Introduction
Seabirds are globally declining (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012). Particularly, albatrosses (Diomedeidae) and large petrels (Macronectes and Procellaria spp.) have increasingly roused concerns about their sustainability (Croxall and Gales Reference Croxall, Gales, Robertson and Gales1998, Woehler et al. Reference Woehler, Cooper, Croxall, Fraser, Kooyman, Miller, Nel, Patterson, Peter, Ribic, Salwicka, Trivelpiece and Weimerskirch2001, Cooper et al. Reference Cooper, Baker, Double, Gales, Papworth, Tasker and Waugh2006). This group comprises some of the world’s most endangered species of birds, with rapidly decreasing populations and their conservation status markedly deteriorating in recent years (Paleczny et al. Reference Paleczny, Hammill, Karpouzi and Pauly2015, Phillips et al. Reference Phillips, Gales, Baker, Double, Favero, Quintana, Tasker, Weimerskirch, Uhart and Wolfaardt2016). Incidental mortality in fisheries (hereafter “bycatch”) has been well documented and identified as a major threat to these species (Tuck et al. Reference Tuck, Polacheck, Croxall and Weimerskirch2001, Baker et al. Reference Baker, Gales, Hamilton and Wilkinson2002, Lewison et al. Reference Lewison, Crowder and Read2004, Rolland et al. Reference Rolland, Barbraud and Weimerskirch2009, Reference Rolland, Weimerskirch and Barbraud2010, Jimenez et al. Reference Jacobs, Deguchi, Perriman, Flint, Gummer and Uhart2014). However, invasive alien species, degradation or loss of nesting habitat, human disturbance, and marine pollution or plastic ingestion are also significant factors in population declines (Phillips et al. Reference Phillips, Gales, Baker, Double, Favero, Quintana, Tasker, Weimerskirch, Uhart and Wolfaardt2016).
Notably, less is known about the threat that albatrosses and petrels face from infectious disease, even though pathogens have the potential to cause rapid declines and extinction in vulnerable vertebrate populations (Smith et al. Reference Smith, Acevedo-Whitehouse and Pedersen2009, Delahay et al. Reference Delahay, Smith, Hutchings, Delahay, Smith and Hutchings2009, Heard et al. Reference Heard, Smith, Ripp, Berger, Chen, Dittmeier, Goter, McGarvey and Ryan2013). It is likely that most albatross and large petrels are immunologically naïve to infectious diseases due to evolutionary and current geographic isolation (Phillips et al. Reference Phillips, Gales, Baker, Double, Favero, Quintana, Tasker, Weimerskirch, Uhart and Wolfaardt2016). This, coupled with their highly gregarious breeding habits, make them particularly susceptible to opportunistic pathogens and disease epidemics (Descamps et al. Reference Descamps, Jenouvrier, Gilchrist and Forbes2012). Dramatic evidence of this are the recurrent chick mortalities and reproductive failure from avian cholera presently affecting two albatross species from Amsterdam Island in the Indian Ocean, and threatening the ‘Critically Endangered’ and endemic Amsterdam Albatross Diomedea amsterdamensis (Weimerskirch Reference Weimerskirch2004, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015). Furthermore, the avian cholera agent Pasteurella multocida is closely linked to poultry and human dispersion, and has caused the most significant epizootics in locations as isolated as Antarctica (Woods et al. Reference Woods, Jones, Watts, Miller, Shellam, Kerry and Riddle2009).
As pathogen transmission dynamics evolve rapidly with globalisation and climate change (Morse Reference Morse1995, Altizer et al. Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013, Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014), threats from disease will likely increase exponentially. Moreover, the synergistic effects of disease with other highly negative impact factors, such as interactions with fisheries and environmental pollution, may become determinants for species extinction and further accelerate this irrevocable process (Rolland et al. Reference Rolland, Barbraud and Weimerskirch2009, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013). An additional concern is that albatrosses and large petrels spend most of their life at sea and return to land only to breed, thus the impact of diseases is perhaps more difficult to detect than in coastal species (Weimerskirch Reference Weimerskirch2004).
The Agreement on the Conservation of Albatrosses and Petrels (ACAP) has recognized the potential impact of diseases on this group of seabirds, and has rightfully encouraged actions to improve knowledge and management of diseases of concern; i.e. “…review evidence for impacts of pathogens and parasites on ACAP species and effectiveness of mitigation measures” (ACAP AC7 2013); “… implement long-term disease surveillance programs” and “…thoroughly investigate albatross disease/mortality events when they occur” (ACAP AC8 2014).
Here we provide a compilation of all available pathogen and health-related information in albatross and large petrel species listed under ACAP as a starting point to enable a thorough evaluation of the overall threat posed by disease. In order to expand the utility of this information, we also summarised meaningful context for data interpretation (e.g. location of studies, study type, potential reservoirs, vectors or modes of transmission). Finally, we discuss the significance of findings and when possible, suggest recommendations for filling current knowledge gaps.
Methods
Literature review
The species-level taxonomy and nomenclature used in our review is based on BirdLife checklist v. 8.0 (http://www.birdlife.org/datazone/info/taxonomy). We used Google Scholar and Pubmed databases to conduct an extensive search of peer-reviewed journal papers on any types of pathogen and/or clinical disease reported in albatross and large petrel species included in ACAP (henceforth ACAP species). We also included health assessments reporting exposure as evidenced by blood antibodies, as well as detection of pathogen DNA/RNA by molecular methods. In addition, we provide data from unpublished “grey” literature (including presentations at scientific conferences) reporting on any of the above when peer-reviewed data were not available. Finally, existing disease information in ACAP database (available at www.acap.aq) and in ACAP Population and Conservation Status Working Group meetings (http://acap.aq/en/working-groups/population-and-conservation-status-working-group) was likewise collated. Weblinks to these online documents are provided in the references section.
To provide meaningful context for data interpretation, we extracted information about geographical distribution of pathogen reports, tissues tested, tests performed and, when available, whether studies were conducted on apparently healthy animals or on individuals with signs of disease. Author comments or hypothesis on potential reservoirs, vectors or modes of transmission for reported pathogens were also included.
Results
Publications and species covered
We identified a total 53 studies reporting on pathogen isolation, molecular identification (e.g. by PCR) or pathogen-specific antibody searches (including those which yielded negative results) in 18 of the 31 (58%) albatross and large petrel species listed under ACAP. Henceforth, all results and discussion refer to these 18 species (ACAP species subset). Summarised information includes references for 10 (56%) species considered as threatened (Critically Endangered, Endangered or Vulnerable) by IUCN. The remaining species in this review are categorised as ‘Near Threatened’ (four) or have a ‘Least Concern’ status (three) (BirdLife International 2015) (Tables 2–7).
Publications cover six genera, namely Diomedea (two species): exulans, amsterdamensis; Macronectes (two species): giganteus, halli; Thalassarche (five species): melanophrys, chrysostoma, cauta, carteri, chlororhynchos; Phoebastria (four species): albatrus, immutabilis, irrorata, nigripes; Phoebetria (two species): fusca, palpebrata; and Procellaria (three species): aequinoctialis, cinerea, parkinsoni.
The number of publications per species was highly variable. Black-browed Albatross Thalassarche melanophrys (Near Threatened) and Southern Giant Petrel Macronectes giganteus (Least Concern) with 15 and 17 papers, respectively, were the two species with a higher number of health or pathogen-related publications. In contrast, Amsterdam Albatross, Atlantic Yellow-nosed Albatross Thalassarche chlororhynchos, Black Petrel Procellaria parkinsoni, Grey Petrel Procellaria cinerea, Short-tailed Albatross Phoebastria albatrus, Sooty Albatross Phoebetra fusca, Indian Yellow-nosed Albatross Thalassarche carteri, Waved Albatross Phoebastria irrorata, Black-footed Albatross Phoebastria nigripes and Northern Giant Petrel Macronectes halli had three publications or less each, and with exception of the last two species, are all included in the IUCN Red List (BirdLife International 2015).
Type of study
Most research on pathogens or pathogen-specific antibodies in the ACAP species subset has resulted from disease/mortality investigations (15/53) and baseline studies (17/53) (28% and 32%, respectively). Three additional studies investigated the role of ACAP species as vectors or dispersers of pathogens of human origin (e.g. gastrointestinal bacteria), and five others were targeted pathogen searches (Borrelia sp., Edwardsiella sp., Chlamydophila sp., Poxvirus avium and influenza A virus). On the other hand, the information on type of study was not inferable in 13 studies of the reviewed literature.
Temporal and spatial distribution of reports
The number of studies reporting on pathogens or health assessments in ACAP species has increased over time. Referenced publications go as far back as the 1940s (Johnston and Mawson Reference Johnston and Mawson1942, Lent and Freitas Reference Lent and Freitas1948). Up to 1970 there were nine publications, 14 were added from 1981 to 2000, and 30 from 2001 to the present.
The location of studies surveying (including antibodies, isolation, recovery and molecular characterization of DNA or RNA) pathogens in the ACAP species subset is diverse, and covers circumpolar as well as tropical locations. In Table 1 we summarise the number of studies reporting on different pathogen taxa by site, classified as Antarctic (> 61°S), Subantarctic (48–61°S) and “other” locations (those not included in the two previous categories). Overall, there are fewer studies in Subantarctic areas (Table 1). Distribution of reports is likely reflective of albatross and petrel reproductive site location (BirdLife International 2004), as this is where most sampling has occurred for all species.
Table 1. Number of studies in ACAP species reporting on specific pathogen findings by geographic region. Studies reporting indirect evidence of exposure (i.e. antibodies) between brackets. Note that some papers report on more than one pathogen group, therefore the total pathogen findings (65) differ from the total number of reports collated (53).

* Antarctic region: extends from the South Pole to the Antarctic Convergence (higher than 61°S latitude).
** Subantarctic region: located immediately north of the Antarctic region and adjacent to the Antarctic Convergence (48-61°S latitude).
*** Other: locations not included in the other two categories (i.e. lower latitudes, such as Hawaii and Galápagos islands).
Isolation of pathogens, direct detection (i.e. pathogen DNA or RNA) and indirect evidence of exposure (i.e. antibodies)
Detailed results separated by pathogen group (viruses, bacteria and fungi, protozoa, gastrointestinal parasites and ectoparasites) and host species are provided in tables 2–6. In addition, for viral, bacterial and fungal isolations and direct detection via PCR, we present data on type of sample tested and diagnostic tests performed in Table 7.
Table 2. Summary of reports on viral pathogens (exposure antibodies, viral isolation and/or direct detection), including those yielding negative results, in albatrosses and large petrels in ACAP species subset. References in brackets. Location: SA: Subantarctic, A: Antarctic, Other (see Table 1 for definitions). NA: not available or not applicable.
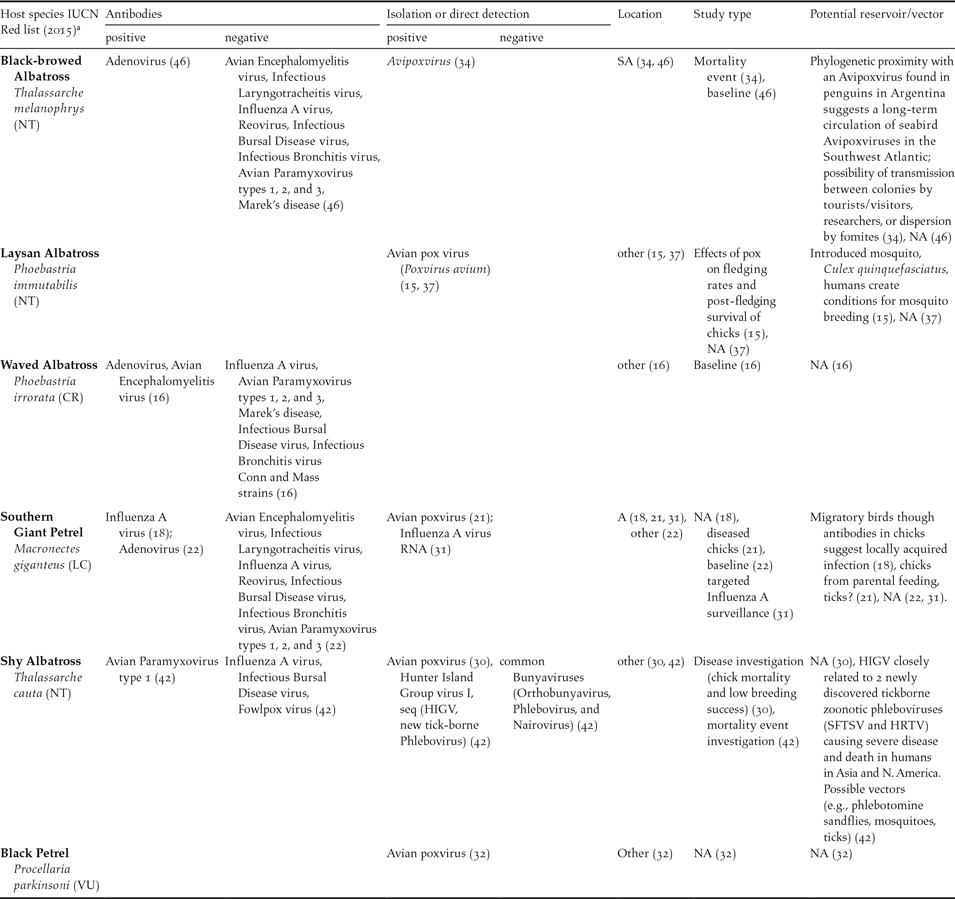
a IUCN Status: CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern. <www.iucnredlist.org>.
Table 3. Summary of reports on bacterial and fungal pathogens (exposure antibodies, isolation, direct detection), including those yielding negative results, in albatrosses and large petrels in ACAP species subset. References in brackets. Location: SA: Subantarctic, A: Antarctic, Other (see Table 1 for definitions). NA: not available or not applicable.

* Padilla et al. (Reference Padilla, Huyvaert, Merkel, Miller and Parker2003): Samples were tested for antibodies to avian cholera by microagglutination at the Diagnostic Laboratory of the University of Missouri–Columbia, College of Veterinary Medicine, Columbia, Missouri 65211, USA.
** Chlamydial bacteria underwent several taxonomic miss-classifications in the past. It is likely that the authors refer to the bacteria Chlamydophila psittaci a common avian pathogen, or another pathogen within the Chlamydophila genera (Nunes and Gomes Reference Nunes and Gomes2014).
*** Weimerskirch (2004, ref n°12) monitored 200 pairs of Yellow-nosed Albatrosses (Diomedea chlororhynchos) annually since 1979 at Pointe d’Entrecasteaux, on the western coast of Amsterdam Island (37°S, 70°E). Diomedea chlororhynchos (Sibley and Monroe 1990, 1993) has been divided into chlororhynchos and carteri and both placed in the genus Thalassarche (Brooke Reference Brooke2004). T. chlororhynchos or Atlantic Yellow-nosed Albatross breeds on Atlantic Ocean islands (Tristan da Cunha and Gough Island). T. carteri or Indian Yellow-nosed Albatross is the species that breeds in Indian Ocean (Amsterdam Island). Therefore we refer to the individuals included in reference No 12 (Weimerskirch Reference Weimerskirch2004) as Indian Yellow-nosed Albatross (Thalassarche carteri).
a IUCN Status: CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern. <www.iucnredlist.org>.
Table 4. Summary of reports on Protozoa in albatrosses and large petrels in ACAP species subset. References in brackets. Location: SA: Subantarctic, A: Antarctic, Other (see Table 1 for definitions). NA: not available.

* Ippen R. and Henne D. (1989). Information on source of pathogen (muscle, blood, other tissue) not available.
a IUCN Status: CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern. <www.iucnredlist.org>.
Table 5. Summary of reports on helminths in albatrosses and large petrels in ACAP species subset. References in brackets. Location: SA: Subantarctic, A: Antarctic, Other (see Table 1 for definitions). NA: not available. Dx: diagnosis.

a IUCN Status: CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern. <www.iucnredlist.org>.
Table 6. Summary of reports on ectoparasites in albatrosses and large petrels in ACAP species subset. References in brackets. Location: SA: Subantarctic, A: Antarctic, Other (see Table 1 for definitions). NA: not available.

a IUCN Status: CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern. <www.iucnredlist.org>.
Table 7. Details on type of sample tested and diagnostic method used for direct detection or identification of bacterial, viral and fungal isolates in albatrosses and large petrels in ACAP species subset. References in brackets.

a IUCN Status: CR = Critically Endangered, EN = Endangered, VU = Vulnerable, NT = Near Threatened, LC = Least Concern. <www.iucnredlist.org>.
NA: not available.
* Munro (Reference Munro2007) report on outbreak of avian poxvirus in Gentoo Penguins, but include unpublished data from BBA pox isolate from Malvinas/Falkland Islands.
** Weimerskirch (2004, ref n°12) monitored 200 pairs of Yellow-nosed Albatrosses (Diomedea chlororhynchos) annually since 1979 at Pointe d’Entrecasteaux, on the western coast of Amsterdam Island (37° S, 70° E). Diomedea chlororhynchos (Sibley and Monroe 1990, 1993) has been divided into chlororhynchos and carteri and both placed in the genus Thalassarche (Brooke, Reference Brooke2004). T. chlororhynchos or Atlantic Yellow-nosed Albatross breeds on Atlantic Ocean islands (Tristan da Cunha and Gough Island). T. carteri or Indian Yellow-nosed Albatross is the species that breeds in Indian Ocean (Amsterdam Islands). Therefore we refer to the individuals included in reference n° 12 (Weimerskirch Reference Weimerskirch2004) as Indian Yellow-nosed Albatross (Thalassarche carteri).
References: (1) Peirce and Prince (Reference Peirce and Prince1980), (2) Mironov and Stefan (Reference Mironov and Stefan2013), (3) Palma and Horning (Reference Palma and Horning2002), (4) Zlotorzycka and Modrzejewska (Reference Zlotorzycka and Modrzejewska1992), (5) Clay and Moreby (Reference Clay and Moreby1970), (6) Tsurumi et al. (Reference Tsurumi, Kawabata and Sato2002), (7) Palmgrem et al. (Reference Palmgrem, McCafferty, Aspan, Broman, Sellin, Wollin, Bergstrom and Olsen2000), (8) Goff et al.(Reference Goff, Sievert and Sileo1989), (9) Bergstrom et al. (Reference Bergstrom, Haemig and Olsen1999b), (10) Bergstrom et al. (Reference Bergstrom, Haemig and Olsen1999a), (11) Olsen et al. (Reference Olsen, Duffy, Jaenson, Gylfe, Bonnedahl and Bergström1995), (12) Weimerskirch (Reference Weimerskirch2004), (13) Wilson (Reference Wilson1970), (14) Work et al. (Reference Work, Smith and Duncan1998), (15) Young and Vanden Werf (Reference Young and Vanden Werf2008), (16) Padilla et al. (Reference Padilla, Huyvaert, Merkel, Miller and Parker2003), (17) Leotta et al. (Reference Leotta, Rivas, Chinen, Vigo, Moredo, Coria and Wolcott2003), (18) Baumeister et al. (Reference Baumeister, Leotta, Pontoriero, Campos, Montalti, Vigo, Pecoraro and Savy2004), (19) Ippen and Henne (Reference Ippen and Henne1989), (20) Jorge et al. (Reference Jorge, Najle and Montalti2002), (21) Shearn-Boschler et al. (Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008), (22) Uhart et al. (Reference Uhart, Quintana, Karesh and Braselton2003), (23) Zdzitowiecki and Drozdz (Reference Zdzitowiecki and Drozdz1980), (24) Whitehead et al. (Reference Whitehead, Burton, Bell, Arnould and Rounsevell1991), (25) Leotta et al. (Reference Leotta, Cerda, Coria and Montalti2001), (26) Leotta et al. (Reference Leotta, Piñeyro, Serena and Vigo2009), (27) Munday (Reference Munday1972), (28) Bonnedahl et al. (Reference Bonnedahl, Broman, Waldenström, Palmgren, Niskanen and Olsen2005), (29) Mironov (Reference Mironov1991), (30) Woods (Reference Woods and Woods2004), (31) de Souza Petersen et al. (Reference de Souza Petersen, Petry, Durigon and Araújo2015), (32) Bell et al. (Reference Bell, Sim and Scofield2007), (33) Johnstone et al. (Reference Johnstone, Milledge and Dorward1975), (34) Munro (Reference Munro2007), (35) Murray et al. (Reference Murray, Palma, Pilgrim, Shaw, Marchant and Higgins2003), (36) Anderson and Fortner (Reference Anderson and Fortner1988), (37) Sileo et al. (Reference Sileo, Sievert and Samuel1990), (38) Gilardi et al. (Reference Gilardi, Gilardi, Frank, Goff and Boyce2001), (39) Gauthier-Clerc et al. (Reference Gauthier-Clerc, Jaulhac, Frenot, Bachelard, Monteil, Le Maho and Handrich1999), (40) Woods et al. (Reference Woods, Jones, Watts, Miller, Shellam, Kerry and Riddle2009), (41) Chastel et al. (Reference Chastel, Demazure, Chastel, Genevois, Legrand, Grulet, Odermatt and Le Goff1993), (42) Wang et al. (Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014), (43) Tham et al. (Reference Tham, Purcell and Schultz1974), (44) Jaeger et al. (Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013), (45) Iwaki et al. (Reference Iwaki, Yokohata, Kajigaya, Sato and Hiraoka2006), (46) Uhart et al. (Reference Uhart, Karesh, Cook, Huin, Lawrence, Guzman and Mörner2004), (47) Jiménez-Uzcátegui et al. (Reference Jiménez-Uzcátegui, Sarzosa, Encalada, Sevilla and Huyvaert2015), (48) Johnston and Mawson (Reference Johnston and Mawson1942), (49) Lent and Freitas (Reference Lent and Freitas1948), (50) Isaksson et al. Reference Isaksson, Christerson, Blomqvist, Wille, Alladio, Sachse, Olsen, Gonzalez-Acuña and Herrmann2015, (51) Demay et al. (Reference Demay, Barbraud, Delord and Weimerskirch2013), (52) Weimerskirch (Reference Weimerskirch2016), (53) Jaeger et al. (Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015).
In summary, pathogens found in the subset of ACAP species (including isolation, recovery and molecular characterisation of DNA or RNA) included bacteria in seven species (39%), viruses in five (28%), protozoa in four (22%), helminths in nine (50%), ectoparasites in 13 (72%) and fungi in one species (5%). For some species (39%, 7/18), pathogen descriptions are limited to parasites. Namely, ectoparasites in Northern Giant Petrel, Grey Petrel and White-chinned Petrel Procellaria aequinoctialis; ectoparasites plus helminths in Light-mantled Albatross Phoebetria palpebrata and Atlantic Yellow-nosed Albatross; protozoa, ectoparasites and helminths in Wandering Albatross Diomedea exulans; and only helminths in Short-tailed Albatross.
Regarding bacterial and viral isolation, 17 different bacteria were reported in six species, most commonly Pasteurella multocida (six reports in four different species) (Weimerskirch Reference Weimerskirch2004, Reference Weimerskirch2016, Leotta et al. Reference Leotta, Rivas, Chinen, Vigo, Moredo, Coria and Wolcott2003, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015) and Salmonella sp. (four strains in two different species) (Work et al. Reference Work, Smith and Duncan1998, Palmgrem et al. Reference Palmgrem, McCafferty, Aspan, Broman, Sellin, Wollin, Bergstrom and Olsen2000). Only two viruses were isolated from the ACAP species subset, namely pox viruses (six reports in five different species) (Sileo et al. Reference Sileo, Sievert and Samuel1990, Young and Vanden Werf Reference Young and Vanden Werf2008, Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008, Woods Reference Woods and Woods2004, Bell et al. Reference Bell, Sim and Scofield2007, Munro Reference Munro2007) and a newly discovered Phlebovirus (HIGV) in Ixodes eudyptidis ticks collected from Shy Albatrosses Thalassarche cauta from the Hunter Island Group in Tasmania (Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014).
Bacterial DNA for P. multocida and E. rhusiopathiae was found via Polymerase Chain Reaction (PCR) in cloacal and oropharyngeal swabs of apparently healthy Amsterdam Albatross chicks in 2011–2012 by Jaeger et al. (Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015). Information on total number of chicks sampled, number of positives, age of chicks at sampling, and type of PCR performed are not included in the reports. An unclassified Chlamydiaceae-like bacteria was identified by real-time PCR from fresh Southern Giant Petrel faeces (1/6) in the Antarctic Peninsula (Isaksson et al. Reference Isaksson, Christerson, Blomqvist, Wille, Alladio, Sachse, Olsen, Gonzalez-Acuña and Herrmann2015). I. uriae ticks (3/41) from Black-browed Albatross breeding in Campbell Island, New Zealand, were positive for the vector-borne bacteria Borrelia garinii DNA by PCR (Olsen et al. Reference Olsen, Duffy, Jaenson, Gylfe, Bonnedahl and Bergström1995). Recently (2010/11 austral summer), Influenza A virus RNA was detected by Reverse Transcription PCR (RT-PCR) in swabs from one (1/299) male Southern Giant Petrel of undescribed age from Elephant Island, Antarctica (de Souza Petersen et al. Reference de Souza Petersen, Petry, Durigon and Araújo2015). It is unknown whether the isolate was from a cloacal or oral swab. No further characterisation (i.e. serotype) of the virus is provided by the authors.
According to indirect evidence of exposure, virus and bacteria-specific antibodies were reported in four (22.2%, Table 2) and two (11.1%, Table 3) of the ACAP species subset (n = 18), respectively. Southern Giant Petrel was the species with the highest exposure to infectious agents inferred by antibodies against four pathogens (Avian Adenovirus, Avian Influenza, Salmonella spp., Chlamydophila spp.) (Munday Reference Munday1972, Uhart et al. Reference Uhart, Quintana, Karesh and Braselton2003), followed by Waved Albatross with antibodies to two viruses (Avian Encephalomyelitis virus and Avian Adenovirus) (Padilla et al. Reference Padilla, Huyvaert, Merkel, Miller and Parker2003).
Spatial distribution of pathogen isolates
Poxviruses were isolated in all but two cases from locations in the “other” category, mostly in the North Pacific (Sileo et al. Reference Sileo, Sievert and Samuel1990, Woods Reference Woods and Woods2004, Bell et al. Reference Bell, Sim and Scofield2007, Young and Vanden Werf Reference Young and Vanden Werf2008). The remaining poxvirus isolates were recovered from a Southern Giant Petrel in Antarctica (Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008) and a Black-browed Albatross in a Subantarctic location (Malvinas/Falkland Islands; Munro Reference Munro2007). A Phlebovirus (HIGV) was isolated from Shy Albatross ticks collected in Tasmania (Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014) and Influenza A virus RNA was found in a Southern Giant Petrel from Antarctica by PCR (de Souza Petersen et al. Reference de Souza Petersen, Petry, Durigon and Araújo2015) (Tables 1 and 2). Bacteria were recovered in all geographical sites in similar numbers (Tables 1 and 3). In addition, a single fungal isolate was recovered in an “other” type location, from Grey-headed Albatross Thalassarche chrysostoma (Tham et al. Reference Tham, Purcell and Schultz1974). Helminths were mostly reported in “other” type locations (Tables 1 and 5), while ectoparasites were found in decreasing order in “other”, Antarctic and Subantarctic sites (Tables 1 and 6).
Discussion
Albatrosses and large petrels are among the world’s most endangered birds, with their status dramatically deteriorating in recent years (Brooke Reference Brooke2004, Cooper et al. Reference Cooper, Baker, Double, Gales, Papworth, Tasker and Waugh2006, Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012, Cherel et al. Reference Cherel, Jaeger, Alderman, Jaquemet, Richard, Wanless, Phillips and Thompson2013, Paleczny et al. Reference Paleczny, Hammill, Karpouzi and Pauly2015). While disease has been identified as a substantial risk in some of the most vulnerable species (e.g. Amsterdam Albatross) (Phillips et al. Reference Phillips, Gales, Baker, Double, Favero, Quintana, Tasker, Weimerskirch, Uhart and Wolfaardt2016), information on pathogens and, more importantly associated morbidity and mortality, is limited. This review of pathogen and health-related information in albatross and large petrel species listed under ACAP will enable a thorough evaluation of the overall threat posed by disease, identifying critical gaps and facilitating targeted conservation actions.
Species coverage
Available reports comprise nearly 60% of the species listed in ACAP (18/31), with no information on the remaining 40%. A notable asymmetry exists in species focus, with the Black-browed Albatross (Near Threatened) and the Southern Giant Petrel (Least Concern), concentrating most health or pathogen-related publications. Furthermore, the Southern Giant Petrel is the species from which the highest number of pathogens has been recovered. However, most are gastrointestinal bacteria isolated from healthy adults during targeted surveys, and considered clinically insignificant (Jorge et al. Reference Jorge, Najle and Montalti2002, Leotta et al. Reference Leotta, Piñeyro, Serena and Vigo2009). The only allegedly meaningful pathogens isolated from these two species are Pasteurella multocida in Southern Giant Petrel (Leotta et al. Reference Leotta, Rivas, Chinen, Vigo, Moredo, Coria and Wolcott2003) and avian poxvirus in both species (Munro Reference Munro2007, Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008). Interestingly, both Southern Giant Petrel isolates are from Antarctica.
On the contrary, there are three or less publications per species for most (7/10) albatross and petrel species in critical conservation status. Yet in this case, some reports are highly relevant as they refer to the pathogenic bacteria P. multocida and E. rhusiopathiae in Amsterdam Island species (Sooty, Indian Yellow-nosed and Amsterdam Albatross) (Weimerskirch Reference Weimerskirch2004, Reference Weimerskirch2016, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015); and avian poxvirus in Black Petrels breeding in New Zealand (Bell et al. Reference Bell, Sim and Scofield2007). Notwithstanding the vulnerable condition of these species, however, existing descriptions exclude relevant information to assess the extent to which these pathogens may be impacting their populations, such as number of animals dying from infection annually, numbers of survivors, sex and age categories affected, etc.
In the remaining threatened species within the ACAP subset, reports are limited to findings of parasites, namely protozoa, helminths and ectoparasites (with the exception of a serosurvey in Waved Albatross, see below). Included are reports of the tick Ixodes kerguelenensis in Wandering Albatross (Critically Endangered), and White-chinned Petrels (Vulnerable) (Gauthier-Clerc et al. Reference Gauthier-Clerc, Jaulhac, Frenot, Bachelard, Monteil, Le Maho and Handrich1999).
Finally, indirect evidence of pathogen exposure via bacteria and virus-specific antibodies is rarely reported in ACAP species (five studies in four species; Munday Reference Munday1972, Uhart et al. Reference Uhart, Quintana, Karesh and Braselton2003, Reference Uhart, Karesh, Cook, Huin, Lawrence, Guzman and Mörner2004, Padilla et al. Reference Padilla, Huyvaert, Merkel, Miller and Parker2003, Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014). Only one of these studies was conducted in a Critically Endangered species (Waved Albatross; Padilla et al. Reference Padilla, Huyvaert, Merkel, Miller and Parker2003).
Pathogen isolation
Viruses
Avian pox is a relatively slow-developing disease, characterized by wart-like lesions on featherless areas of the body (i.e. feet, head). Infections are usually mild and rarely result in death, except when they affect the eyelids, mouth and upper respiratory tract (van Riper and Forrester Reference van Riper, Forrester, Thomas, Hunter and Atkinson2007). All avian poxvirus reports in ACAP species subset relate to findings in clinically ill or dead animals, mostly chicks or fledglings, and were often associated with mortality or low fledging success. In some cases, the immediate cause of death was not poxvirus, but a secondary bacterial infection (i.e. Clostridium perfringens; Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008). Poxvirus outbreaks seem to be recurrent in some locations and species (i.e. Laysan Albatross Phoebastria immutabilis on the Hawaiian Islands, and Shy Albatross on Albatross Island, Indian Ocean). In many cases, morbidity seems to have exceeded mortality, at least while chicks were under parental care (Sileo et al. Reference Sileo, Sievert and Samuel1990, Woods Reference Woods and Woods2004, Young and Vanden Werf Reference Young and Vanden Werf2008). Adults appear, for the most part, to be immune or capable of overcoming infection. Recovery has been reported in sick chicks (Young and Vanden Werf Reference Young and Vanden Werf2008) and adults (Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008, Young and Vanden Werf Reference Young and Vanden Werf2008), and was found to be highly dependent on health status and exposure to additional stressors such as environmental conditions and parasitism (Woods Reference Woods and Woods2004). Most reported cases refer exclusively to cutaneous pox (Sileo et al. Reference Sileo, Sievert and Samuel1990, Bell et al. Reference Bell, Sim and Scofield2007, Young and Vanden Werf Reference Young and Vanden Werf2008). The more severe form of the disease, diphtheritic pox, was only described in a Southern Giant Petrel chick that died during an outbreak in Antarctica (Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008). All these characteristics agree with what is observed in many avian species infected with pox viruses (van Riper and Forrester Reference van Riper, Forrester, Thomas, Hunter and Atkinson2007, Kane et al. Reference Kane, Uhart, Rago, Pereda, Smith, Van Buren, Clark and Boersma2012), yet differs from some island birds for which avian pox has been a major driver of extinction (i.e. Hawaiian forest birds; van Riper et al. Reference van Riper, Van Riper and Hansen2002). Notwithstanding, because the virus is transmitted mechanically by arthropod vectors or by contact with pox-infected particles, it is highly contagious (Woods Reference Woods and Woods2004). This implies that outbreaks often occur in clusters within colonies, but also that it can be spread to remote locations through bird travels and migration, human visitors, and as importantly, reintroduction programmes (Gyuranecz et al. Reference Gyuranecz, Foster, Dan, Ip, Egstad, Parker, Higashiguchi, Skinner, Höfle, Kreizinger, Dorrestein, Solt, Sós, Jun Kim, Uhart, Pereda, González-Hein, Hidalgo, Blanco and Erdelyi2013). Therefore, strict biosecurity is recommended during outbreaks, and poxvirus-specific screening should be included in translocation and reintroductions risk assessments (Jacobs et al. Reference Jacobs, Deguchi, Perriman, Flint, Gummer and Uhart2014). Dispersal of poxvirus with translocated albatrosses to remote islands could threaten not only the reintroduced flock, but any native bird species there (van Riper and Forrester Reference van Riper, Forrester, Thomas, Hunter and Atkinson2007). Furthermore, avian pox outbreaks often coincide with weather-induced increases in vector populations (van Riper et al. Reference van Riper, Van Riper and Hansen2002, Young and Vander Werf Reference Young and Vanden Werf2008). Thus, monitoring the behaviour of this disease over time, particularly in areas subject to influences from global climate change, is recommended (Kovats et al. Reference Kovats, Campbell-Lendrum, McMichael, Woodward and Cox2001).
The only other virus isolated from ACAP species subset is a novel tick-borne phlebovirus, named Hunter Island Group Virus (HIGV; Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014). It was identified recently by next-generation sequencing from samples collected during the investigation of a disease outbreak of Shy Albatrosses in Tasmania. The HIGV is closely related to two newly discovered tick-borne zoonotic phleboviruses (SFTSV and HRTV) that were responsible for severe disease and death in humans in four countries in Asia and North America (Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014). However, and as reported by the authors of the study, this is probably an incidental finding and not particularly related to the disease event in the Shy Albatrosses.
Bacteria
Due to their capacity to produce acute systemic disease followed by death, the most significant isolates from the ACAP species subset are Pasteurella multocida and Erysipelothrix rhusiopathiae. P. multocida is a contagious avian pathogen that is known to infect over 190 species of birds and causes major and recurrent epizootics of avian cholera in waterfowl in North America (Samuel et al. Reference Samuel, Botzler, Wobeser, Thomas, Hunter and Atkinson2007). Transmission occurs from contact between birds and by ingestion or inhalation of bacteria within a contaminated environment. Diseased birds and rotting carcasses are important sources of contamination and the bacteria can be carried between infected sites by migrating or carrion scavenging birds (Botzler Reference Botzler1991, Friend Reference Friend, Friend and Franson1999, Samuel et al. Reference Samuel, Shadduck, Goldberg and Johnson2005, Wille et al. Reference Wille, McBurney, Robertson, Wilhelm, Blehert, Soos, Dunphy and Whitney2016). Therefore, removing carcasses is the recommended method for reducing environmental loads and controlling avian cholera outbreaks (Friend Reference Friend, Friend and Franson1999, Samuel et al. Reference Samuel, Shadduck, Goldberg and Johnson2005, Blanchong et al. Reference Blanchong, Samuel, Goldberg, Shadduck and Lehr2006). On the other hand, E. rhusiopathiae typically appears to be a secondary pathogen affecting individuals, not populations (Wolcott Reference Wolcott, Thomas, Hunter and Atkinson2007). When it is the primary pathogen, death occurs acutely from a septic process with few pre and post-mortem signs (Wolcott Reference Wolcott, Thomas, Hunter and Atkinson2007). Therefore, it is probably under-detected and under-reported in wild birds. Potential sources or vectors of erysipelas are unclear but may include fish, marine mammals, human handlers and ectoparasites (Wolcott Reference Wolcott, Thomas, Hunter and Atkinson2007).
At least two mortality events from P. multocida infections have affected several seabird species in Antarctica (Leotta et al. Reference Leotta, Chinen, Vigo, Pecoraro and Rivas2006) in addition to an isolated case in an adult Southern Giant Petrel (Leotta et al. Reference Leotta, Rivas, Chinen, Vigo, Moredo, Coria and Wolcott2003). P. multocida and E. rhusiopathiae have been reported (including isolation and/or molecular detection) in three threatened albatross species breeding on Amsterdam Island, namely the Indian Yellow-nosed, Sooty and Amsterdam Albatross (Weimerskirch Reference Weimerskirch2004, Reference Weimerskirch2016, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015). Mortality attributed to disease in Indian Yellow-nosed Albatross extends to the 1980s, and in Sooty and Amsterdam Albatross to the 2000s (Weimerskirch Reference Weimerskirch2004, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013). However, the first bacterial isolates from dead Indian Yellow-nosed Albatrosses were not obtained until 1996 (chicks - E. rhusiopathiae) and 1999 (adults and chicks - P. multocida) (Weimerskirch Reference Weimerskirch2004). No co-infections were reported. More recently (in 2012–2013), P. multocida has been isolated from Indian Yellow-nosed and Sooty Albatross chick carcasses (Demay Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015, Weimerskirch Reference Weimerskirch2016). Notably, Amsterdam Albatross chicks in apparent good health were PCR positive for P. multocida and E. rhusiopathiae in 2011–2012 (Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015). Notwithstanding, without information on the age of the positive chicks, and given recent studies demonstrating extended duration of maternal antibodies in a procellariform (Garnier et al. Reference Garnier, Ramos, Staszewski, Militao, Lobato, González-Solís and Boulinier2011), it is impossible to discern whether this implies survival due to resistance or to passive immunity. Some albatrosses might be resistant to, or able to overcome the disease, as has been seen in other species such as swans and Common Eiders Somateria mollissima (Samuel et al. Reference Samuel, Shadduck, Goldberg and Johnson2005, Descamps et al. Reference Descamps, Jenouvrier, Gilchrist and Forbes2012). Or it may well be that, as was described in Cory’s Shearwater Calonectes diomedea, the presence of maternal antibodies over the first 3-4 weeks of life enable chick survival (Garnier et al. Reference Garnier, Ramos, Staszewski, Militao, Lobato, González-Solís and Boulinier2011). In either case however, these asymptomatically infected birds could then serve as healthy carriers or reservoirs (long-term sources) for the bacteria and initiate new outbreaks (Botzler Reference Botzler1991, Samuel et al. Reference Samuel, Shadduck, Goldberg and Johnson2005). Moreover, of additional high concern are P. multocida isolates obtained from co-inhabiting Subantarctic Skuas Catharacta antarctica lonnbergi (adults and chicks) at this location, during the same season (Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Weimerskirch Reference Weimerskirch2016). Skuas scavenge in all albatross colonies, connecting otherwise isolated sections of the Island where the different albatross species breed (Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Weimerskirch Reference Weimerskirch2016). Thus, the role of skuas as carriers/mechanical vectors of P. multocida and/or other pathogens in this confined island situation is likely to be significant (Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Weimerskirch Reference Weimerskirch2016, Wille et al. Reference Wille, McBurney, Robertson, Wilhelm, Blehert, Soos, Dunphy and Whitney2016).
Recent studies show phylogenetic resemblance between P. multocida isolates from albatrosses at Amsterdam Island and poultry in Europe (Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015, Weimerskirch Reference Weimerskirch2016). This suggests that the bacteria may have been introduced by live poultry kept at the island navy station, and spread to the albatross colonies via skuas, humans, rats or other means (Weimerskirch Reference Weimerskirch2004, Reference Weimerskirch2016, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015). Strict biosecurity has been implemented since 2010 for the Amsterdam Albatross colony, and since 2013 for Yellow-nosed and Sooty Albatross colonies as well, to minimise disease spread between colonies and species by researchers (Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Jaeger et al. Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015, Wemerskirch Reference Weimerskirch2016). Notwithstanding, spread by other means may still occur (e.g. skuas and rats). Of concern, due to the inaccessibility of most albatross colonies, and particularly that of Amsterdam Albatrosses, carcasses are not removed and remain as infectious foci. Vaccination trials using a P. multocida strain from Amsterdam Island are currently being conducted in-situ on Yellow-nosed Albatross chicks and adults (Bourret et al. Reference Bourret, Tornos, Jaeger, Gamble, Ramos, Kada, Bazire, Le Berre, Giraud, Thibault, Gantelet, Thiebot, Barbraud, Delord, Weimerskirch, Garnier and Boulinier2016). Given the critical condition of Amsterdam Albatrosses and the epidemiological scenario on the Island, such interventions are timely. Their success, however, will be influenced by vaccine efficacy and the proportion of the population inoculated (Plumb et al. Reference Plumb, Babiuk, Mazet, Olsen, Pastoret, Rupprecht and Slate2006). While this method is not practical for immunizing large numbers of free-ranging birds, it has been used successfully in smaller groups such as captive propagation flocks of Canada Goose Branta canadensis (Friend Reference Friend, Friend and Franson1999).
Based on currently available information, several factors make the behaviour of P. multocida in albatrosses from Amsterdam Island unusual and merit further investigation. For example, its biased impact on recently hatched chicks, its apparent self-limitation, its somewhat erratic recurrence (i.e. random years with high or very low mortality), and the fact that albatrosses seem to be suffering proportionately greater mortality than other species on the island even though potentially susceptible waterfowl are also present (Botzler Reference Botzler1991, Friend Reference Friend, Friend and Franson1999, Weimerskirch Reference Weimerskirch2004, Demay et al. Reference Demay, Barbraud, Delord and Weimerskirch2013, Wille et al. Reference Wille, McBurney, Robertson, Wilhelm, Blehert, Soos, Dunphy and Whitney2016). As has been indicated by Jaeger et al. (Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015) and Weimerskirch (Reference Weimerskirch2016), the situation on Amsterdam Island requires urgent and detailed research on the epizootiology of P. multocida infection to better understand the ecology of the disease and accordingly define mitigation and prevention methods. Future studies should include host-specific factors affecting susceptibility to infection (e.g. sex, age, genetic variation or behavioural differences, immunity, contact with other species at breeding and foraging grounds) and environmental factors influencing the occurrence of these epizootics (i.e. proximity to human dwellings, weather, presence of carrier species, etc.) (Botzler Reference Botzler1991, Descamps et al. Reference Descamps, Gilchrist, Bety, Buttler and Forbes2009).
Beyond the pathogens described above, a number of additional bacterial infections have been implicated in chick mortalities of the subset of ACAP species. However, they do not seem to be extended or sustained problems. Three strains of Salmonella sp. and compatible histopathological lesions were described in Laysan Albatross chicks dying from necrotizing enteritis (Work et al. Reference Work, Smith and Duncan1998). The authors suggest a similar problem may have caused earlier mortality in this species (Sileo et al. Reference Sileo, Sievert and Samuel1990). In both cases death was associated with dehydration, and potentially linked to lead poisoning. Infections with Nocardia asteriodes were reported in dead Laysan Albatross chicks with mild fibrinous airsacculitis (Sileo et al. Reference Sileo, Sievert and Samuel1990). Nocardiosis is uncommon in birds and was suggested by the authors to deserve further study. However, no follow-up studies have been published to date. Enterotoxemia from Clostridium perfringens toxins was the ultimate cause of death in a Southern Giant Petrel nestling with cutaneous and diphtheritic pox (Shearn-Boschler et al. Reference Shearn-Boschler, Green, Converse, Docherty, Thiel, Geiz, Fraser and Patterson-Fraser2008). Pox infections in this case were considered significant stressors triggering clostridial overgrowth in the gut. Finally, Escherichia coli (E. coli) was cultured from a single adult Southern Giant Petrel dying from P. multocida infection in Antarctica, but was considered a secondary finding (Leotta et al. Reference Leotta, Rivas, Chinen, Vigo, Moredo, Coria and Wolcott2003).
Given scant information available on morbidity and mortality triggers in ACAP species, accompanying pathogen isolation findings with histopathology, particularly when investigating disease or mortality, is highly recommended. This might be hindered, however, by the extended time lag between the onset of mortality and investigations, a common occurrence as inferred from this review. Furthermore, several reported mortality events in ACAP species remain undiagnosed, revealing weaknesses in current outbreak response and investigation capacities. This most likely reflects the remoteness and inaccessibility of albatross breeding sites, but also highlights the need for establishing early warning systems, particularly at sensitive locations (i.e. where critically endangered species congregate). Determining cause of death will improve knowledge on disease pathogenesis and virulence, allow for evaluation of the potential population-level impact of diseases, and enable adequate mitigation and preventative measures.
All other reported bacterial isolates in ACAP species were recovered during targeted assessments of contamination by human waste. In this context, gastro-intestinal bacteria were cultured from rectal swabs of apparently healthy Southern Giant Petrel adults (including E. coli; Jorge et al. Reference Jorge, Najle and Montalti2002, Leotta et al. Reference Leotta, Piñeyro, Serena and Vigo2009) and one Black-browed Albatross chick (1/240) (S. newport; Palmgrem et al. Reference Palmgrem, McCafferty, Aspan, Broman, Sellin, Wollin, Bergstrom and Olsen2000) and are likely of little clinical significance. These reports do however show that albatrosses regularly shed bacteria and can therefore act as carriers to distant locations. This might be particularly relevant in carrion-eating species such as the Southern Giant Petrel.
Fungi
Fungal nephritis caused by Aspergillus flavus-oryzae group was reported in a moribund and later euthanised Grey-headed Albatross (Tham et al. Reference Tham, Purcell and Schultz1974). Based on the chronicity of histological lesions however, it was considered more likely that co-infection with Proteus sp., a common urinary tract bacteria (Guentzel Reference Guentzel and Barron1996), was responsible for the debilitated condition of the animal. Of note, Aspergillus genus consists of several hundred species undergoing taxonomical changes with the advent of genome sequencing (Bennett Reference Bennett, Machida and Gomi2010). Therefore, the classification of the fungus in Tham et al. (Reference Tham, Purcell and Schultz1974) might be presently inaccurate.
Parasites
The majority of parasite papers for the ACAP species subset are taxonomic reports, and very few provide information on pathology or impacts on host species. Papers reporting ectoparasites (20) greatly outnumber those reporting helminths (7) and protozoa (2). This likely reflects visibility and ease of collection and preservation of external parasites. Only one hematozoan, Hepatozoon albatrossi, potentially transmitted by the tick I. uriae or a laelapid mite (Woods et al. Reference Woods, Jones, Watts, Miller, Shellam, Kerry and Riddle2009), was described in Grey-headed, Wandering and Black-browed albatross at Islas Georgias del Sur/South Georgia and not associated to clinical disease (Peirce and Prince Reference Peirce and Prince1980). Numerous helminths have been described, yet most are from opportunistic collections in Australia and Tasmania and confined to a single report by Johnston and Mawson (Reference Johnston and Mawson1942). The most relevant helminth report is a recent survey of faecal parasites from apparently healthy adult and juvenile Critically Endangered Waved Albatross in Galápagos Islands (Jiménez-Uzcátegui et al. Reference Jiménez-Uzcátegui, Sarzosa, Encalada, Sevilla and Huyvaert2015). Findings included a nematode, a cestode and a trematode, all in genera previously described in fish-eating birds, including albatrosses, and not considered particularly pathogenic (Jiménez-Uzcátegui et al. Reference Jiménez-Uzcátegui, Sarzosa, Encalada, Sevilla and Huyvaert2015). It is very likely that available reports are not at all comprehensive of the endoparasite biodiversity in albatrosses and large petrels, so much research remains to be done in this field. Similarly, the impacts of internal parasite burdens on their host’s health remain to be explored.
Only infestations with ticks in Black-browed Albatross (Ixodes uriae; Bergstrom et al. Reference Bergstrom, Haemig and Olsen1999a, Reference Bergstrom, Haemig and Olsenb) and Shy Albatross (Ixodes spp. and I. eudyptidis; Johnstone et al. Reference Johnstone, Milledge and Dorward1975, Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014, respectively); and mites Myialges nudus (Gilardi et al. Reference Gilardi, Gilardi, Frank, Goff and Boyce2001) and Womersia midwayensis (Sileo et al. Reference Sileo, Sievert and Samuel1990) in Laysan Albatross have been linked to disease or death in the ACAP species subset. Harassment by Aedes taeniorhynchus mosquitoes (Anderson and Fortner Reference Anderson and Fortner1988), on the other hand, was considered responsible for nest abandonment in the Waved Albatross. With the exception of ticks which have been described in numerous seabird species in circumpolar locations, most ectoparasite infestations seem to be restricted to “other” type sites (i.e. tropics), presumably due to warmer climate conditions at lower latitudes. Notwithstanding, changes in parasite and associated vector-borne pathogen distribution can be expected with variations in climate conditions (Kovats et al. Reference Kovats, Campbell-Lendrum, McMichael, Woodward and Cox2001, Antoniazzi et al. Reference Antoniazzi, Manzoli, Rohrmann, Saravia, Silvestri and Beldomenico2011, Altizer et al. Reference Altizer, Ostfeld, Johnson, Kutz and Harvell2013), and should be a monitoring priority in the near future.
Pathogen-specific antibodies
Few studies report antibodies to viruses in ACAP species. Findings are restricted to antibodies specific for Adenoviruses in serosurveys involving Black-browed Albatross (Uhart et al. Reference Uhart, Karesh, Cook, Huin, Lawrence, Guzman and Mörner2004), Waved Albatross (Padilla et al. Reference Padilla, Huyvaert, Merkel, Miller and Parker2003) and Southern Giant Petrel (Uhart et al. Reference Uhart, Quintana, Karesh and Braselton2003), Avian Encephalomyelitis virus in Waved Albatross (Padilla et al. Reference Padilla, Huyvaert, Merkel, Miller and Parker2003), Avian Influenza virus in Southern Giant Petrel (Baumeister et al. Reference Baumeister, Leotta, Pontoriero, Campos, Montalti, Vigo, Pecoraro and Savy2004), and Paramyxovirus type 1 in Shy Albatross (Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014). Four different studies (Uhart et al. Reference Uhart, Quintana, Karesh and Braselton2003, Reference Uhart, Karesh, Cook, Huin, Lawrence, Guzman and Mörner2004, Padilla et al. Reference Padilla, Huyvaert, Merkel, Miller and Parker2003, Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014) explored a suite of additional viral diseases but were unable to find indication of exposure, even though in one occasion samples were collected from animals during a mortality event investigation (Wang et al. Reference Wang, Selleck, Yu, Ha, Rootes, Gales, Wise, Crameri, Chen, Broz, Hyatt, Woods, Meehan, McCullough and Wang2014).
In the case of antibodies to bacterial agents, two studies report positives for Chlamydophila spp. in Southern Giant Petrel (Munday Reference Munday1972) and Black-browed Albatross (Uhart et al. Reference Uhart, Karesh, Cook, Huin, Lawrence, Guzman and Mörner2004), and for Salmonella spp. in Southern Giant Petrel (Uhart et al. Reference Uhart, Quintana, Karesh and Braselton2003). Given taxonomic misclassifications in the past and cross-reactivity of serological assays, it is unknown whether Munday (Reference Munday1972) refers to Chlamydophila psittaci, or another pathogen within the Chlamydophila genera (Nunes and Gomes Reference Nunes and Gomes2014). While available serosurvey reports suggest albatrosses and large petrels are occasionally exposed to common avian viruses and bacteria, in the absence of sequenced sampling to examine change over time, or indication of clinical disease in the individuals sampled, their utility remains limited. Furthermore, in some cases they might represent cross-reactivity with other pathogens given the lack of validation of the serological tests used, a common limitation in disease surveillance via serology in wild species (Gardner et al. Reference Gardner, Hietala and Boyce1996). Notwithstanding, implementing long-term and adequately designed disease surveillance programmes via measurement of antibodies in blood is recommended because antibodies are typically easier to detect and persist longer than the inciting infectious agents (Gilbert et al. Reference Gilbert, Fooks, Hayman, Horton, Müller, Plowright, Peel, Bowen, Wood, Mills, Cunningham and Rupprecht2013). In addition, antibody prevalence data provide information about the cumulative exposure history of the population (Gilbert et al. Reference Gilbert, Fooks, Hayman, Horton, Müller, Plowright, Peel, Bowen, Wood, Mills, Cunningham and Rupprecht2013). This is particularly significant for risk assessments (i.e. serosurveys of source stock) preceding reintroductions and translocations, an increasingly frequent conservation strategy for depleted ACAP species (Gardner et al. Reference Gardner, Hietala and Boyce1996, Jacobs et al. Reference Jacobs, Deguchi, Perriman, Flint, Gummer and Uhart2014). Finally, to ensure that reliable and meaningful data are obtained, serosurveys would benefit from modelling prior to field sampling, greater consideration of pathogenesis and age structure in the population, investment in longitudinal studies whenever possible, and standardized sample collection, storage and testing protocols (Gilbert et al. Reference Gilbert, Fooks, Hayman, Horton, Müller, Plowright, Peel, Bowen, Wood, Mills, Cunningham and Rupprecht2013).
Direct detection of pathogen DNA/RNA
There are three reports on pathogen shedding diagnosed by PCR. Two are based on swabs from apparently healthy chicks including Amsterdam Albatross (sample size unknown) for P. multocida and E. rhusiopathiae (Jaeger et al. Reference Jaeger, Bastien, Lebarbenchon, Tortosa, Thiebot, Marteau and Weimerskirch2013, Reference Jaeger, Lebarbenchon, Thiebot, Delord, Marteau, Dellagi, Barbraud, Boulinier, Tortosa and Weimerskirch2015), and one Southern Giant Petrel for Influenza A virus (de Souza Petersen et al. Reference de Souza Petersen, Petry, Durigon and Araújo2015). The potential significance of the finding in apparently healthy Amsterdam Albatross chicks has been discussed above, yet without further details, it remains speculative at best. On the other hand, Influenza A-specific antibodies have previously been described in several Antarctic seabirds, including Southern Giant Petrels (Baumeister et al. Reference Baumeister, Leotta, Pontoriero, Campos, Montalti, Vigo, Pecoraro and Savy2004, Barbosa and Palacios Reference Barbosa and Palacios2009). To date however, disease or health impacts associated to this virus have not been reported in albatrosses and petrels. Seabirds, and particularly Charadriiformes, are natural reservoirs of influenza viruses, which have been described in more than 105 avian species in 13 orders worldwide (Olsen et al. Reference Olsen, Munster, Wallensten, Waldenstrom, Osterhaus and Fouchier2006). The third study reported 1/6 Southern Giant Petrel positive for bacteria of the order Chlamydiales by real-time PCR targeting the 23S rRNA gene from fresh faeces collected in Antarctica (Isaksson et al. Reference Isaksson, Christerson, Blomqvist, Wille, Alladio, Sachse, Olsen, Gonzalez-Acuña and Herrmann2015). Knowledge about the diversity and distribution of Chlamydiaceae-like bacteria is limited and evolving, with reports in 460 wild and domestic avian species in 30 orders (Kaleta and Taday Reference Kaleta and Taday2003). While commonly found in Sphenisciformes and Lariformes (Kaleta and Taday Reference Kaleta and Taday2003), at this time, the ecological and health relevance of the finding reported by Isaksson et al. (Reference Isaksson, Christerson, Blomqvist, Wille, Alladio, Sachse, Olsen, Gonzalez-Acuña and Herrmann2015) is unknown. In addition, DNA of vector-borne bacteria, Borrelia garinii, was found in I. uriae ticks from Black-browed Albatross breeding in Campbell Island, New Zealand (Olsen et al. Reference Olsen, Duffy, Jaenson, Gylfe, Bonnedahl and Bergström1995). It is now known that B. garinii is maintained in an enzootic cycle in seabirds by I. uriae at their nesting sites over a wide circumpolar area (Smith et al. Reference Smith, Muzaffar, Lavers, Lacombe, Cahill, Lubelczyk, Kinsler, Mathers and Rand2006). The significance of this finding relates to public health as B. garinii causes neurologic Lyme disease in Europe. However, exchange between the marine enzootic cycle of B. garinii and Borrelia spp. terrestrial cycles has not been described to date (Smith et al. Reference Smith, Muzaffar, Lavers, Lacombe, Cahill, Lubelczyk, Kinsler, Mathers and Rand2006).
Conclusion
In this summary we note that even though the number of health-related studies has increased in recent years, there are still obvious gaps in species and geographical coverage and likely under-reporting due to remoteness, accessibility and sporadic monitoring. Deficiencies in investigations of disease and mortality events add to the mix. Current insufficient knowledge may be hampering effective protection and management of populations at risk. Specifically, the threat posed by avian cholera for the most vulnerable albatross species, merits urgent attention.
Acknowledgements
The authors gratefully acknowledge R. Botzler for helpful comments and A. Jaeger, T. Boulinier, and H. Weimerskirch for contributions which helped us improve and complete the information provided in this manuscript. This review stems from ACAP’s awareness of diseases as threats for albatrosses and petrels. Support for this study was provided by the Agreement on the Conservation of Albatrosses and Petrels (F.Q. and M.U., grant ID 2013–20), Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (F.Q., Senior Researcher; L.G Postdoctoral Fellowship) and the University of California, Davis (M.U Senior Veterinarian).









