Introduction
At present there is no readily accessible and up-to-date synthesis of the conservation status of the world’s seabirds. Why is this important, given that only some 350 species (i.e. 3.5% of all birds) are entirely dependent on marine habitats for at least part of their life cycle? Although relatively few in number, seabirds as a group occur in all seas and oceans worldwide, and their role as potential indicators of marine conditions is widely acknowledged (e.g. Boyd et al. Reference Boyd, Wanless and Camphuysen2006, Piatt et al. Reference Piatt, Sydeman and Wiese2007, Parsons et al. Reference Parsons, Mitchell, Butler, Ratcliffe, Frederiksen, Foster and Reid2008). Many studies use aspects of seabird biology and ecology, especially productivity and population trends, to infer and/or correlate with aspects of the marine environment, particularly food availability. Nevertheless, despite the importance of seabirds as indicators, both regionally and globally, of many aspects of the functioning of marine systems, the most important current challenge is to ensure the survival and improve the status of the many seabird species which are already globally threatened with extinction and to maintain the remainder in favourable conservation status. Compared with other groups of equivalent role in marine systems, seabirds are exceptionally well-studied (Schreiber and Burger Reference Schreiber, Burger and 2001). Consequently, knowledge of their conservation status is more comprehensive and reliable than for any comparable group of marine organisms (Vie et al. Reference Vie, Hilton-Taylor, Stuart and 2008). Therefore, both intrinsically and because the status of seabirds is likely to reflect the underlying state of important parts of the coastal and oceanic systems of the world, we should take particular interest in how seabirds are faring, how and why this status has changed in recent times, what actions are needed to address the main current threats and what kind of baseline exists against which to measure future change.
In this paper we provide a brief global overview of the status of seabird species (focusing particularly on globally threatened and Near Threatened species), especially in relation to jurisdictional responsibility. We identify the main reasons why seabirds are threatened and review priority actions to address the main threats. Finally, we indicate priorities for research and monitoring and identify particular knowledge gaps. Our analyses are global in scale and should be supplemented by reviews and identification of priorities at the regional level (but using global Red List categories, as assessments of extinction risk at the regional scale are largely lacking).
Methods
Our analyses are based on BirdLife International’s assessments for the 2010 IUCN Red List (available at www.birdlife.org/datazone/species and in summary form at www.iucnredlist.org) and data on IBAs held in BirdLife’s World Bird Database (WBDB; available at www.birdlife.org/datazone/sites). Summary data are provided in Table S1 in the online Supplementary Materials. Both species and IBA data are compiled and regularly updated from reviews of published and unpublished literature as well as information provided by a network of over 100 BirdLife Partner organisations, hundreds of other institutions, and thousands of scientists, conservationists, birdwatchers and local or species experts.
We follow the taxonomy of BirdLife International (2010a) and define seabirds as species for which a large proportion of the total population rely on the marine environment for at least part of the year. With this circumscription, 346 species qualify, of which 282 meet a stricter definition (excluding ducks, loons, etc.) used in some earlier reviews (e.g. Croxall et al. Reference Croxall, Evans, Schreiber and 1984). We subdivided seabirds into three groups. ‘Pelagic seabirds’ are those that primarily use marine pelagic deep water (sea above open ocean, typically > 200 m in depth) and/or marine neritic pelagic continental shelf water (sea above continental shelf or around near-shore oceanic islands, typically < 200 m in depth) excluding species that may occasionally use these habitats, but that are more typical of coastal inshore waters. ‘Coastal seabirds (year-round)’ are those that primarily use coastal inshore water (sea along coasts, typically < 8 km from the shoreline) throughout the year, excluding species that may occasionally use this habitat, but do not do so typically. ‘Coastal seabirds (non-breeding season)’ are those that primarily use coastal inshore water during the non-breeding season, excluding species that may occasionally use this habitat but do not do so typically.
Species on the IUCN Red List are placed into categories of extinction risk (ranging from Least Concern, to Near Threatened, Vulnerable, Endangered, Critically Endangered, and Extinct) based on quantitative criteria using information on population and range size, structure and trends (IUCN 2001, 2010). Vulnerable, Endangered and Critically Endangered species are referred to collectively as ‘threatened’ (IUCN 2001). Species for which there is insufficient information to apply the criteria are classified as Data Deficient (IUCN 2001, Butchart and Bird Reference Butchart and Bird2009). A small number of Critically Endangered species are tagged as Possibly Extinct if they are, on the balance of evidence, likely to be extinct, but for which there is a small chance that they may be extant and thus should not be listed as Extinct until adequate surveys have failed to find the species and unconfirmed reports have been discounted (IUCN 2010, Butchart et al. Reference Butchart, Stattersfield and Brooks2006).
In analyses of the numbers of seabird species by country we exclude vagrant records (as defined in relevant national checklists, field guides and handbooks; generally used for species for which there are few records or that only occur sporadically and infrequently), but include those species whose occurrence in a particular country is coded as uncertain (because maps of confirmed distribution indicate that they are likely to occur in territorial waters, but no published records have been traced). We consider confirmed resident or breeding species separately. We used GIS and BirdLife’s digitised species’ distribution maps to determine occurrence of species in national Exclusive Economic Zones (EEZs), Large Marine Ecosystems (LMEs; Sherman et al. Reference Sherman, Alexander, Gold and 1993) and areas of application of different Regional Fisheries Management Organisations (RFMOs). Boundaries for EEZs were taken from VLIZ (2010), for LMEs from National Oceanic and Atmospheric Administration (2010), and for RFMOs from each of the individual organisations’ websites. Jurisdictions are as listed by the International Organisation for Standardization (ISO; www.iss.org) at July 2010; note that subsequently Tristan da Cunha (with Gough Island) was accorded a status separate from St Helena.
Direction of current population trend was coded as increasing, stable, fluctuating, decreasing or unknown. Threats to species were classified using the IUCN/Conservation Measures Partnership (CMP) threats classification scheme (Salafsky et al. Reference Salafsky, Salzer, Stattersfield, Hilton-Taylor, Neugarten, Butchart, Collen, Cox, Master, O’Connor and Wilkie2008) with threats from all invasive alien species identified to species level where possible, and threat magnitude calculated from scores for timing, scope and severity following BirdLife International (2010b). Analyses of threats were based on data for threatened species only (i.e. excluding Extinct, Near Threatened, Least Concern and Data Deficient species). Priority conservation and research actions were coded following the IUCN/CMP Actions classification scheme (Salafsky et al. Reference Salafsky, Salzer, Stattersfield, Hilton-Taylor, Neugarten, Butchart, Collen, Cox, Master, O’Connor and Wilkie2008).
We assessed trends in extinction risk using the IUCN Red List Index (RLI; Butchart et al. Reference Butchart, Stattersfield, Bennun, Shutes, Akçakaya, Baillie, Stuart, Hilton-Taylor and Mace2004, Reference Butchart, Resit Akçakaya, Chanson, Baillie, Collen, Quader, Turner, Amin, Stuart and Hilton-Taylor2007) for 1988–2008 (the period between comprehensive assessments of all bird species for the IUCN Red List), updated using current knowledge. The RLI is calculated from the number of species in each Red List category and the number changing categories between assessments as a result of genuine improvement or deterioration in status (category changes owing to improved knowledge or revised taxonomy are excluded). RLI values relate to the proportion of species expected to remain extant in the near future without additional conservation action. An RLI value of 1.0 equates to all species being categorised as Least Concern, and hence that none are expected to go extinct in the near future. An RLI value of zero indicates that all species have become Extinct.
Important Bird Areas (IBAs) are key sites for the conservation of the world’s birds (e.g. BirdLife International 2011). IBAs are places of international significance for the conservation of birds and are identified using a standardised set of data-driven criteria and thresholds based on (1) globally threatened bird species, (2) restricted-range bird species (those with ranges smaller than 50,000 km2), (3) biome-restricted assemblages (communities of birds characteristic of a distinct biome) and (4) congregations (large aggregations of one or more species, e.g. migratory waterbirds or breeding seabirds). IBAs are delimited so that, as far as possible, they: (a) are different in character, habitat or ornithological importance from surrounding areas; (b) provide the requirements of the ‘trigger’ species (those for which the site qualifies) while present, alone or in combination with networks of other sites; and (c) are or can be managed in some way for conservation. Terrestrial IBAs have been identified in almost all countries of the world, but for the analyses presented here, data were incomplete and therefore omitted for 21 countries: American Samoa, Argentina, Chile, Cook Islands, French Guiana, Guyana, Kiribati, Kyrgyzstan, Nauru, New Caledonia, New Zealand, Niue, Papua New Guinea, Paraguay, Samoa, Solomon Islands, Tokelau, Tuvalu, USA, Vanuatu and Wallis and Futuna Islands. Marine IBA identification, i.e. of important areas in coastal waters and on the High Seas (Areas Beyond National Jurisdiction (ABNJs)) for feeding and aggregation, is ongoing (BirdLife 2010e, Lascelles et al. 2012).
We examined growth in coverage of IBAs by nationally designated protected areas following the approach of Butchart et al. (Reference Butchart, Walpole, Collen, van Strien, Scharlemann, Almond, Baillie, Bomhard, Brown, Bruno, Carpenter, Carr, Chanson, Chenery, Csirke, Davidson, Dentener, Foster, Galli, Galloway, Genovesi, Gregory, Hockings, Kapos, Lamarque, Leverington, Loh, McGeoch, McRae, Minasyan, Morcillo, Oldfield, Pauly, Quader, Revenga, Sauer, Skolnik, Spear, Stanwell-Smith, Stuart, Symes, Tierney, Tyrrell, Vié and Watson2010). Protected Area coverage data was taken from the WBDB and is based on GIS overlays of IBA polygons with those nationally designated PAs for which a boundary polygon was included in the 2010 release of the World Database on Protected Areas (WDPA; www.wdpa.org), excluding internationally designated PAs and all sites with a status other than ‘designated’. Where multiple protected areas overlapped an IBA, the date of designation of the earliest protected area was used. Where data in the WDPA were incomplete or inaccurate, estimates of protected area coverage of IBAs were updated by BirdLife Partners. For 257 protected areas (14% of all PAs) with an unknown date of establishment, and for 88 IBAs (4.8%) known (from national experts) to be partially protected but to an unknown extent, we randomly assigned a date or proportion protected from another site in that country (doing this 10,000 times and plotting the mean and 95% CIs to capture the uncertainty introduced by lack of data for a subset of sites); where < 2 sites with known date/proportion protected occurred in the country we randomly selected from all sites. We plotted trends in mean percentage area protected and number of sites completely covered by protected area(s). Trends for Central America and Oceania were omitted as seabird IBA identification is still very incomplete in these regions.
Results and Discussion
Status
Of the 346 seabird species considered here (Table S1), 97 (28%) are globally threatened, 17 (5%) in the highest category of Critically Endangered and a further 10% Near Threatened (Figure S1). Only four species, all storm-petrels (White-vented Oceanites gracilis, Markham’s Oceanodroma markhami, Matsudaira’s O. matsudairae and Ringed O. hornbyi) are regarded as Data Deficient. Three species are considered Extinct (Great Auk Pinguinus impennis, Large St Helena Petrel Pterodroma rupinarum, Small St Helena Petrel Bulweria bifax) and two other species are Possibly Extinct (Guadalupe Storm-petrel O. macrodactyla and Jamaica Petrel Pterodroma caribbaea; included in Critically Endangered in Figure S1). Seabirds are more threatened than all other groups of birds with similar numbers of species: 26% of parrots (Psittacidae; 374 species), 19% of pigeons/doves (Columbidae; 318 species), and 18% of raptors (Accipitridae; 238 species) are threatened; all other similarly speciose bird families are equally or less threatened than the global average (12%). Furthermore, dividing seabirds into pelagic and coastal species and accounting separately for those species which only visit marine habitats outside their breeding season (Figure 1) shows that pelagic seabird species are considerably more threatened than coastal resident seabirds and that both are an order of magnitude more threatened than non-breeding coastal seabirds. This likely reflects that pelagic species tend to have small clutch sizes relative to coastal species, reducing their capacity to absorb human-induced mortality and slowing recovery following cessation of impacts.

Figure 1. Proportion of species in each IUCN Red List category for pelagic species, coastal residents and coastal non-breeding visitors. Figures give number of species (for totals > 5).
Reviewing the pattern taxonomically (Figure 2) reveals that, of the main families (which together account for 87% of species), the most threatened are the penguins and albatrosses/ petrels. These two orders (Sphenisciformes and Procellariiformes) represent nearly one half (43%) of all seabirds and contain many pelagic species. After albatrosses, Diomedeidae, whose conservation benefits considerably from the Agreement on the Conservation of Albatrosses and Petrels (ACAP; www.acap.aq), by far the most threatened group of seabirds are the gadfly petrels of the genera Pterodroma and Pseudobulweria (and a special conservation internet forum has recently been established to promote priority conservation action for these: Gadfly Petrel Conservation Group; www.gadflypetrel.ning.com). The primary reasons for the classification of seabird species as threatened (or Near Threatened), based on the IUCN Red List criteria they trigger, are summarised in Figure 3. Thus, of the 132 threatened/Near Threatened species, 70 (53%) qualify by virtue of very small population/range and a similar number (66; 50%) by reason of rapid decline. Of particular concern are those where small range or population is combined with decline (64 species; 48%). Noteworthy examples are six penguins (two Eudyptes and two Spheniscus), 17 gadfly petrels and eight cormorants. Throughout, pelagic species are disproportionately represented in all categories in comparison with coastal species.

Figure 2. Percentage of species in each IUCN Red List category for the major seabird families. Figures give number of species.

Figure 3. The number of seabird species listed as threatened or Near Threatened for different reasons (note that some species are listed for multiple reasons).
As a broad generalisation, seabirds tend to have particularly small total breeding population sizes, with 20% estimated to have fewer than 5,000 breeding pairs and about one half fewer than 50,000 pairs (Figure S2). Furthermore, 76 (23%) of these population estimates date from 2000 or earlier (see Table S1), so this may be an optimistic portrayal of the current situation. The 15 species whose global population estimates predate 1996 are: Royal Penguin Eudyptes schlegeli, Buller’s Shearwater Puffinus bulleri, Short-tailed Shearwater P. tenuirostris, Auckland Island Shag Phalacrocorax colensoi, Lava Gull Larus fuliginosus (all pre-1991), Emperor Penguin Aptenodytes forsteri, Adélie Penguin Pygoscelis adeliae, Northern Royal Albatross Diomedea sanfordi, Black-winged Petrel Pterodroma nigripennis, Cape Verde Shearwater Calonectris edwardsii, Jouanin’s Petrel Bulweria fallax, Australasian Gannet Morus serrator, Stewart Island Shag Phalacrocorax chalconotus and Kerguelen Tern Sterna virgata. Most of these are Australasian in distribution and many are restricted to single islands or island groups. In addition, even recent population estimates are often of relatively low quality, with broad bands of uncertainty.
Thus most seabirds, especially pelagic ones, typically have small breeding populations and many are likely to be in decline, demographic characteristics which severely limit their rate of recovery, and a restricted number and range of breeding sites; this makes them disproportionately vulnerable amongst birds to a wide range of threats.
Trends
Nearly half (47%; 52% of those with known trends) of seabird species are known or suspected to be experiencing population declines (Figure 4a). Nevertheless, 57 species (17%) are increasing; many, such as the 17 gull species, doubtless due to their abilities to exploit close links with human activities. This probably also accounts for increases in Northern Fulmar Fulmarus glacialis, some Morus spp. gannets and possibly Black-footed Phoebastria nigripes and Campbell Albatrosses Thalassarche impavida, although the last two are still recovering from past declines. Encouragingly, a few species are increasing (e.g. Spectacled Petrel Procellaria conspicillata), often as a result of long-term targeted conservation action (e.g. Fea’s Pterodroma feae, Bermuda P. cahow and Magenta P. magenta Petrels and Amsterdam and Short-tailed Albatrosses Diomedea amsterdamensis and Phoebastria albatrus). As expected, pelagic species are disproportionately more likely to be declining than coastal ones (52% versus 33%) but, perhaps surprisingly, the non-breeding visitors to coastal waters have a similar proportion of decreasing species (46%) to pelagic ones (Figure 4b); they also have a higher proportion of increasing species (25% versus 19% coastal and 12% pelagic).

Figure 4. Current direction of trend for (a) all seabirds (n = 346 species); (b) pelagic species, coastal residents and coastal non-breeding visitors. Figures give number of species.
Precise quantified rates of population decline or increase are available for very few species. A broader, but less sensitive, measure of overall trends is provided by the Red List Index (RLI, Butchart et al. Reference Butchart, Stattersfield, Bennun, Shutes, Akçakaya, Baillie, Stuart, Hilton-Taylor and Mace2004, Reference Butchart, Resit Akçakaya, Chanson, Baillie, Collen, Quader, Turner, Amin, Stuart and Hilton-Taylor2007), which measures trends in extinction risk and is virtually the only trend indicator currently available for seabirds on a worldwide and/or regional basis. The RLI is based on the movement of species through IUCN Red List categories owing to genuine improvement or deterioration in status (i.e. re-categorisations owing to improved knowledge or revised taxonomy are excluded). It shows (Figure 5a) that, over the last 20 years, seabirds have had a substantially poorer conservation status than non-seabirds and that they have deteriorated faster over this period. Seabirds are more threatened than a number of other similarly speciose groups (e.g. raptors, pigeons, gamebirds and waterbirds), and are marginally more threatened than parrots. However, among seabirds, pelagic species are more threatened and have deteriorated faster than coastal species, and this difference is particularly pronounced for the albatrosses and large petrels that are covered by ACAP (Figure 5b). Table S2 summarises the background and evidence relating to the 26 cases, involving 21 species, that qualified for reclassification to a higher or lower Red List category during 1988-2008 (and also presents all changes that occurred during this period, including as a result of improved knowledge or revised taxonomy).

Figure 5. Red List Indices for (a) seabird and non-seabird species; (b) coastal and pelagic species and those listed in the Agreement on the Conservation of Albatrosses and Petrels (ACAP). Figures are for non-Data Deficient extant species in 1988.
National jurisdictional responsibility
The most important countries, in terms of the number of breeding seabird species and the total number of species recorded within EEZ waters, are shown Figure 6a. In general terms, the outcomes are rather similar; however, Japan, Mexico and several South American countries (Argentina, Brazil, Peru, Ecuador), which are adjacent to important marine current systems supporting large numbers of seabirds on migration and in winter, are in the top 10 (Japan, Mexico) or top 20 (rest) overall but not in the top 20 for breeding species. In the top 10 of both categories are USA, Canada, Russia, Australia, New Zealand, Chile and South Africa. If Overseas Territories are included with the mainland jurisdiction, then France (with French Southern Territories) and UK (with Pitcairn [Henderson], St Helena [Tristan da Cunha and Gough] and with or without the Falkland Islands [Islas Malvinas]) would both be in the top five of both categories.

Figure 6. The countries supporting the largest numbers of (a) seabird species; (b) endemic breeding seabird species; (c) seabird species of conservation concern (breeding and non-breeding species combined).
If we focus on seabirds endemic or near-endemic (only in two, usually adjacent, countries) as breeding species (Figure 6b), then a similar outcome results, albeit with New Zealand pre-eminent. For single-country endemics (Table 1), the most important countries are New Zealand (33 species), UK (eight, mainly on the Tristan da Cunha islands), Mexico (five), Ecuador (five, all in Galapagos), Chile (four), Australia (four) and USA (three but with 21 species shared with either Mexico or Canada). Even if we focus on threatened species (Figure S3), New Zealand retains “pole position”, having more than double the number of threatened species of any other country. However, Chile and South Africa hold the next largest number of threatened species, followed by France (including French Southern Territories and French Polynesia), UK (including Tristan da Cunha, South Georgia [Islas Georgias del Sur] and Falkland Islands [Islas Malvinas]). Australia (including Heard and Macquarie Islands), followed by USA, Mexico, Peru and Russia complete the top 10. If non-breeding species are included (Figure 6c), the distribution is somewhat more even and South American countries more prominent, but the basic pattern is similar.
Table 1. Seabird species endemic to single countries/jurisdictions.

Therefore, to protect a considerable majority of the world’s seabirds, especially globally threatened species, either when breeding or when foraging within EEZ waters, priority attention should be given to the geographical areas represented by New Zealand (with Australia), Chile (with Peru, Ecuador), USA (with Canada, Japan and Russia), South Africa (with Namibia), UK and France (only in respect of their overseas territories), Mexico, Brazil and Argentina (see Table 2). Delivery of effective conservation of breeding sites and of EEZ waters in these nine regions (16 countries) would take account of most of the needs of the 25% of seabird species which are completely restricted to these areas and make a major contribution to the 92% of seabird species whose ranges are included, at least in part.
Table 2. Priority countries for seabirds, ranked according to total numbers of (a) breeding and non-breeding species, (b) globally threatened and Near Threatened species, and (c) endemic species (restricted to one or two countries). Overall rank is derived from the sum of ranks for the three parameters. Total number of countries / territories = 239.

Threats
Assessing threats to seabirds is a complex and somewhat subjective task. BirdLife International has compiled an inventory, using the published literature and an extensive network of correspondents. Some illustrations of the general conclusions from this are provided below (Figures 7a–7c); it must be emphasised that this part of the review is confined to threatened species, i.e. excluding Near Threatened, Least Concern and Data Deficient species.

Figure 7. Threats to threatened (a) seabirds (n = 346 species); (b) pelagic seabirds (n = 197 species); (c) coastal seabirds (n = 146 species).
Globally, of the top 10 threats to threatened seabirds (Figure 7a), invasive species (invariably acting at the breeding site) potentially affect 73 species (75% of all threatened seabird species and nearly twice as many as any other single threat, although in some cases the threat is of a potential future impact). The remaining threats are fairly evenly divided between those acting mainly at the breeding site: problematic native species (31 species, 32%), human disturbance (26 species, 27%), infrastructure/commercial/residential development (14 species, 14%) and those acting mainly at sea in relation to foraging, moulting or migration areas/aggregations: bycatch (40 species, 41%), pollution (30 species, 31%), overfishing or inappropriate spatial management of fisheries (10 species, 10%). Hunting and trapping (23 species, 24%) and energy production/mining (10 species, 10%) affect both domains, the former more at breeding sites, the latter more in relation to foraging areas, flight paths and flyways. Climate change and severe weather (39 species, 40%), as presently assessed, largely reflects adverse weather and climatic events at breeding sites and the potential impact of sea level rise but is clearly an important driver of change that is increasingly affecting seabirds in many ways, albeit mainly in the medium to long term (i.e. at timeframes mostly outside those of relevance to IUCN Red List criteria). The relative importance of threats is largely similar when only those of high impact are considered, although bycatch becomes almost as significant as the impacts of invasive alien species.
If pelagic seabirds are considered alone (Figure 7b), the pattern is generally similar, although bycatch assumes a higher priority (particularly when comparing high-impact threats only). For coastal species (Figure 7c) however, disturbance and hunting and trapping assume greater significance, with overfishing of food resources, disturbance and pollution important if considering high-impact threats only. The absence of bycatch from the top 10 coastal threats, however, may simply reflect the fact that the impacts of inshore/coastal gillnets and of artisanal fishing are almost completely undocumented, although likely widespread and important (Zydelis et al. Reference Zydelis, Bellebaum, Österblom, Vetemaa, Schirmeister, Stipniece, Dagys, van Eerden and Garthe2009). Overall, it is important to note that some threats, especially bycatch, coastal pollution and overfishing, are assessed to have higher impact on a larger proportion of the species they affect, considerably increasing their overall importance.
Although these assessments of threats are based on data only for threatened species (97 in total), there is no reason to believe that the pattern is greatly different for the 35 Near Threatened and 207 Least Concern species. The diversity of threats testifies to the vulnerability of seabird species to substantial actual and potential threats at all stages of their annual and life cycles. For Near Threatened and Least Concern species it is likely that the relative importance of human disturbance, development and consumption (hunting/trapping) would increase markedly, particularly for tropical species, for which major reductions in populations and/or breeding sites are increasingly indicated but seldom quantified, especially across the whole range of the many wide-ranging tropical seabird species.
Conservation action
In terms of recommended conservation actions, some indication of the main priorities for threatened and Near Threatened species are provided in the BirdLife World Bird Database (based on a review of the conservation literature and expert opinion; Figure 8). The classes used are rather broad and the data may not be consistent and comprehensive for all species, as they are based on information collated for IUCN Red List assessments. Notwithstanding these caveats, the main priorities are: a) control/eradication of invasive alien species; b) increased and enhanced site/area protection (i.e. formal protected area designation or other forms of recognised protection plus effective implementation of appropriate management plans); c) improved legislation/regulation/best-practice standards and effective implementation/enforcement of these (especially in marine contexts). Other, more generic actions, such as education/awareness and accompanying stakeholder involvement are also high priorities, as are some more species-specific activities, such as harvest management, reintroductions and species recovery (as defined by Salafsky et al. Reference Salafsky, Salzer, Stattersfield, Hilton-Taylor, Neugarten, Butchart, Collen, Cox, Master, O’Connor and Wilkie2008).

Figure 8. Priority conservation actions needed for threatened, Near Threatened and Data Deficient seabirds.
Although it is relatively straightforward to derive these generic recommendations for conservation action, it is usually costly and difficult (practically and/or politically) to implement them effectively and at a sufficient scale to make a difference to the conservation status of seabird species. However, considerable progress has been achieved in recent years in terms of the three highest priority actions: protecting key sites (encompassing many more specific interventions), eradicating/controlling invasive alien species and addressing seabird bycatch. In contrast, less progress has been made in ensuring that ecosystem approaches underpin implementation of fisheries management.
Site protection
One index of the most general level of protection afforded to seabird breeding sites is the coverage by nationally designated protected areas of IBAs identified for seabird species. Thus, of the 1,820 terrestrial IBAs currently identified for seabirds (major areas currently incomplete are Antarctica, many Pacific Islands, USA, Mesoamerica, Russian Arctic, East Asia, South-east Asia, parts of the Indian Ocean and West Africa; however, there is no reason to believe that the properties of IBAs in these areas will be substantially different than in the rest of the world), on average, 38% of the area of these IBAs is covered by protected areas and 28% is completely covered (Figure 9a). This is an improvement of about one order of magnitude since the 1950s–1960s and an approximate doubling of protection since the mid-1980s. It is concerning, however, that the rate of increase appears to have reduced substantially since about 2000, perhaps because remaining unprotected IBAs are in areas with the greatest land-use conflicts, but possibly partly because of time-lags between countries identifying IBAs, designating protected areas and providing data on these to the WDPA.

Figure 9. Protected Area coverage of Important Bird Areas identified for (a) seabirds worldwide (n = 1,820 sites); (b) seabirds in different regions (showing mean percentage area protected). Figures indicate number of sites.
Trends in the coverage of seabird IBAs by protected areas in different regions are shown in Figure 9b. It is perhaps unsurprising that Australasia, with a number of very large marine parks, and Europe, with many important seabird breeding sites long protected at some level, achieve the highest levels of protection. It is interesting, however, that both Asia (with relatively many sites) and South America (with the fewest sites of any region) come next and that both are ahead of North America and the Caribbean. Particularly in the Caribbean – but probably in North America generally – designation of coastal protected areas has been severely constrained by the priority accorded to human recreational and commercial development of coastal areas.
Finally, examining protection of IBAs at a national scale (Figure 10), on average, more than two-thirds of the extent of IBAs is protected in France (including French Southern Territories), UK, Ecuador (chiefly by virtue of the Galapagos National Park), Netherlands, Denmark and Egypt. Among the countries protecting more than 50% of the extent of their IBAs, Japan and Australia both stand out in respect of the large number of breeding sites involved. At the other end of the scale, countries with at least 10 seabird IBAs, for which preliminary data indicate that a mean of < 25% of IBA extent is covered by protected areas, include the Bahamas, Brazil, Estonia, Faroe Islands, Iceland, Morocco, Oman, Saudi Arabia, Ukraine and Yemen.

Figure 10. Countries with the highest proportion of their seabird Important Bird Areas protected. Figures indicate number of sites.
Further analysis of these data (beyond the scope of this review) is needed to assess the main gaps in protection for the most important terrestrial IBAs for seabirds as well as an assessment of the practical effectiveness of this protection. The present analyses include protected areas in all IUCN categories (IUCN and UNEP 2010) but for many, if not most, the actual protection may be purely nominal. Indeed, an important challenge is to categorise each protected area according to the level, nature and effectiveness of protection actually afforded to the seabirds in the IBA, including whether any management plan (of relevance to seabirds) exists and whether this plan is being implemented. Only when this is undertaken, in conjunction with monitoring the status of the seabirds in the IBA, will a meaningful assessment of the level of protection accorded to breeding seabirds at national, regional and global levels be feasible. Doing this is at least as high a priority as any of the research actions noted below.
The foregoing discussion has dealt almost exclusively with protection of breeding sites. Protection of key feeding and aggregation (e.g. for moult and on migration) areas is the essential complementary conservation action. In order to address this, BirdLife International recently extended its global IBA programme to include marine areas, especially seaward extensions around breeding colonies, sites of coastal congregations of non-breeding birds, migration bottlenecks and key pelagic sites. All these sites are likely to represent priority sites for protection and/or management and more than 40 national BirdLife Partners are currently actively engaged in work related to marine IBA identification and protection.
Some parts of the foraging ranges of seabirds are afforded protection by existing marine protected areas at some IBAs. However, in nearly all cases, the size of the area included is too small or inappropriately located to include the resources required by breeding seabirds (BirdLife International 2010c). It is currently very difficult to estimate the number and proportion of marine IBAs effectively protected, as marine IBA coverage is still patchy and incomplete on a global scale. The process of marine protection for seabirds is perhaps the most developed globally in the European Union (EU), under its Birds Directive, but even here only around 1.5% of the EEZs of EU member states have Special Protected Area (SPA) status. It is likely that the percentage is similar or smaller for the majority of coastal nations globally.
Given that marine protected areas cover only about 1.17 % of the ocean (comprising 4.32% of continental shelf areas but only 0.91% in off-shelf waters), i.e. an order of magnitude less than the equivalent value (c. 10%) for terrestrial areas (Toropova et al. Reference Toropova, Meliane, Laffoley, Matthews and Spalding2010, UN 2010), it is hardly surprising that establishing better protection, as well as better regulation and management of relevant threats at sea, is the highest priority of all for those marine areas of greatest importance to seabirds. Nevertheless the fundamental differences between protected areas on land and at sea, particularly in relation to the large range of pelagic species and the dynamic nature of many of the key habitat features that such species exploit, need to be recognised. Effective protected areas in marine systems will need to be large and their management is likely to focus more on management of threatening processes (particularly those of resource exploitation) than outright prohibition of such activities.
In relation to marine areas of greatest importance for seabirds, relative priorities in terms of EEZs are shown in Figure S4, based on data (as total species) in Figure 6a. The most important countries are USA (147 species overall, 11 threatened), Mexico (109/14), Chile (103/22), Canada (100/9), Australia (97/23), New Zealand (96/38), Japan (92/9), Russia (91/7), South Africa (82/16) and Argentina (74/14). Other countries whose EEZs support 50 or more seabird species are China, Peru, Brazil, Spain , France (and French Polynesia), UK (and Falkland Islands [Islas Malvinas] and South Georgia [Islas Georgias del Sur]), Ireland, Portugal, Denmark, Ecuador, Colombia, Costa Rica and India.
An important suite of marine areas are Large Marine Ecosystems (LMEs; Sherman et al. Reference Sherman, Alexander, Gold and 1993), 63 areas that have been recognised as constituting discrete and coherent marine regions, particularly from a resource-management perspective. Many of these are coastal, but most overlap the EEZs of more than one country and/or extend into the High Seas. The overlap between the distribution of seabirds and LMEs is illustrated in Figure S5 and summarised in Table S3. It is not surprising that the LMEs of most importance to seabirds include the Humboldt Current (with 17% more species than any other area), California Current, New Zealand Shelf, East Central Australian Shelf, Agulhas Current, Pacific Central-American Coastal, Kuroshio Current, Patagonian Shelf, Southeast Australian Shelf and West Bering Sea (all supporting > 70 seabird species), with the Gulf of Alaska, Benguela Current and East Bering Sea being the other LMEs supporting > 60 species. Taken together, appropriate management of the marine environment in these LMEs would make a substantial contribution to the conservation of at least 275 seabird species (80% of the total), including 62 (64%) of the globally threatened species.
For marine areas and habitats largely or exclusively on the High Seas, the main relevant jurisdictions are the areas of application of the various RFMOs. The overlap between these and seabird species is summarised in Table S3. This emphasises the potential importance of appropriate environmental management in the vast areas where members of these RFMOs operate: the top eight RFMOs all support more seabird species than any individual EEZ or LME, with 223 species in the area of the Western and Central Pacific Fisheries Commission (WCPFC), more than double the numbers in the Humboldt Current LME. The five main tuna RFMOs are all in the top six RFMOs for seabirds.
To address priority seabird conservation issues in the marine environment will therefore require approaches that combine and coordinate actions in EEZs and on the High Seas (with particular focus on those LMEs that straddle EEZs and High Seas). However without effective action by the RFMOs with High Seas jurisdictions and responsibilities, many threats to seabirds cannot be adequately addressed. Ensuring that sites/areas for seabirds are well represented within proposed candidate Ecologically and Biologically Sensitive Areas (EBSAs) under the Convention on Biological Diversity will be vital.
Eradication or control of invasive alien species
Over the last two decades, improved materials and techniques and considerable effort have led to the successful removal of alien invasive species from many islands of substantial importance for breeding seabirds. Thus, of the 25 most important sites identified in 1982 (Croxall et al. Reference Croxall, Evans, Schreiber and 1984), several have been successfully cleared of at least some alien invasive species (e.g. feral cats removed from Isla de la Plata, Ascension, Marion and Macquarie islands; feral goats from Isla de la Plata and South Trindade) and appropriate plans are well advanced for several others. The success in removing rats Rattus spp. from Campbell Island has stimulated the development of rodent removal plans for numerous other islands. Those for Macquarie Island, Henderson (Pitcairn group) and South Georgia are currently being implemented while plans are well developed for Palmyra, Wake, several islands in the Gambier group and, subject to final feasibility studies, for house mouse Mus musculus at Gough Island, arguably the world’s most important site (and for general biodiversity as well as for seabirds) for which alien eradication is the top priority (Wanless et al. Reference Wanless, Angel, Cuthbert, Hilton and Ryan2007).
Many other islands of national and/or regional importance for seabirds have had rats, cats, dogs, pigs, goats, rabbits and cattle removed in the last decade or so (e.g. Nogales et al. Reference Nogales, Martin, Tershy, Donlan, Veitch, Puerta, Wood and Alonso2004, Angel et al. Reference Angel, Wanless and Cooper2009). Indeed, by late 2006, 332 successful rodent eradications (35 failed, 20 unknown outcome) had been undertaken, with invasive rodents eradicated from 284 islands (Howald et al. Reference Howald, Donlan, Galván, Russell, Parkes, Samaniego, Wang, Veitch, Genovesi, Pascal, Saunders and Tershy2007). As techniques and materials are further improved, it is probable that, notwithstanding funding and political constraints, most of the remaining top priority sites for seabirds could be cleared of relevant important invasive aliens over the next decade or so. A list of seabird sites requiring urgent attention, involving some 73 islands and 20 jurisdictions, is provided in Table 3. This list is inevitably incomplete, with some key areas and islands still unsurveyed, especially in the Pacific, but it does represent a synthesis of current expert knowledge and opinion. For many sites, to develop further towards implementation will require consideration of potential benefits to other vertebrate taxa (especially mammals and reptiles) and to wider biodiversity. It will usually be important to consider the full range of issues and opportunities, especially involving island restoration and including translocations to former breeding sites, that often need to be assessed before appropriate eradication decisions can be made (Mulder et al. Reference Mulder, Anderson, Towns and Bellingham2011).
Table 3. A list of priority islands where eradication of invasive alien vertebrates would benefit globally threatened seabirds or major multi-species colonies. (CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern, PE = Possibly Extinct).

Note 1. Cat, Pig, Goat, Reindeer, Dog, Donkey, Cattle refer to feral animals. Scientific names: House Mouse Mus musculus; Black Rat Rattus rattus; European Rabbit Oryctolagus cuniculus; Masked Owl Tyto novaehollandiae; Feral cat Felis catus; Feral pig Sus domesticus; Feral goat Capra hircus, Pacific Rat Rattus exulans; Feral reindeer Rangifer tarandus; Brown Rat Rattus norvegicus; Northern Raccoon Procyon lotor; Feral dog Canis familiaris; Southern Coati Nasua nasua; Lesser Rice-field Rat Rattus losea; Donkey = Feral donkey Equus asinus; Feral cattle Bos taurus; Weka Gallirallus australis; Rhesus Macaque Macaca mulatta; Garden Dormouse Eliomys quercinus; Pine Marten Martes martes; Arctic Fox Alopex lagopus; Red Fox Vulpes vulpes
Note 2. See Lord Howe Board (2009).
Note 3. Additional colony-specific details made available from Conselleria de Medi Ambient, Govern de les Illes Balears via P. Arcos and M. McMinn (in litt., 2011).
Thus, while it is relatively straightforward to provide indicative lists of important sites where eradication of alien species would benefit seabirds, and feasible to review and analyse these and similar lists for other taxa in order to derive conservation-related priorities, implementation of successful eradications still remains challenging, especially from islands with resident human populations (Oppel et al. Reference Oppel, Beaven, Bolton, Vickery and Bodey2011). In these circumstances the biological priorities rapidly become subordinate to the socio-economic and political (including land tenure) realities, at least in terms of building the support from stakeholder partnerships that will be essential for any eradication implementation to be feasible or successful. Implementation of appropriate bio-security procedures, especially following successful eradications, is also a top priority.
Seabird bycatch
This issue has only been apparent for about two decades (Brothers Reference Brothers1991, Croxall Reference Croxall2008). Nevertheless, seabird bycatch is the most pervasive and immediate threat to many albatross and petrel species in both coastal waters and on the High Seas. The problem is largely being tackled in four complementary ways. These involve: a) using long-term demographic studies of relevant seabird species, linked to observational and recovery data to identify the cause of population declines (e.g. Croxall et al. Reference Croxall, Prince, Rothery, Wood, Robertson and Gales1998, Tuck et al. Reference Tuck, Polacheck, Croxall, Weimerskirch, Ryan, Nel, Wayte, Bulman and Tuck2004, Poncet et al. Reference Poncet, Robertson, Phillips, Lawton, Phalan, Trathan and Croxall2006); b) risk assessments, based on spatio-temporal overlap between seabird species susceptible to bycatch and effort data for fisheries likely to catch them (e.g. Waugh et al. Reference Waugh, Baker, Gales and Croxall2008; Filippi et al. Reference Filippi, Waugh and Nicol2010; Reference Filippi, Waugh and NicolTuck et al. in press); c) working with multinational and international bodies (e.g. FAO and RFMOs) to develop and implement appropriate regulations for the use of best-practice techniques to reduce or eliminate seabird bycatch (see below) and; d) working with fishers (and national fishery organisations) to assist cost-effective implementation of these mitigation techniques (see below). In terms of point b), the use of modern data on seabird distribution, derived from remote-recording studies (satellite tracking and geolocators) has been essential, with the BirdLife Global Procellariiform Tracking Database (BirdLife International 2004) being a crucial tool for identifying actual and potential bycatch ‘hotspots’ in coastal waters and on the High Seas.
In relation to point c), the most important organisations include the Food and Agriculture Organisation of the United Nations (FAO; particularly for best-practice advice for addressing bycatch within the context of implementation of the FAO International Plan of Action for Reducing Incidental Catch of Seabirds in Longline Fisheries - see www.fao.org/fishery/ipoa-seabirds/npoa/er) and ACAP (whose Seabird Bycatch Working Group has rapidly become a leading forum for technical advice on the implementation of specific mitigation measures to eliminate seabird bycatch, further developing the pioneering work of the Working Group on Incidental Mortality Associated with Fishing of the Convention for the Conservation of Antarctic Marine Living Resources (CCAMLR)).
However, despite attempts to reduce the level of seabird bycatch by some tuna RFMOs, the extent of implementation of effective measures remains largely inadequate. The following measures are required to improve fishery performance and reduce seabird bycatch in all RFMOs, especially those involved in management of tuna and related species: a) universal adoption and implementation of best-practice scientific advice on mitigation measures to reduce seabird bycatch; b) improved data collection through at-sea observer programmes; and c) full use of appropriate monitoring, surveillance and compliance measures. Whereas in 2004 none of the five tuna RFMOs had enacted seabird bycatch conservation measures, by 2010 four of the five had at least one such measure in place. So, while much improved implementation is still needed to reduce and document seabird bycatch levels, there has been some progress in recent years towards better management of key fisheries in respect of non-target species.
At the practical level (and in terms of point d) above), BirdLife International’s Albatross Task Force (ATF), the world’s first international team of bycatch mitigation instructors, was established in 2006 to meet an urgent need for skilled practitioners to work at the ‘grassroots level’ with fishers on-shore and at-sea to reduce seabird bycatch to negligible levels. The ATF currently works in seven countries in South America and southern Africa and has demonstrated in South Africa and Chile that bycatch reductions of > 80% can be achieved in pelagic longline and trawl fisheries with the adoption of cost-effective bycatch mitigation measures (BirdLife Global Seabird Programme 2010). The ATF is actively involved in the development and at-sea trialling of new mitigation measures that when exported to and adopted by RFMO fisheries have the potential to make major contributions to reducing seabird bycatch in coastal and High Seas pelagic longline fisheries.
Other actions
Most of the other actions necessary to protect seabirds are considerably more difficult, either practically or politically, to achieve. Thus, implementation of ecosystem approaches to fishery management is still entirely inadequate, especially in fisheries whose target species are over-exploited or fully exploited; similarly, significant reduction/elimination of seabird mortality due to hydrocarbon pollution requires continuing, coordinated national and international action to achieve anything resembling best-practice regulation and management, even in territorial waters. Coastal habitats, both on land and at sea are under threat as never before. Accelerating development, both industrial and recreational (and including energy generation and aquaculture), combined with growing and ubiquitous chronic pollution (oil, pesticides, etc), the acute impacts of ever more frequent environmental accidents and, in many areas, increasing depredations for human sustenance, are putting many populations and species of seabird at increasing risk. Where effective protection of breeding and feeding sites can be achieved, hope remains; in many places, however, where seabirds breed or feed on and close to coasts accessible to humans, the prognosis appears bleak.
In addition to some of the above initiatives, which are operating at the scale of entire islands and/or habitats – and therefore usually addressing simultaneously threats to several seabird species – effective progress may often be achieved and coordinated through developing and implementing appropriate Species Actions Plans. At least 87 seabird species (25% of the total and 43% of all globally threatened species) have had recent action/recovery plans (or their close equivalents) developed (Table 4).
Table 4. Global or regional Species Action Plans (or close equivalents) for seabirds. (Note that this list does not include brief outline plans, such as those for all Australian birds in Garnett and Crowley (Reference Garnett and Crowley2000) nor more generic national plans, such as Environment Australia (2001). Red List category abbreviations follow Table 3).

Notes
1. The Action Plan for Newell’s Shearwater and Hawaiian Petrel is in preparation by a group of biologists at the State of Hawaii Division of Forestry and Wildlife (DOFAW) and the U.S. Fish and Wildlife Service (H. Freifeld and N. Holmes in litt. 2011)
2. Revised and updated at http://www.birdlifeforums.org/WebX/.2cba720f
Research priorities
The main research actions recommended (from the BirdLife World Bird Database, based on a review of the conservation literature and expert opinion) as a basis for, or complement to, conservation action are summarised in Figure 11. While recognising that these data would benefit greatly from further expert scrutiny and evaluation, particularly to ensure consistent treatment between areas and species, four generalisations are feasible.
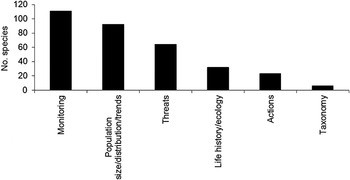
Figure 11. Priority research topics for threatened, Near Threatened and Data Deficient seabirds.
First, it is self-evident that to understand the trends in seabird populations and species, whether on national, regional or global bases, more and better coordinated monitoring is badly needed, as a minimum to permit evaluation of population size and trends for as many species as possible, particularly those already in adverse conservation status. All existing data should be collated, standardised where feasible and made widely and freely available.
Second, for a number of species, the threats they face need to be identified or much better understood before any remedial action is feasible. Thus causes of decline for species like Steller’s Eider Polysticta stelleri, Kittlitz’s Murrelet Brachyramphus brevirostris, Northern and Southern Rockhopper Penguins Eudyptes moseleyi and E. chrysocome and Sooty Shearwater Puffinus griseus, are little understood, nor are the threats facing many poorly known species, such as numerous storm-petrel species and Ivory Gull Pagophila eburnea. In more specific cases, the magnitude of threat from invasive alien predators needs assessing for species like Phoenix and Tahiti Petrels Pterodroma alba and Pseudobulweria rostrata (generally in the Pacific), Magenta Petrel Pterodroma magentae (Chatham Islands), Gould’s Petrel P. brevipes (New Caledonia), Grey Petrel Procellaria cinerea (Gough Island), Heinroth’s Shearwater Puffinus heinrothi (Solomon Islands) and doubtless many others.
The impact of light pollution is a particular concern for Gould’s Petrel Pterodroma leucoptera (in New Caledonia), Barau’s and Mascarene Petrels P. baraui and Pseudobulweria aterrima (Reunion), Newell’s Shearwater Puffinus newelli (Hawaii) and Ashy Storm-petrel Oceanodroma homochroa and may need investigating for a number of other species. Fisheries interactions are deemed potentially important to investigate for a wide range of species, but particularly so for some cormorant species in pot fisheries (e.g. Chatham Islands Shag Phalacrocorax onslowi) and for many species in gillnet fisheries, especially alcids and murrelets (notably Kittlitz’s, Xantus’s Synthliboramphus hypoleuca, Craveri’s S. craveri and Japanese S. wumizusume) and several Spheniscus penguin species. Assessing the impact of direct exploitation by humans is a particular concern for tropical seabird species generally, especially at the few remaining major multi-species colonies in South-east Asia, as well as wherever inshore artisanal fisheries are being undertaken.
Third, for other species, the priority is a better understanding of aspects of life history, distribution and ecology in order to understand their potential vulnerability to particular threats. This is particularly mentioned in respect of demographic studies for species like Northern and Southern Rockhopper Penguins, several Pterodroma petrels and Newell’s Shearwater and for relatively unstudied restricted-range species like Black-faced Cormorant Phalacrocorax fuscescens and Kerguelen Tern Sterna virgata. Better knowledge of foraging distribution is required for many species, especially those susceptible to bycatch but also for boobies (especially Abbott’s Booby Papasula abbotti) and frigatebirds, as well as most Pterodroma petrels. Studies outside the breeding season, particularly of long-distance migrants and of juveniles of almost all species, are of particular importance.
Fourth, for some species groups, modern taxonomic and genetic research is vital to understand the nature of gene flow between populations and the influence this may have on taxonomic ranking and on consequent conservation action. Particular candidates for such work are the species complexes involving Little and Audubon’s Shearwaters, Puffinus assimilis and P. lherminieri, Collared and Gould’s Petrels Pterodroma brevipes and P. leucoptera, Trindade and Herald Petrels Pterodroma arminjoniana and P. heraldica, Fregetta storm-petrels and the Leucocarbo group of Southern Hemisphere cormorants.
Finally, for a very few seabird species, their breeding colonies still remain to be discovered. Prime examples are New Zealand Storm-petrel Oceanites maorianus, Fiji Petrel Pseudobulweria macgillivrayi and Ringed Storm-petrel Oceanodroma hornbyi.
Overall, some of the more wide-ranging and important needs for research and associated activities relate to assessing the severity of the threat and the status of seabird species potentially at high risk because of: a) predation from invasive alien species; b) direct exploitation by humans; and c) direct and indirect impact of artisanal fishing practices, particularly gillnets.
In many cases, appropriate action plans may need developing, but most outcomes should be directly linkable to actions already being undertaken as part of the three major ongoing activities: site protection, invasive alien species eradication and bycatch mitigation.
In addition, enhanced research is desirable on the potential effects on seabirds of:
a) acute mortality events, including those caused by pollution and harmful algal blooms;
b) aquaculture. This is possibly a lower priority for seabirds than for other, less mobile, marine taxa;
c) energy generation in coastal and offshore marine habitats. There is much work to be done, drawing on and generalising from existing experience to inform marine spatial planning, not least in areas where seabird research is less well advanced and coastal development is accelerating;
d) climate change. The main priority is to identify the seabird species and populations that are likely to be most susceptible to sea level rise, as this may have immediate relevance to existing and developing plans for management actions in relation to protected areas, alien invasive species eradications and translocations (e.g. Bermuda Petrel). In respect of potential changes in ocean dynamics, which may have substantial effects on seabird populations worldwide, one perspective is that if we do not act effectively now to counter all the other threats confronting seabirds, many populations will not be around by the time these changes come into effect! Nevertheless, understanding the seabird communities, species and populations most likely at risk from major shifts in ocean conditions might assist in developing management actions for those that we have some prospect of saving and sustaining. This should include research on impacts of ecosystem-level changes that alter predator-prey and competitive interactions between species.
Conclusions
To address the multiplicity of problems confronting the global ocean and the seabirds dependent on it – which are amongst the most visible and iconic of its inhabitants (as well as the best studied) – will require concerted endeavour at all levels from research to policy, complemented by the exceptional effort needed to implement actions to address the conservation and management priorities. For the global seabird community, it is imperative to share and combine resources, data and expertise more effectively. In particular: 1) a high priority is to develop effective intercommunication networks between existing databases to underpin a range of conservation and management objectives. Particular goals should include: a) creating a World Seabird Colony Register (which would complement the BirdLife IBA database); b) developing interoperable databases for seabird monitoring studies, permitting instantaneous overviews of status and productivity at sites worldwide; c) linking seabird at-sea distribution data from remote tracking and at-sea surveys; and d) establishing a new database for seabird mortality events (whether of unknown cause or due to e.g. oil pollution or harmful algal blooms); 2) all available data on seabird distribution need to contribute to the identification of candidate sites for marine protected areas (and for best-practice marine-managed areas) both within national EEZs and especially on the High Seas. Ensuring that sites/areas for seabirds are well represented within proposed candidate EBSAs under the Convention on Biological Diversity will be vital; 3) improved access to information on habitat restoration, especially for seabird islands, including developing an agreed register of priority sites for eradication of alien invasive species, together with advice on best-practice techniques is urgently needed; and 4) enhanced worldwide collaboration is needed to address seabird-fishery interactions, especially bycatch, noting the likelihood of increasing problems from gillnet bycatch and from commercial exploitation of forage fish (anchovies, krill, etc.).
Even if we are unable to deliver all of this, we probably already know enough to: a) to implement the research and conservation priorities already identified; b) scope the data gaps that are priorities for addressing tomorrow’s challenges; c) establish mechanisms for advocating and resourcing the implementation of these priorities; and d) ensure that seabirds are thoroughly linked to the other main initiatives seeking to understand better the dynamics of the ocean and to conserve (and manage sustainably where appropriate) its biodiversity.
In many cases, seabirds will be exemplary models, as well as flagships, for such endeavours. If we cannot generate the commitment and momentum to establish new levels and orders of collaborative interaction on behalf of seabirds and oceans, then we can only expect to watch their destruction from the sidelines.
Supplementary Material
The supplementary materials for this article can be found at journals.cambridge.org/bci
Acknowledgements
We are grateful to the thousands of individuals and organisations, in particular the BirdLife Partners, who contribute to BirdLife’s Red List assessments and documentation of the status of seabirds and/or to the identification, monitoring and conservation of IBAs for seabirds. We thank the many colleagues who provided comments on our text or on the data on which it is based. A draft of the paper was circulated to all 800 attendees at the World Seabird Conference; we are most grateful for the many responses (too many to acknowledge individually) and particularly appreciate the thorough reviews by Jez Bird, Ian Nisbet, Richard Phillips and Cleo Small. In respect of alien invasive species we are much indebted to researchers at the University of California, Santa Cruz (Don Croll, Erin McCreless, Kelly Newton, Dena Spatz, Bernie Tershy) and Island Conservation (Karl Campbell, Nick Holmes) whose input, especially to the table of sites, was invaluable. For additional comments we thank also Steve Creswell, Esteban Frere, Peter Hodum, Guillermo Luna, Thierry Micol, Steffen Oppel, Ivan Ramirez, Mayumi Sato, Alejandro Simeone, Clare Stringer, Ross Wanless and Carlos Zavalaga. We also thank Jörn Scharleman for help with preparation of Figure 7, Sue Patterson for assistance throughout the development of the paper and Rory McCann for help with data compilation. We are most grateful to the reviewers of the final draft and to Peter Ryan for extensive editorial comment and advice.

















