Introduction
The Orinoco Goose Neochen jubata, a once common South American sheldgoose of the Anatidae family (Subfamily Tadorninae), is now a ‘Near-threatened’ species with a patchy distribution throughout its range (BirdLife International 2012). Although poorly assessed, the extant population is currently estimated at 10,000–25,000 individuals and believed to be declining (Delany and Scott Reference Delany and Scott2006, BirdLife International 2012). The remaining strongholds for the species are a few sites in Venezuela (e.g. Esteros de Mantecal), Colombia (e.g. La Primavera), the Bení region of Bolivia and the Central Araguaia river region in Brazil (Hilty Reference Hilty2003, Kriese Reference Kriese2004, Pinheiro and Dornas Reference Pinheiro and Dornas2009). The species occurs east of the Andes, with only a single report from west of the Andes to date (Aranzamendi et al. Reference Aranzamendi, Booker and Bailey2010). In the lowland Amazon Basin, where the Orinoco Goose is one of the most threatened bird species (Stotz et al. Reference Stotz, Lanyon, Schulenberg, Willard, Peterson and Fitzpatrick1997, Whittaker Reference Whittaker2004, Trolle and Walther Reference Trolle and Walther2004, Davenport et al. Reference Davenport, Bazán and Erazo2012), it is found at low densities along the Amazon and Orinoco rivers and tributaries. These populations are thought to be smaller and more fragmented than those in the Llanos region and in other open wetland habitats found in the Bení and the Araguaia river basins (Kriese Reference Kriese2004, Whittaker Reference Whittaker2004, Brewer and Kriese Reference Brewer, Kriese and Kear2005, Schulenberg et al. Reference Schulenberg, Stotz, Lane, O’Neill and Parker III2007).
Little is known about the natural history of the Orinoco Goose. The species is a terrestrial grazer, but seems to be invariably associated with areas providing immediate access to freshwater bodies, such as wet savannas and margins of large freshwater wetlands (Hilty Reference Hilty2003, BirdLife International 2012). The species is a secondary-cavity nester requiring large trees (DBH > 30 cm) with cavities for the species to breed successfully (Newton Reference Newton1994, Kriese Reference Kriese2004).
Due to its large body size and preference for open habitats, the Orinoco Goose is a conspicuous target-species for hunters and hunting is currently suggested to be the most important driver of population declines (BirdLife International 2012). For this reason, effective measures to control the impacts of game harvesting in areas where it occurs are highly desirable.
The increasing number of large protected areas (hereafter, PAs) created over the last two decades within the Orinoco Goose’s geographic range (ARPA 2010) is expected to potentially improve the conservation status of the species. Yet, most of these existing reserves consist of human-occupied PAs that support the livelihoods of either indigenous (Indigenous Territories) or non-indigenous populations (Extractive Reserves). These human populations are typically dependent on the local wildlife to supply their daily protein needs (Silvius et al. Reference Silvius, Bodmer and Fragoso2004). To overcome this issue, most existing PAs along major tributaries of the Amazon were created as sustainable-use PAs, where community-based management of extractive resources have few restrictions compared to strictly protected reserves (Peres and Zimmerman Reference Peres and Zimmerman2001).
In PAs that fall into the sustainable-use category, local human populations are expected to follow a set of management guidelines that attempt to combine extractive activities with the long-term persistence of exploited populations. Such measures may favour the creation of zoning systems, or areas under varying hunting restrictions, potentially providing critical wildlife refugia for exploited populations (Novaro et al. Reference Novaro, Redford and Bodmer2000). Despite the underlying assumption that multiple-use PAs can potentially prevent declines in hunted populations, the effectiveness of such PAs has been the subject of intense debate (Peres Reference Peres2011). Here, we examine the conservation status and habitat occupancy of an Orinoco Goose population across a wide habitat mosaic under varying levels of subsistence hunting and extractive restrictions. We investigate spatio-temporal changes in the abundance of this species in relation to different levels of protection and other socioeconomic and environmental variables. We also provide information on the seasonal variation in the occurrence, habitat use, and reproduction of this species throughout our large study area.
Methods
Study Area
The study was conducted from March 2008 to August 2011 along the Rio Juruá, one of the main tributaries of the Rio Solimões (Amazon) located in western Brazilian Amazonia (Figure 1). The region is subjected to a well-defined seasonal rainfall regime, with a mean annual rainfall of 2,400–2,800 mm (Sombroek Reference Sombroek2001). There is a strong seasonal oscillation in the river water discharge, with the period of low water level occurring from July to October (Figure 2). The region consists of both seasonally flooded forest (várzea) and areas of upland forest (terra firme). We selected a 392-km section of the Rio Juruá, ranging from the southernmost limit of the Carauari municipal boundary to the nearest point along the river from the municipal urban centre (Figure 1). The urban centre concentrates 77% of the entire municipal population of ∼25,800 inhabitants, with the remaining population living in small rural villages spread mainly along the Rio Juruá and its major tributaries (IBGE 2010). The surveyed section of the river also intersects two sustainable-use forest reserves, the Médio-Juruá Extractive Reserve and the Uacari Sustainable Development Reserve (Figure 1). Both of these PAs have land-use zoning systems implemented within their areas. The most strictly protected sites in the region include nine sandy beaches along the main river, the purpose of which is to protect nesting sites for three freshwater turtle species (Podocnemis spp.) that are heavily persecuted by locals for their meat and eggs (Kemenes and Pezzuti Reference Kemenes and Pezzuti2007). In addition, four tributaries of the Rio Juruá were also surveyed (Figure 1): three tributaries located partially (Rio Eré) or fully (Rio Anaxiqui and Paraná do São Raimundo) within the Uacari Sustainable Development Reserve, and the Rio Xeruã, encompassed by two Indigenous Lands, the Deni Indigenous Territory and the Kanamari Indigenous Territory (Figure 1).

Figure 1. Map of the study area showing: the municipal urban centre of Carauari (white triangle), protected areas (white polygons), villages (gray circles) and protected Podocnemis turtle nesting sites (black icons); The inset map depicts the main tributaries of the rio Juruá surveyed (top right) and a small section of the surveyed area showing the river cut-bank (1) and sandy beach (2) sites formed along the meandering river (bottom right).
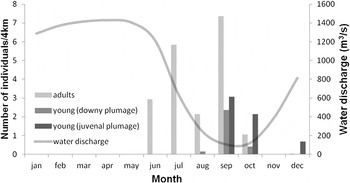
Figure 2. Monthly encounter rates of Orinoco Goose within the study area, and water discharge of the Rio Juruá measured at Porto Gavião for 2008-2010 (Source: Petrobras S.A).
Field Surveys
We surveyed Orinoco Geese by searching for and counting all individuals along both margins of the rivers. The surveys were conducted during all months of the year, except for November, using motorized boats with at least two observers carrying out the counts. The censuses were conducted between 06h30 and 18h30, but were discontinued whenever visibility was impaired by low light conditions or heavy rainfall. Individuals seen on the margins of the river or tributaries, flying, or on the water were recorded and their locations georeferenced. Observations were aided by a set of 10x42 binoculars. For large flocks or crèches of immature individuals, a digital SLR camera equipped with a 200 mm zoom lens was used to take reference photos, which were used as additional documentation to reliably count the total number of individuals, to discriminate adults from immature individuals, and to identify the plumage stage of each immature individual. All individuals close to each other (< 100 m) were counted as a single flock.
Adults were distinguished from immature individuals by their body size, plumage pattern and leg colour, with adults having more reddish legs than immature individuals. Brood size was assessed by counting the number of immature individuals clustered within a single flock and accompanied by at least one adult. We also separated goslings into two different age classes: 1) downy juveniles – recognized by their small body size and pale buff plumage, and 2) fledgling juveniles – individuals with their first flying plumage, similar to that of adults (Figure S1 in the online Supplementary Material).
The meandering nature of the river and the seasonal changes in water level create a strong process of erosion and sediment deposition along the river margins and the seasonally flooded riverbeds. Two broad but clearly distinct habitat categories can thus be observed along the river. Sandy beaches (Figure S1, top) are formed in areas with intense alluvial deposition, consisting of a marked gradient of early successional plant communities (Salo et al. Reference Salo, Kalliola, Häkkinen, Mäkinen, Niemelä, Puhakka and Coley1986). The river cut-bank, on the other hand, consists of areas with a recent history of erosion activity and is characterized by denuded clay soils and steeper slopes bordering late successional forests. To assess the preference of individuals for any of these two particular habitats we recorded the location of each individual encountered according to these habitat types. For all records we also noted whether the geese were found along the main river or its tributaries, and if found along tributaries, how far from the Rio Juruá they were.
Human effects on goose abundance
To assess the effect of human activities on goose abundance, we measured the fluvial distance along the river from the urban centre to the mid-point of each surveyed location (see Data Analysis). Additionally, we assessed the effects of nearby rural villages by measuring the distances along the river, stream or used path to every village found within a 5, 10 and 20-km radius from the mid-point of the same surveyed river section. Village size, defined in terms of number of inhabitants, was also used to evaluate the effects of local human population density on goose abundance. To examine if goose encounter rates were higher within areas under greater hunting restrictions, we compared the abundance of geese in areas subjected to three use restriction levels found across the study area: 1) sites outside any of the existing PAs and therefore potentially most exposed to hunting activities, 2) areas within any of these PAs and used exclusively by the villages found within their boundaries or adjacent areas, 3) strictly protected turtle nesting sites where all extractive activities were prohibited.
In addition, we also documented the number of Orinoco Geese killed by local people using weekly surveys deployed at 220 households belonging to 25 villages across the study area during the period of March 2008 to September 2010. All geese killed and consumed during this study period were recorded using a standardised questionnaire addressing all game species harvested at each household (see Newton et al. Reference Newton, Endo and Peres2012, for a general description of these surveys).
Data Analyses
We evaluated differences in Orinoco Goose population abundance along the river by dividing the surveyed area into 98 sequential 4-km fluvial segments and considering each segment as a sampling unit. Although this procedure involves a certain degree of spatial correlation, this was explicitly accounted for in the analysis by including the linear distance from the urban centre as one of the covariates in the models.
For each fluvial segment, a human population density (HPD) index was calculated as a proxy for the aggregate effect of local village density and size on goose abundance. This index is based on both the size and distance of each village found within any given buffer area around each fluvial segment, and can be described as following:
Where VS = village size (number of inhabitants), BR = buffer radius and VD = distance from the village.
To relate changes in species abundance to the explanatory variables we used generalized linear mixed models (GLMMs) with a Poisson error structure, appropriate for count data. Distance along the river to the urban centre, HPD and site protection status were considered as fixed variables. We also included as a covariate the total extent of beaches, which was measured within each fluvial segment using Landsat images. Finally, month and year were included in the models as nested random variables. These analyses were carried out using the lme4 package for R (Bates and Maechler Reference Bates and Maechler2011).
To calculate the best possible models for the study we used a multi-model inference approach, comparing second-order Akaike Information Criterion (AICc) values, more appropriate for small sample sizes, for each possible candidate GLMM derived from the global model. We ranked the models by comparing each candidate model with the model with the lowest AICc, and considering models with ∆AICc > 2 as poor candidates (Burnham and Anderson Reference Burnham and Anderson2002). Finally, we also calculated the Akaike weights, which provide an overall indication of model likelihood of being the best candidate compared to all other possible models (Burnham and Anderson Reference Burnham and Anderson2002). The MuMIn package (Barton Reference Barton2012) was used to run the analysis in R (R Core Team 2012).
Since the distance to the urban centre and protection status of fluvial segments were strongly correlated (r s = 0.768; P <0.001; Spearman’s rank correlation coefficient) we conducted separate analyses by replacing each of these two variables and selecting the global model with lower AIC value using a multimodel inference framework. We also conducted the analysis using village effect variables with three different buffer radii (5, 10 and 20 km) and selecting the most parsimonious model.
Results
Population abundance
In total, 7,145 km of surveys were conducted along the Rio Juruá and its tributaries. Orinoco Geese were entirely absent between January and May, but frequently recorded between June and December. This corresponds to the months of low water level (Figure 2). The mean encounter rate of adults along the Rio Juruá was of 7.18 ± 2.45 ind./4 km (mean ± SD) in June–September, the months when they were most frequently encountered (Figure 2, Figure 3, Table 1). Encounter rates were considerably lower along the tributaries, where only 0.55 ± 0.34 ind./4 km were seen in June–October. Moreover, all individuals observed along the tributaries were located close to the confluence with the Rio Juruá (mean = 2.4 ± 1.7 km) with no record beyond 5 km from the margin of the river. Additionally, all these individuals were restricted to the portion of the tributaries embedded within seasonally flooded forest.

Figure 3. Encounter rate of Orinoco Goose along the middle rio Juruá between June and October. Colour coding is expressed as the total number of individual recorded per 4-km section of the river.
Table 1. Mean (± SD) monthly encounter rates of Orinoco Goose in the middle Juruá region, western Brazilian Amazonia.
Most adults recorded were seen in pairs (n = 610; 52.8%) or small flocks up to six individuals (28.5%), with a mean flock size of 3.58 ± 4.17 (range = 1–37) individuals (Figure 4). Immature birds were encountered between July and December. Goslings were more frequently observed during months of lowest water level (Figure 2, Table 1), with an encounter rate of 2.02 ± 2.38 ind./4 km. While downy juveniles were found as early as August, fledgling juveniles were only seen from September to December. The mean brood size of downy juveniles was 7.19 ± 6.75 (n = 24; range = 2–35), while fledglings had a mean brood size of 8.2 ± 4.93 (n = 39; range = 1–24), but this difference was not significant (P = 0.16; Wilcoxon rank sum test).
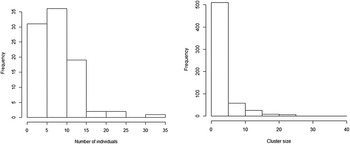
Figure 4. Brood size distribution of Orinoco Goose (mean = 8.59; SD = 5.25; left) and flock size (mean = 3.58; SD = 4.17 adults; right), in the middle Rio Juruá, western Brazilian Amazonia.
Habitat use
Sandy beaches (Figure S1) were more intensively used as resting and foraging sites compared to river cut-banks; 94.1% of all 438 records were of individuals on sandy beaches, whereas only 6% involved individuals on river cut-banks.
The vast majority of individuals observed were either resting or foraging on the ground (n = 449; 94.4%), with only a few seen on the water (2.9%) or flying (2.7%). Most individuals observed on the water (n = 13; 85.6%) were immature individuals or adults with their brood. No individuals were seen perched in trees.
Hunting pressure
Orinoco Geese were killed and consumed in 11 of the 25 villages monitored. A total of 27 individuals were killed during the study period, resulting in a rate of 0.09 ± 0.13 individuals killed per household per year. Geese were killed exclusively by hunters living in villages along the margin of the main river. No geese were seen during the surveys in May, but four kills were recorded by hunters during this month.
Predictors of species abundance
A single model including all the explanatory variables used in the analysis was the best candidate model from the 16 possible combinations to explain the abundance of adult geese along the Rio Juruá (Table 2). The results show that the encounter rate is positively associated with the linear extent of sandy beaches, the availability of protected turtle nesting sites, and distance from the urban centre. Conversely, the encounter rates were negatively related to the HPD index. Finally, the HPD index with a BR constant of 5 km was the most significant candidate predictor among the three BR values.
Table 2. Summary of generalized linear mixed model selection based on 16 candidate models predicting encounter rates of Orinoco Goose. Only the most parsimonious model (ωi = 1) and the two illustrative subsequent models are shown. LL = log-likelihood; K = number of parameters, AICc = Akaike’s information criterion score corrected for small sample sizes; ∆AICc = difference between a given model and the best model, in units of AICc; ωi = Akaike weight for each model. Explanatory variables are coded as following, dst: distance from the municipal urban centre, bch: proportion of sandy beaches within each fluvial segment, trt: strictly-protected turtle nesting site, HPD: human population density.

Discussion
We show that the Middle Juruá region of western Brazilian Amazonia is an important breeding area for several hundred Orinoco Geese, possibly supporting one of the largest populations known for the entire Amazon Basin to date. This region is therefore a key stronghold for the Orinoco Goose which has until now been largely overlooked. Sporadic visits to areas upriver of the surveyed section of the river suggest that goose abundance may be similar (W. Endo, pers. obs.), likely increasing the total number of individuals in the middle portion of the Rio Juruá to a few thousand individuals. Previous surveys in the upper portion of the Rio Juruá (Whittaker and Oren Reference Whittaker and Oren1999) resulted in no records of geese and the absence of sightings during other surveys carried out in areas close to the junction of the Rio Juruá with the Rio Solimões (ICMBio 2009, R. Czaban in litt. 2013) may indicate that the species is restricted to the middle reaches of the Rio Juruá. Further studies are, nonetheless, required in the upper and lower portions of the Rio Juruá in order to properly estimate the Orinoco Goose population for this entire river basin.
The várzea floodplain forest is associated with a large number of oxbow lakes that remain partially unconnected to the Rio Juruá channel and its tributaries during the dry season. Observations of Orinoco Geese on the margins of lakes connected to the surveyed tributaries suggest that these lakes also serve as important foraging and brood rearing habitat for this species. Reports from local villagers also indicate that these lakes and lake margins are important nesting sites. The inclusion of such habitats in future surveys may therefore boost the population estimates for the region. The fact that a few geese were killed by hunters in May, when our surveys failed to record any individuals, support the notion that we are likely underestimating the total number of Orinoco Geese found in the study area.
The seasonal occurrence of Orinoco Geese in the study area suggests that this population migrates to other regions during the wet season. This migratory behaviour contrasts with most studies to date, which describe the species as non-migratory (del Hoyo et al. 1992, Stotz et al. Reference Stotz, Lanyon, Schulenberg, Willard, Peterson and Fitzpatrick1997, Kriese Reference Kriese2004, Brewer and Kriese Reference Brewer, Kriese and Kear2005). However, reports of a longitudinal migration of Orinoco Geese between southern Peru and northern Bolivia (Davenport et al. Reference Davenport, Bazán and Erazo2012) and the seasonal occurrence of another population in the Central Araguaia river region (De Luca et al. Reference De Luca, Develey and Olmos2006) indicate that migratory behaviour is far more widespread than the Juruá population. The seasonal flood pulse which inundates important grazing sites may be one of the primary reasons for the evolution of such migratory behaviour among Orinoco Goose populations, and indeed other waterbirds (e.g. Black Skimmer Rynchops niger and Large-billed Tern Phaetusa simplex) dependent on riverine beach habitats that become flooded in the high-water season. Competition for suitable nesting sites has also been suggested as a potential factor leading birds to migrate to areas with a higher abundance of trees (Davenport et al. Reference Davenport, Bazán and Erazo2012). These observations, combined with the fact that all migratory populations are apparently restricted to the Amazon Basin, suggest that seasonal migration is the rule, rather than the exception, in this region.
The foraging constraints imposed by the seasonal flood pulse and river dynamics seem to be important determinants of habitat use in this species. The growing number of planned or approved hydroelectric projects within the species’ geographic range is therefore likely to impact on the occurrence of this species in newly modified areas due to critical changes in inundation patterns (Tollefson Reference Tollefson2011, Finer and Jenkins Reference Finer and Jenkins2012).
The existence of large transient populations, such as that in our study area, suggests that populations previously considered to be independent are, in fact, the same populations that seasonally occupy different breeding areas. The occurrence of migratory populations may also pose a greater risk for the species (Davenport et al. Reference Davenport, Bazán and Erazo2012), potentially exposing these populations to a higher number of threats (Kirby et al. Reference Kirby, Stattersfield, Butchart, Evans, Grimmett, Jones, O’Sullivan, Tucker and Newton2008). Further studies are clearly required to better understand the migratory behaviour of these transient populations in order to design an improved protection strategy for the species.
The fact that all individuals surveyed were distributed exclusively along sections of the river dissecting floodplain forests, coupled with the strong preference for sandy beach sites, make the Orinoco Goose a relatively selective bird in terms of habitat requirements. The Rio Juruá is one of the most meandering tributaries of the Amazon river (Latrubesse Reference Latrubesse2008). The high number of river bends with extensive sandy beaches may be one of the reasons facilitating the species’ occurrence throughout the region. Our results also show that the Orinoco Goose mainly uses the main Rio Juruá channel as its preferred foraging and brood-rearing area, being conspicuously absent along most surveyed tributaries. This underlines the difficulty of providing satisfactory protection for the species. Várzea floodplain forests are, since pre-colonial times, some of the most intensively exploited Amazonian ecosystems (Roosevelt Reference Roosevelt, Padoch, Ayres, Pinedo-Vasquez and Henderson1999). Margins of the main navigable rivers are also of high socio-economic importance for local semi-subsistence populations, being important areas for extractive and agricultural activities (Pinedo-Vasquez and Sears Reference Pinedo-Vasquez, Sears, Pinedo-Vazquez, Ruffino, Padoch and Brondízio2011, Newton et al. 2011). Consequently, any protection measures that impose constraints on local communities to exploit such areas are likely to meet local political resistance and be doomed to fail if not properly planned and implemented.
The brood size recorded here is consistent with the values of 6–10 goslings reported elsewhere (Brewer and Kriese Reference Brewer, Kriese and Kear2005). Moreover, records of broods with considerably larger numbers of goslings confirm the common occurrence of intraspecific nest parasitism for the species (Kriese Reference Kriese2004) or the voluntary capture of immature individuals from other parents (Williams Reference Williams1974). The stable values of brood size for goslings in different stages of development also indicate that the mortality rate is low during this life stage. The larger brood size recorded for older goslings, however, indicates that our surveys failed to obtain complete counts of downy fledglings. Additionally, the results showed a high proportion of fledgling juveniles compared to adults in December. This unbalanced proportion was partially due to observations of fully grown juveniles unassisted by their parents. However, this should be more carefully investigated in order to understand whether these individuals were in fact abandoned by their parents and able to migrate without parental guidance, or if we simply failed to detect the adults during surveys.
Orinoco Goose encounter rates increased significantly both in areas enjoying greater protection from hunting and areas farther from the urban centre of Carauari. The greater abundance of geese in areas under a higher level of protection suggests that these sustainable-development PAs have been effective in providing better protection for foraging and brood rearing.
The significantly higher encounter rate of geese in the surveyed segments including protected turtle nesting sites indicates that these sites may also benefit species other than freshwater turtles. Other vertebrate species known to use turtle nesting sites, such as wading birds (Caputo et al. Reference Caputo, Canestrelli and Boitani2005) and iguanas (Hirth Reference Hirth1963), could therefore benefit by such enforcement measures. However, effectively protected turtle nesting sites are small and sparsely distributed, and are therefore insufficient to fully protect the Orinoco Goose. The fate of the population will therefore continue to depend on the level of exploitation of surrounding areas, unless a larger number of beach nesting sites can be protected.
Despite records of Orinoco Geese being harvested by hunters in the study area, the low number of kills suggests that the species is hunted only opportunistically by local inhabitants. The number of geese killed represents a small fraction (1.1%) of the total number of game vertebrate kills recorded (n = 2,515) during the study period (W. Endo et al. unpubl. data). Yet, the continued exposure of this species to hunters converging on its preferred habitat renders this seasonal offtake an important issue shaping the future conservation status of the population. This mortality risk is further increased during the breeding season when adults display a more sedentary behaviour while rearing their brood along the margins of the river.
In summary, our results indicate that the Orinoco Goose is very habitat specific. While the preferred habitat is located in areas subjected to intense anthropogenic activities, our metric of abundance consistently showed that the geese were primarily restricted to the most sparsely settled and best protected areas. These findings, together with the transient behaviour displayed by the Juruá population, indicate that more refined and effective measures to protect the species are needed. The creation of local community-based management and bi-national agreements between countries at both ends of migration routes are some of the measures that will likely bring desirable conservation outcomes.
Supplementary Material
The supplementary materials referred to in this article can be found at journals.cambridge.org/bci
Acknowledgements
We are grateful to the Norwegian University of Life Sciences (Department of Ecology and Natural Resource Management) and a Darwin Initiative Project (DEFRA – UK, Ref. No.16-001) for financial support. We are also indebted to the Secretaria do Estado do Meio Ambiente e Desenvolvimento Sustentável do Estado do Amazonas (SDS-CEUC) and Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis (IBAMA) for granting permission to conduct the research. We thank Lisa Davenport and Leonardo Fleck for providing insightful comments on the manuscript and Almir R. Lima for field assistance. This is publication no. 07 of the Médio Juruá Project series on resource management in Amazonian sustainable use reserves (see http://www.tropicalforestresearch.org/projects/jurua.aspx).








