zealand: flax and coastal scrub at Boulder Beach, and coastal forest at Hinahina Cove (Figure 1). Boulder Beach comprises c.55 ha of vegetated land extending up to 250 m inland and situated along c.2 km of mixed gravel-sandy beach and some cliffs on the south coast of the Otago Peninsula. The area was used for sheep grazing until the mid-1980s when it was fenced and a re-vegetation programme was established (Seddon et al. Reference Seddon, van Heezik and Darby1989). Vegetation cover consists of varying patches of a native coastal scrub Hebe elliptica and flax Phormium tenax interspersed amongst larger areas of grasses (mainly Ammophila arenaria and Poa species) and exotic scrub species (primarily Lupinus arboreus and Ulex europaeus). Also present are small patches of native broadleaf trees (Cordyline australis and Myoporum laetum), shrubs (e.g. Solanum laciniatum), vines (e.g. Muehlenbeckia australis), bracken fern Pteridium esculentum, and rushes. The flax habitat was dominated by Phormium tenax, and included occasional Hebe elliptica scrub, Solanum laciniatum, Blechnum fern species, and grasses. Scrub habitat consisted primarily of Hebe elliptica and/or exotic Ulex europaeus, and also included some Myoporum laetum, Lupinus arboreus, Muehlenbeckia australis, and Solanum laciniatum. The flax and scrub habitats at Boulder Beach were mapped using orthorectified colour aerial photographs taken in September 2006, and validated with observations recorded during data collection.

Figure 1. Locations of the two study areas, Boulder Beach and Hinahina Cove, on the south-east coast of the South Island of New Zealand. Aerial imagery of the study areas are overlaid with the locations of active Yellow-eyed Penguin Megadyptes antipodes nest sites and randomly selected unused sites sampled in 2006–2007. The extents of flax and scrub nesting habitats at Boulder Beach in 2006–2007 are also indicated.
Hinahina Cove lies c.100 km south-southwest of Boulder Beach and has a rocky coastline along sheer cliffs. Hoiho access the area via a rock platform at the mouth of Hinahina Stream and nest within native coastal forest that extends c.2 km inland along the stream and on a steep slope to the north. Open grazed pasture lie on a gradually rising slope to the south of the stream. The forest covers c.565 ha, while the area used by hoiho for nesting is considered to be c.25 ha (Seddon et al. Reference Seddon, van Heezik and Darby1989). The forest canopy consists of Melicytus lanceolatus, Weinmannia racemosa, and Myoporum laetum near the coast, changing inland to podocarp tree species such as Metrosideros umbellata, Podocarpus ferrugineus, and Dacrydium cupressinum. Much of the area beneath the forest canopy is relatively open, which may be partly due to cattle grazing that occurred until 1987, when the area was designated a reserve (Seddon et al. Reference Seddon, van Heezik and Darby1989), and may also reflect the presence of deer and pigs (New Zealand Department of Conservation 2013). Nevertheless, crown ferns Blechnum discolor cover much of the forest floor, and other scattered patches of sub-canopy vegetation consist of broadleaf trees (e.g. Griselinia littoralis, Myrsine australis, and Pseudopanax crassifolius), fern trees (e.g. Dicksonia species), the liane Ripogonum scandens, and shrubs (e.g. Coprosma species). Logs, stumps and snags of dead or fallen trees are also scattered throughout the forest.
Data collection
The study areas were thoroughly searched for active nest sites beginning in October 2006, and periodically throughout the breeding season, until January 2007. Active nest site locations were recorded with a professional grade GPS (Leica Geosystems GS20 Professional Data Mapper), with which we obtained sub-metre accuracy following the differential correction of coordinates. At Hinahina Cove we examined all 14 active nest sites found, whilst at Boulder Beach, the number of examined nest sites was limited to 31 of the 55 found because of resource and time constraints, the difficulty accessing some nests due to cliffs or steep, slippery slopes, and the exclusion of two nests located in man-made structures (i.e. a nest box and the remnants of a small stone hut).
We established locations of unused sites to compare with active sites in each habitat using a random point-generating algorithm in a GIS, excluding points that occurred within 5 m of each other or an active nest site (based on the minimum distance between nests reported by Seddon and Davis (Reference Seddon and Davis1989). When in the field, if the randomly generated location of an unused site did not occur on level ground, or occurred outside of the designated habitat type (e.g. in an open, unvegetated or grass covered area), then the position of the site was relocated towithin the nearest habitat patch matching the designated type (flax, scrub, or forest). We ensured as much as possible that unused sites were not very different from active nest sites (i.e. they were within or immediately surrounded by vegetation cover within the first metre above the ground, could be accessed by hoiho, and usually had a backing structure). Table 1 provides a summary of the number of active nest and random unused sites examined in each habitat type.
Table 1. Sample sizes for: (1) the original datasets of active Yellow-eyed Penguin Megadyptes antipodes nests and randomly selected unused sites; (2) the datasets used in analyses of variables influencing nest site selection, and (3) the dataset for the assessment of the mean minimum inter-nest distance, in flax and scrub habitats at Boulder Beach, and forest habitat at Hinahina Cove, New Zealand, 2006–2007.

To minimise disturbance to breeding adults and chicks, we collected measurements of the mean maximum distance of visibility (hereafter referred to as “visibility”), and the mean amount of protection from insolation (“insolation cover”) of active nests and unused sites in February 2007, when nests had been recently vacated. For the assessment of visibility, we assumed that human vision was not significantly different from that of hoiho vision on land. This was based on findings that penguin visual acuity appears to be nearly emmetropic in air (Sivak and Millodot Reference Sivak and Millodot1977, Sivak et al. Reference Sivak, Howland and McGill-Harelstad1987), and the physiology of the penguin retina is considered well adapted to the spectral properties of both deep water and terrestrial environments (Bowmaker and Martin Reference Bowmaker and Martin1985, Suburo and Scolaro Reference Suburo and Scolaro1999).We assessed visibility with a profile pole, a device for measuring the amount of visual obstruction of vegetation and/or other habitat structures (Robel et al. Reference Robel, Briggs, Cebula, Silvy, Viers and Watt1970, Griffith and Youtie Reference Griffith and Youtie1988, Higgins et al. Reference Higgins, Oldemeyer, Jenkins, Clambey, Harlow and Bookhout1996). We used a profile pole constructed of a 100 cm x 5cm plastic tube divided into 10 alternating black and white sections, and fitted with a metal spike in a cap on the bottom to anchor it in the ground. At each active and unused site, we placed the pole in the centre, and at the height of a standing adult hoiho - approximately 60–65 cm based on Darby and Seddon (Reference Darby, Seddon, Davis and Darby1990) and Marchant and Higgins (Reference Marchant and Higgins1990) - we recorded measurements of the percentage of each 10 cm section of the pole visible from set distances along three bearings. The first bearing was determined by a random number between 0 and 359; the second and third bearings were 120 degrees to the east and west of the first bearing. Along each bearing we measured visibility beginning at 0.5 m from the pole, then at 1m, 2m, and every subsequent 2 m until less than 5% of the pole could be seen. We defined the maximum distance of visibility as the set distance immediately preceding that where less than 5% of the pole was visible. We therefore collected three measurements of the maximum distance of visibility at each site, one for each bearing, and used the mean of the three measurements in analyses. The top 20 cm of the pole were excluded from the assessment as this portion extended above the canopy at several sites.
To assess the amount of insolation cover of active and unused sites, we used the LAI-2000 Plant Canopy Analyser (LI-COR Inc. 1990) to obtain estimates of the fraction of gaps in site canopies. The LAI-2000 estimates parameters of canopy structure by comparing measurements of diffuse solar radiation recorded in a nearly hemispheric “view” (i.e. both overhead and laterally) above (or outside) and beneath a canopy (LI-COR Inc. 1990, Welles and Norman Reference Welles and Norman1991). An estimate of the fraction of gaps in a canopy is obtained with the diffuse non-interceptance parameter ( τ ), which is the probability of diffuse radiation above a canopy penetrating to a particular location beneath the canopy (LI-COR Inc. 1990, Welles and Norman Reference Welles and Norman1991). At each active and unused site, we collected one above-canopy recording and a set of three beneath-canopy recordings taken approximately 10 cm above the ground at the same position near the centre of the site. We collected above-canopy recordings of the open sky for sites in the flax and scrub habitats at Boulder Beach, whilst at Hinahina Cove we collected above-canopy recordings under the main forest canopy, where we assumed an even level of light intensity due to the relative completeness of the canopy. The LAI-2000 divided the average of the beneath-canopy recordings by the above-canopy recordingto obtain a single τ value that ranged from 0 (no gaps in the site canopy = assumed complete insolation cover) to 1 (little or no site canopy = assumed minimal insolation cover) (LI-COR Inc. 1990). For example, a τ value of 0.47 would indicate gaps in an average of 47% of a site canopy (LI-COR Inc. 1990), which we would assume to indicate approximately 53% insolation cover. Subsequently, for data analyses we converted τ to % insolation cover, i.e. 100 * (1- τ ).
Data analysis
To assess the relative importance of visibility and insolation cover in hoiho nest site selection, we conducted a three-part analysis that included univariate, correlation test, and logistic regression components. The sample sizes for these analyses were determined after excluding outlying values that had a significant effect on the distribution and variance of a dataset (defined by habitat and site type, e.g. forest habitat - active sites; Table 1).
We first used univariate ANOVA and Mann-Whitney tests (for non-normally distributed datasets) to separately compare the mean visibility and percentage insolation cover recorded for active sites with the mean values recorded for unused sites in each habitat, and to compare habitats in terms of the means of each variable recorded for active sites. However, we did not compare the mean percentage insolation cover at forest active sites with that at flax and scrub active sites due to the different conditions in which above-canopy measurements were recorded. To further test the significance of any differences, we compared the means of the observed data with five thousand bootstrap samples of each data set.
We anticipated that percentage insolation cover could affect visibility, and therefore assessed this by evaluating Spearman's correlations between the visibility and percentage insolation cover of active sites in each habitat. Thirdly, to assess the relative importance of visibility and insolation cover in hoiho nest site selection, we evaluated logistic regression models (binomial family with logit link function), with a dependent variable of 1 = active nest site, or 0 = unused site. The analysis of logistic regression models has been recommended for evaluating the relative importance of multiple variables in comparisons of used (active nests) and available (random unused) units (Manly et al. Reference Manly, McDonald, Thomas, McDonald and Erickson2002). We considered five possible models for each habitat (Table 2) and we evaluated the relative evidential support of the models, and the relative importance of visibility and percentage insolation cover based on the difference of the second-order (i.e. corrected for small sample sizes) Akaike's Information Criterion scores (∆AICc) from the smallest AICc score, and the associated Akaike weights (w i ) (Burnham and Anderson Reference Burnham and Anderson1998, Johnson and Omland Reference Johnson and Omland2004, Wagenmakers and Farrell Reference Wagenmakers and Farrell2004, Burnham et al. Reference Burnham, Anderson and Huyvaert2011).
Table 2. Logistic regression models (binomial family with logit link function) evaluated in an analysis of the relative importance of the maximum distance of visibility (V), and the percentage insolation cover (IC) in the selection of nest sites by the Yellow-eyed Penguin Megadyptes antipodes. Data for the models was collected at active nest sites and randomly selected unused sites in flax and scrub habitats at Boulder Beach, and forest habitat at Hinahina Cove, New Zealand, 2006–2007. Listed for each model are the number of parameters (K), Akaike's Information Criterion value corrected for small sample sizes (AICc), differences (∆AICc) between the AICc of each model and the lowest AICc, and the Akaike weight (w i ). The * symbol represents models that contained an interaction between V and IC, and the + symbol represents models that contained both V and IC. The global model for each habitat fit the data well: Flax (χ2 = 3.98, df = 8, P > 0.8); Scrub (χ2 = 3.29, df = 8, P > 0.9); Forest (χ2 = 0.43, df = 8, P = 1.0).

For the analysis of inter-nest distance, we used ArcGIS to obtain Euclidean distances (to the nearest 0.1 m) between the differentially corrected GPS recorded locations of active sites. To avoid effects resulting from the fragmented distribution of the flax and scrub habitats at Boulder Beach, we limited the inter-nest distance samples of these habitats to include only values for active sites that occurred within the same contiguous habitat patch (Table 1). We used Mann-Whitney tests to compare the mean minimum inter-nest distances in each habitat type, and we used Spearman’s correlation to assess the effect of visibility and percentage insolation cover on inter-nest distance in each habitat.
The analyses were primarily conducted with SPSS 14.0 statistical software, apart from the bootstrap sampling which was run in Microsoft Excel. We conducted AICc model evaluation using the “AICcmodavg” package, version 1.35, in the programme R (Mazerolle Reference Mazerolle2013).
Results
The univariate analysis revealed varied yet significant differences between active and unused sites in all but one comparison. In the assessment of visibility, unused sites in forest were visible from a mean maximum distance of 4.2 m, which was greater than the mean visibility of active sites by more than 2.5 m (F = 26.4, P < 0.001, Figure 2). At Boulder Beach, the mean visibility of unused sites in scrub was greater than that of active sites by 0.9 m (F = 4.2, P = 0.05), while in flax the mean visibility of active and unused sites was nearly equal (Figure 2). In habitat comparisons, active sites in forest were visible from a mean maximum distance that was 0.8 m greater than that of active sites in flax (U = 45.5, P < 0.01) and scrub (F = 5.1, P = 0.03), which were not significantly different in visibility (Figure 2).
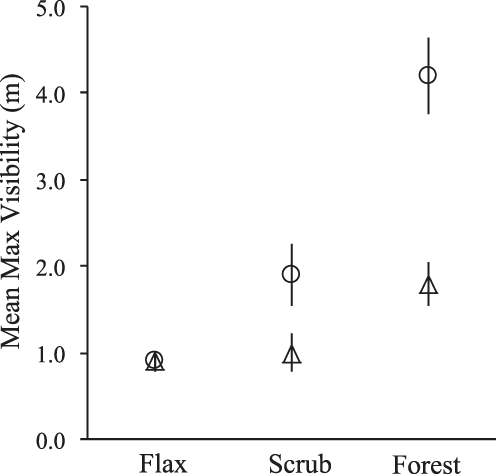
Figure 2. Mean (± SE) maximum distance of visibility measured (to the nearest 0.5 m) at active Yellow-eyed Penguin Megadyptes antipodes nests (triangles) and randomly sampled unused sites (circles) in flax and scrub habitats at Boulder Beach, and forest habitat at Hinahina Cove, New Zealand, 2006–2007. Sample sizes are provided in Table 1.
As with visibility, the difference between active and unused sites in percentage insolation cover was again greatest in the forest habitat, where the mean diffuse non-interceptance values indicated an average percentage insolation cover at active sites that was 35% greater than at unused sites (U = 10.0, P < 0.001; Figure 3a). The mean diffuse non-interceptance values recorded in flax and scrub habitats indicated a relatively high percentage insolation cover at both active and unused sites. However, the average percentage insolation cover at active sites was greater than at unused sites by a relatively small yet statistically significant 5% in both flax (F = 5.9, P = 0.02), and scrub (F = 15.4, P = 0.001; Figure 3b).Similarly, in a comparison between active sites in scrub and flax, the average percentage insolation cover at scrub sites was greater than at flax sites by 5% (U = 50.0, P < 0.001; Figure 3b).

Figure 3. Mean (± SE) % insolation cover (derived from the mean diffuse non-interceptance) recorded at active Yellow-eyed Penguin Megadyptes antipodes nests (triangles) and randomly sampled unused sites (circles) in (a) forest habitat at Hinahina Cove, and (b) flax and scrub habitats at Boulder Beach, New Zealand, 2006–2007. Sample sizes are provided in Table 1.
The correlation analysis showed no relationship between visibility and insolation cover in flax and scrub. Conversely, in the forest habitat a decrease in percentage insolation cover had a relatively strong, positive monotonic effect on the visibility of active sites (r s = 0.66, P = 0.02; Figure 4c). Subsequently, we interpreted the results of the logistic regression analysis with caution, particularly for the forest habitat.

Figure 4. Scatter plots representing the relationship between mean maximum distance of visibility (measured to the nearest 0.5 m) and % insolation cover (derived from the mean diffuse non-interceptance) recorded at active Yellow-eyed Penguin Megadyptes antipodes nest sites in flax and scrub habitats at Boulder Beach, and forest habitat at Hinahina Cove, New Zealand, 2006–2007. The habitat type, Spearman’s correlation coefficient (r s ) and associated significance (P) are indicated above each plot. The significant correlation in plot (c) is indicated in bold font. Sample sizes are provided in Table 1.
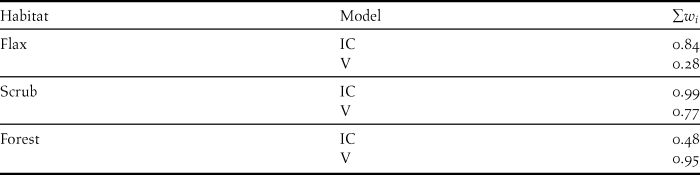
According to the Akaike weights (w i ), the model with the greatest w i for each habitat reflected the univariate analysis results, i.e. both visibility and insolation cover were important in forest (w i = 0.52) and scrub (w i = 0.61), and only insolation cover was important in flax (w i = 0.60; Table 2). However, the probabilities of these models were not particularly strong (i.e. no w i > 0.90), and the differences between the AICc scores of most models and the highest weighted model (i.e. with the lowest AICc score) were small (i.e. ∆AICc < 5), leading to uncertainty and reduced confidence in the interpretation of a single best model (Johnson and Omland Reference Johnson and Omland2004, Burnham et al. Reference Burnham, Anderson and Huyvaert2011). Subsequently, we compared the cumulative weight (∑w i ) of the set of models containing insolation cover with the ∑w i of the set containing visibility to estimate the relative importance of each variable in each habitat (Burnham and Anderson Reference Burnham and Anderson2004, Johnson and Omland Reference Johnson and Omland2004, Wagenmakers and Farrell Reference Wagenmakers and Farrell2004). From this it was apparent that insolation cover was more important for hoiho nest site selection than visibility in flax and scrub, where the ∑w i of models with % insolation cover were 0.84 and 0.99, respectively (Table 3). This was primarily a result of the large difference in ∆AICc and w i of the models containing each variable individually (Table 2). Converse results were observed for the forest habitat, where visibility appeared to be more important than insolation cover (Tables 2 and 3). However, the reliability of this result is uncertain due to the correlation of visibility with insolation cover.
Table 3. Comparison of the cumulative Akaike weights (∑w i ) of models containing insolation cover (IC) with the ∑w i of models containing visibility (V), as part of an analysis of the relative importance of the two variables in the selection of nest sites by the Yellow-eyed Penguin Megadyptes antipodes. The individual w i and associated information of all models are provided in Table 2.
Mean minimum inter-nest distance was greatest in forest (23.4 m), but not significantly greater than in scrub (22.6 m), whereas mean minimum inter-nest distance in flax (10.7 m) was significantly less than in forest (U = 10.0, P < 0.001) and scrub (U = 4.0, P < 0.001). An effect of insolation cover and/or visibility on minimum inter-nest distance was only apparent in the flax habitat, where a Spearman correlation revealed a significant, positive monotonic influence of decreasing insolation cover on minimum inter-nest distance (r s = 0.63, P = 0.03; Figure 5b).

Figure 5. Scatter plots representing the relationships between mean minimum inter-nest distance (measured to the nearest 0.1 m) and the mean maximum distance of visibility (measured to the nearest 0.5 m) and mean % insolation cover (derived from the mean diffuse non-interceptance) recorded at active Yellow-eyed Penguin Megadyptes antipodes nest sites in flax and scrub habitats at Boulder Beach, and forest habitatat Hinahina Cove, New Zealand, 2006–2007. The habitat type, Spearman’s correlation coefficient (r s ) and associated significance (P) are indicated above each plot.The significant correlation in plot (b) is indicated in bold font. Sample sizes are provided in Table 1.
Discussion
Despite the variation between habitats in our results, there was more evidence for the importance of the amount of insolation protection than for visibility, and thus visual isolation, in hoiho nest site selection. While we observed a significant difference in the visibility of active and unused sites in forest and scrub, this did not reflect an importance of visual isolation from conspecifics in hoiho nest site selection in these habitats. This inference is based on three primary outcomes of our study: 1) no relationship between inter-nest distance and visibility, 2) a relatively strong influence of insolation cover on visibility in forest along with evidence of insolation cover having greater importance than visibility in scrub and flax, and 3) an effect of the amount of insolation cover on inter-nest distance in flax. The correlation of visibility with insolation cover in the forest habitat might have also been observed in flax and scrub if insolation cover was similar among habitats. Alternatively, had we been able to measure insolation cover in forest as it was measured in flax and scrub, then we might not have observed a significant correlation of visibility with insolation cover in forest. Nevertheless, our results support the hypothesis that the visual isolation of hoiho nests from conspecifics is at least partly a consequence of selection for nest site features that provide significant protection from insolation.
Like all penguins north of the sub-Antarctic, the hoiho is considered to be over-insulated for the terrestrial environment, and subsequently may require shelter from insolation while on land to avoid heat stress (Stonehouse Reference Stonehouse and Holdgate1970, Seddon and Davis Reference Seddon and Davis1989). Protection from insolation may be most important during the breeding season, when incubating birds are particularly prone to heat stress (Frost et al. Reference Frost, Siegfried and Burger1976, Seddon and Davis Reference Seddon and Davis1989). Therefore, for hoiho, the most important features of a nest site appear to be those that help minimise the risk of negative effects resulting from insolation.
Hoiho indeed appear to be highly selective of the amount of cover at a nest site regardless of the habitat type. This was particularly evident in the forest habitat at Hinahina Cove, where, despite the apparently low risk of insolation due to the intact forest canopy, hoiho primarily selected maximally sheltered nest sites in hollows under logs, stumps, or tree stems. This has also been observed on New Zealand's southern islands, where hoiho nesting areas are covered primarily by indigenous coastal scrub, e.g. as described for Campbell Island by Moore (Reference Moore1992). Seddon and Davis (Reference Seddon and Davis1989) reported that the amount of cover within 50–100 cm of the ground appears to be particularly important for hoiho nest site selection. The hoiho's selection for nest sites with these structural features can be considered analogous to the use of caves and burrows by other penguin species at temperate latitudes (Stonehouse Reference Stonehouse and Holdgate1970, Frost et al. Reference Frost, Siegfried and Burger1976, Williams Reference Williams1995). Along with insolation protection, these sites would also offer shelter from other climatic effects, thereby providing a moderate and stable micro-climate. The selection for these types of sites could also explain the use of a wooden nest box and stone hut remnant we observed (but excluded from analyses) at Boulder Beach. Furthermore, a study on the deployment of nest boxes designed after these typical features of hoiho nest sites showed that they were readily and successfully used by hoiho (Lalas et al. Reference Lalas, Jones and Jones1999).
In contrast, McKay et al. (Reference McKay, Lalas, McKay and McKonkey1999) observed hoiho successfully nesting in a grazed grassland-dominated habitat, where a few nests had little to no overhead and lateral cover. The authors of this study did not specifically state whether hoiho at these nest sites were visible to each other. However, they did report that the grassland nests had a lower success rate than nests in adjacent shrubland habitat, which they considered likely to reflect that the grassland nests had been established by inexperienced breeders (McKay et al. Reference McKay, Lalas, McKay and McKonkey1999). Two important features of the most exposed grassland nest sites observed by McKay et al. (Reference McKay, Lalas, McKay and McKonkey1999) were a solid backing in the form of a clay bank, rock, or rushes, and a south facing aspect. McKay et al. (Reference McKay, Lalas, McKay and McKonkey1999) suggested that the uncovered nest sites with a south-facing aspect were probably not affected by insolation as they were only exposed to sunlight during early morning hours. Marchant and Higgins (Reference Marchant and Higgins1990) also reported observations of hoiho nesting on steep cliffs that faced away from the sun and toward the sea.
A solid backing structure has been reported as an important feature of hoiho nest sites by Seddon and Davis (Reference Seddon and Davis1989), who observed that active nest sites had a backing structure significantly more often than random unused sites in all habitats examined. While we did not assess the importance of a solid backing structure, the likely presence of this feature at active nest sites may have influenced the results of our analyses. For example, in the flax and scrub habitats, the relatively small yet significant difference between active and unused sites in the amount of insolation cover may have reflected a greater occurrence or different composition of a solid backing structure at active nest sites.
In addition to the consequence of visual isolation, the relatively large distances between hoiho nests could also be attributed to the selection for structural micro-habitat features that provide extensive cover within 1 m above the ground. This was reflected in the significant correlation between inter-nest distance and insolation cover in flax, which suggests that a lower ground-level (i.e. up to 1 m) vegetation density could result in a lower density of sites with a suitable amount of cover, and therefore a greater distance between nests. In any nesting habitat, the availability and distribution of suitable nest sites will be influenced primarily by the spatial variation of the preferred features. For example, the distribution of nests in forest habitat may reflect the spatial distribution of logs, stumps and similar features containing the hollows that hoiho seem to prefer. Habitats that do not contain these particular features, yet consist of relatively dense vegetation within 1 m of the ground, may provide suitable nest sites at shorter distances and greater densities. This was apparent in the flax habitat we examined at Boulder Beach. However, we cannot infer from this observation that a nesting habitat dominated by flax may be more suitable for hoiho than other habitat types. More research on the aspects (e.g. micro-climate) of nest sites in flax compared to other habitats is needed. In the forest habitat, we might have observed shorter minimum inter-nest distances if there was a greater density of understorey vegetation (i.e. not modified by introduced mammals such as deer, pigs, and cattle). However, it is unknown whether this would also lead to a greater number of nests at Hinahina Cove as this can be influenced by several other factors that were beyond the scope of our study.
In conclusion, our results provide support for the hypothesis that hoiho nest site selection and distribution appear to be influenced primarily by the location of structural micro-habitat features (e.g. a significant amount of cover 50–100 cm above the ground and a solid backing) that provide optimal protection from insolation, and may help fulfil other potential requirements such as shelter from other climatic effects. Strong selection for these nest site features results in: 1) a high probability of visual concealment, 2) relatively large distances between nests (especially in habitats where suitable nest site features are available at lower densities), and subsequently 3) the typical but non-essential visual isolation of nest sites from conspecifics. The consequential visual concealment of nests may be beneficial for reducing the risk of predation and negative effects of disturbance from other animals and humans, such as nature tourism (Ellenberg et al. Reference Ellenberg, Setiawan, Cree, Houston and Seddon2007), but there is no concrete evidence that visual isolation from conspecifics is an essential requirement. The proximate cause of the nest failures attributed to a lack of visual isolation from conspecifics by Darby (Reference Darby1985) and Lalas (Reference Lalas1985) may have been a detrimental frequency of disturbance. However, rather than visible exposure to neighbouring conspecifics, the ultimate cause of these failures may have been increased disturbance due to a lack of insolation cover (i.e. increased frequency of the incubating adult standing or leaving the nest for shade to relieve heat stress, thereby exposing the eggs to insolation (Seddon and Darby Reference Seddon and Darby1990). Furthermore, perhaps the nesting birds were inexperienced breeders, as was suggested for the reduced breeding success of some of the “open” nests in grazed grassland observed by McKay et al. (Reference McKay, Lalas, McKay and McKonkey1999).
Nesting habitats comprised of relatively dense vegetation and/or other structures within 1 m of the ground may provide conditions that allow for greater nest densities. However, as demonstrated in McKay et al. (Reference McKay, Lalas, McKay and McKonkey1999) and reported in Marchant and Higgins (Reference Marchant and Higgins1990), where dense vegetation or other forms of cover are not available, hoiho can successfully nest in relatively open conditions where the nest backing structure and the aspect may provide enough shelter from insolation, though reduced breeding performance may be a consequence.
Recommendations
Our findings suggest that restoration projects should aim to produce nesting habitats with a relatively high density and diversity of vegetation and solid structures (boulders, banks, etc.), particularly within 1 m of the ground. This may eventually provide an optimal availability and quality of suitable nest sites with adequate insolation cover and a firm backing structure, thereby helping to improve the nesting success and growth of the hoiho population within the South Island range. Lastly, we propose that authoritative texts and other sources of information on hoiho should be amended to reflect that the common visual isolation of nest sites from conspecifics is a consequence of selection for microhabitat features that provide, amongst other possible requirements, a significant amount of protection from insolation.
Acknowledgements
We are grateful to the many volunteers, and University of Otago and Department of Conservation staff who gave invaluable assistance and advice. We thank the Burgess family for providing access to Hinahina Cove. Two reviewers provided thoughtful suggestions that helped greatly improve the manuscript. Animal Ethics approval was granted under University of Otago AEC Protocol 69/06. Some funding for this project was generously provided by the Department of Conservation, under SAF project 2007/1, and by a University of Otago Sciences Division grant to RDC.










