Introduction
Estimates of population size and trends in inter-annual abundance are key data for conservation agencies tasked with managing threatened species. Low population size can have important implications for species persistence and viability. Identifying which sites support larger numbers is also an important parameter for setting conservation priorities for threatened fauna. Clarke et al. (Reference Clarke, Oliver, Boulton, Cassey and Clarke2003) noted that long-term monitoring data are necessary for gauging the ‘status’ of endangered fauna and the effectiveness of recovery actions taken. Such datasets have been given a high priority by recovery planning agencies (Garnett and Crowley Reference Garnett and Crowley2000).
The Capricorn (previously known as Dawson) Yellow Chat (CYC) Epthianura crocea macgregori (Aves: Meliphagidae) is a ground-adapted insectivorous honeyeater which is ‘Critically Endangered’ under the Australian Government’s Environment Protection and Biodiversity Conservation (EPBC) Act 1999. It is one of three subspecies of Yellow Chat - the Inland E. c. crocea occurs patchily throughout inland northern Australia but reaches the coast near Broome; the Alligator Rivers E. c. tunneyi occurs coastally in the Alligator Rivers area of northern Australia; and the Capricorn in coastal central Queensland. Prior to the 1990s, the CYC was considered to be either a rare vagrant (Longmore Reference Longmore1978) or possibly extinct (Schodde and Mason Reference Schodde and Mason1999, Higgins et al. Reference Higgins, Peter and Steele2001). It was known from two general locations – the Rockhampton district and Torilla Plain in the Broad Sound area north of Rockhampton (Campbell Reference Campbell1917, Mack Reference Mack1930). Following the 1917 Torilla Plain sighting, there were no confirmed sightings in the next 75 years. However, in 1992 the subspecies was rediscovered when a small subpopulation of less than 50 birds was found on the marine plain in the north-east part of Curtis Island, east of the Fitzroy River mouth (Arnold et al. Reference Arnold, Bell and Porter1993). Subsequently, its historical range was re-established and the probable reason why it has not been detected determined (Jaensch et al. Reference Jaensch, Houston, Black, Campbell, Elder and McCabe2004, Houston et al. Reference Houston, Porter, Elder, Black and Sheaves2004a, Reference Houston, Porter, O’Neill and Elder2004b, Reference Houston, Black and Elder2013). The CYC occurs in small, seldom-visited, difficult-to-access fragments of suitable habitat totalling less than 7,000 ha in three main areas (north at Broad Sound, south in the Fitzroy River delta and south-east at Curtis Island; Figure 1). The most important breeding habitats are marine plain wetlands, particularly grass-sedge swamps and supratidal saltmarshes (Houston et al. Reference Houston, Black and Elder2013). Breeding was found to be associated with the warmer months following substantial rainfall and wetland inundation (Houston Reference Houston2013). CYC habitat is threatened by mining, industrial expansion, habitat alteration by ponded pasture grasses and introduced grazers, and sea level rise associated with climate change (Houston and Melzer Reference Houston and Melzer2008, Houston et al. Reference Houston, Black and Elder2013). Recent molecular studies have provided a preliminary evaluation of the divergence between the CYC and its nearest subspecies, the widespread Inland Yellow Chat, indicating a time to the most recent common ancestor of 215,000 years or less (Houston et al. Reference Houston, Aspden, Black, Elder, Carruthers, Campbell and Black2015). This estimate coincides with two periods of aridity in the Central Queensland region when populations may have been connected, ∼150 000 years or ∼20 000 years ago (Houston et al. Reference Houston, Aspden, Black, Elder, Carruthers, Campbell and Black2015).
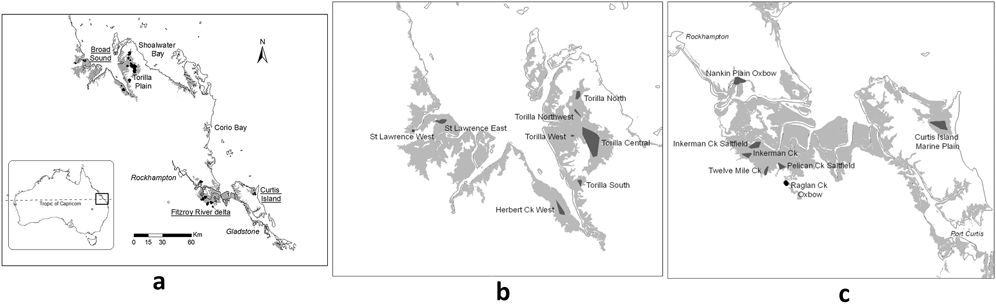
Figure 1. Known Capricorn Yellow Chat sites shown as dark shading (Houston et al. Reference Houston, Black and Elder2013). Grey shading indicates marine plains which are a mixture of grasslands, mangroves and wetlands (i.e. not all suitable CYC areas): (a) overview of all sites (b) sites in the Broad Sound area and (c) sites in the Fitzroy River delta and Curtis Island areas.
Although generally regarded as nomadic and mobile (Ford and Parker Reference Ford and Parker1974, Storr Reference Storr1980, Black et al. Reference Black, Duggan, Pedler and Pedler1983), detailed studies have yet to be done and the movements of Yellow Chats are poorly understood. Others have described them as resident (Jaensch and Vervest Reference Jaensch and Vervest1990) or sedentary (Keast Reference Keast1958), while those in the Alligator Rivers region were thought to undertake local seasonal movements (Deignan Reference Deignan1964). Movements between sites may affect population estimates if the same birds are being counted twice.
Many wetland-dependent mammals and waterbirds show fluctuations in abundance associated with rainfall (Braithwaite and Muller Reference Braithwaite and Muller1997, Kingsford et al. Reference Kingsford, Wong, Braithwaite and Maher1999, Chambers and Loyn Reference Chambers and Loyn2006). While less well-studied, passerines occupying unpredictable rainfall environments have shown fluctuations in abundance with rainfall (Donald et al. Reference Donald, De Ponte, Pitta Groz and Taylor2003, Burbidge and Fuller Reference Burbidge and Fuller2007). Rainfall is known to be an important cue of breeding in birds and other fauna occupying unpredictable rainfall ecosystems such as the arid inland and the wet-dry tropics, ensuring that rearing of dependent young coincides with peaks in availability of food resources (Zann et al. Reference Zann, Morton, Jones and Burley1995, Lloyd Reference Lloyd1999, Shine and Brown Reference Shine and Brown2008, Houston Reference Houston2013). Understanding such drivers of abundance is essential to provide a sound baseline for monitoring changes in abundance and thus detection of declines before a crisis is reached.
This study provides key data for conservation planners: an estimate of population size based on comprehensive repeated survey data and information on fluctuations in population size in relation to climatic drivers such as rainfall. Evaluation of movements at a local scale (i.e. within areas) was also undertaken to expand knowledge of CYC ecology and underpin estimates of population size.
Methods
Study area
The CYC occurs on the marine plains of north-eastern Australia spanning the Tropic of Capricorn between latitudes 22.2oS and 23.7oS on coastal plains to the north, south and east of Rockhampton (Figure 1). Habitat is of limited extent, the majority less than 5 m above sea level and thus vulnerable to long-term rises in sea level (Houston et al. Reference Houston, Black and Elder2013). The climate is hot, seasonally wet-dry but with relatively cool winters; and forms a continuum with more typical wet-dry tropics of northern Australia which have warmer winters. Average maximum summer temperatures are just above 30°C while winter minima average around 10 to 14°C. Annual rainfall is 800–1,000 mm (Figure 2), of which over two-thirds falls between November and March. Rainfall is least in the months of June to September. The region occupied by the CYC is typified by highly variable annual rainfall which is comparable to that of the region occupied by Yellow Chats of inland Australia, ∼800 km to the west (Bureau of Meteorology 2010).
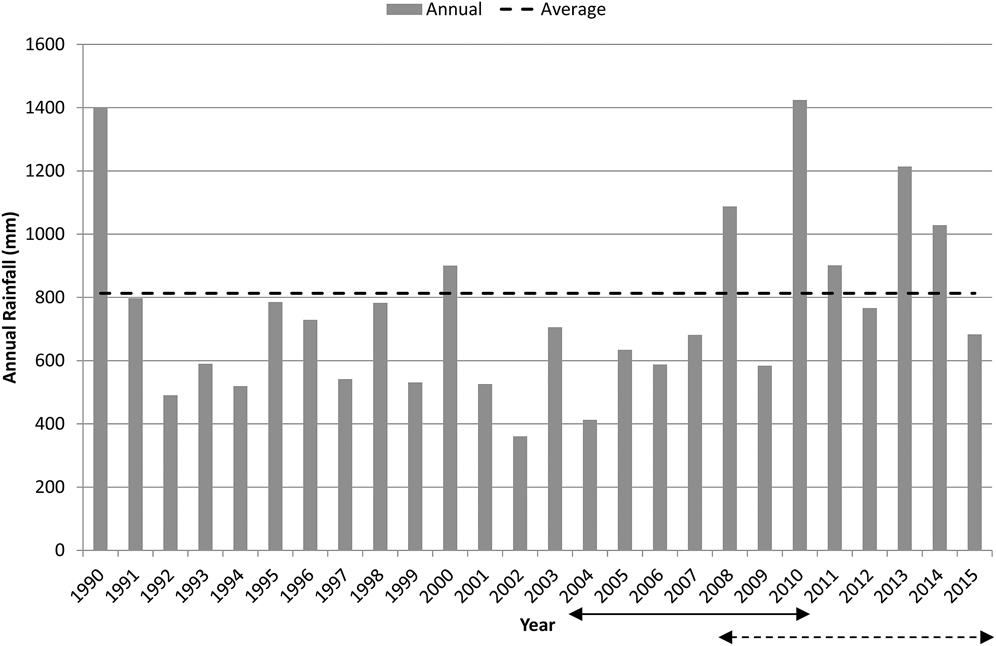
Figure 2. Annual Rainfall at Rockhampton (Bureau of Meteorology Station 039083, Bureau of Meteorology 2016) compared with average rainfall (76 years from 1940 to 2015). The solid arrow denotes the period during which the population size of the CYC was estimated (2004–2010). The dashed arrow indicates the trend evaluation period for the Fitzroy River delta (2008 to 2015) while trend evaluation encompassed the years of 2004-2009 plus 2012, 2013 and 2015 for the Broad Sound area.
Seasonal habitat use
Seasonal habitat use was inferred from regular monthly counts of chat numbers at four of the six known sites in the Fitzroy River delta. These sites were representative of the two main habitat types in the Southern Fitzroy River delta – saltmarshes (Twelve Mile Ck and Inkerman Ck) and salt fields (Inkerman Ck Salt field and Pelican Ck Salt field) (Figure 1c). Monthly surveys were undertaken between September 2008 and August 2010 at each of the four sites. This sequence was extended by sampling at approximately two-month intervals; additional surveys being conducted in October and December 2010 and February, March, May, July, September and December 2011. These provided a longer dataset upon which to base conclusions, giving a total of four wet seasons.
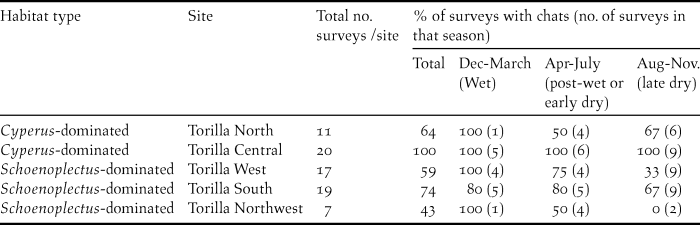
At Broad Sound, three Torilla Plain sites, representative of two habitat types which grade into each other, were sampled regularly: (i) Cyperus-dominated in the upper to mid- marine plain of dominantly Cyperus alopecuroides sedge and Para Grass Urochloa mutica (Torilla Central), and (ii) Schoenoplectus-dominated in the lower marine plain adjacent to tide exclusion banks, a mixture of sedge Schoenoplectus subulatus, previously known as S. litoralis, and supratidal saltmarsh (Marine Couch Sporobolus virginicus and samphire Tecticornia pergranulata) (Torilla West and Torilla South; Figure 1b). These three sites were surveyed at least once in each four-month period (wet: December to March, post-wet: April to July and dry: August to November) between December 2003 and November 2008. In all, there were 20 surveys of 2–4 days duration. There were four exceptions when one of the three sites could not be surveyed: one at Torilla South during the post-wet of 2004 due to vehicle breakdown and three surveys at Torilla West due to weed-related quarantine restrictions on this paddock in post-wet 2006, wet and post-wet 2007. However, the site supporting the most chats and the biggest in area, Torilla Central, representing the Cyperus-dominated habitat type, was sampled each time, as was at least one representative of the Schoenoplectus-dominated habitat type. Although with less comprehensive data, two sites, one Cyperus-dominated in the upper marine plain, Torilla North, and one Schoenoplectus-dominated in the lower marine plain, Torilla Northwest, were added to supplement this dataset.
To assess the influence of season on chat usage of the three Schoenoplectus-dominated sites on Torilla Plain, a Kruskal-Wallis non-parametric equivalent of an ANOVA was undertaken using percentage occurrence of chats at each site. Data were pooled by season for all years of observation.
Population size estimate (2004–2010 dataset)
A census (where all known sites of the target species are surveyed simultaneously or within a relatively short time period to avoid double counting of moving birds) is the preferred approach for estimating population size of threatened species with limited distributions (Clarke et al. Reference Clarke, Oliver, Boulton, Cassey and Clarke2003, Gregory et al. Reference Gregory, Gibbons, Donald, Sutherland, Newton and Green2004). This was not possible in the earlier part of this study because not all sites were known and additional sites were added over the study period from 2004 to 2010 during which surveying was most intensive. For most sites, at least 6–7 years of data were available over this period but a few were based on only three surveys. Many sites were sampled seasonally so there were several counts available in a year but only one was chosen as representative of the census count for that year. Different selection strategies had to be used in each of the three areas where CYCs occur (Broad Sound, Fitzroy River delta and Curtis Island) in order to standardise for the different characteristics of the data available in each (Table 1). Because not all sites were surveyed in the same years, the total population could not be calculated by totalling each year and averaging. Instead the population size of CYC at each site was estimated by averaging values of standard census surveys. These averages were then added to provide an estimate of the total population size. Because standard errors are not additive, it was based on the largest standard error at an individual site.
Table 1. (a) Rationale and years surveyed for long-term estimate of CYC population size and (b) sites surveyed and years surveyed for population trend evaluation based on selected CYC sites.
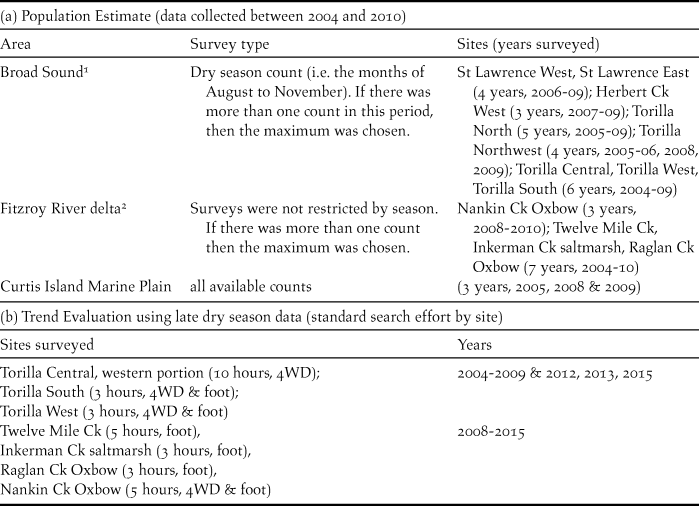
Rationale for selection of survey type: 1Broad Sound: Due to high rainfall and consequent inundation of the marine plain, sites were unable to be accessed during the wet season (and sometimes the following months). Further, counts in the post-wet season frequently included large flocks of immature birds and numbers showed large fluctuations in this period.
2 Fitzroy River delta: Until 2008, chats were rarely found in the drier part of the year in the delta (chats were not found at the two salt field sites until August 2008) so most counts during the study period from 2004 to 2010 where obtained in the wet or post-wet season periods. Only data for the three sites for which breeding has been confirmed were used because the birds migrated between breeding habitat and dry season habitat as shown in this study.
Sites were surveyed either on foot or by vehicle depending on site size. Foot surveys were not possible in larger sites due to their extent (e.g. the suite of sites on Torilla Plain spans 20 km) (Figure 1). In both types of survey, once the extent of chat occurrence within a site had been determined, surveys were standardised for effort (person-hours) and search area (standard track) over the years. In addition, for larger sites such as Torilla Central, the same paddocks were searched each time. Effort per paddock varied slightly because of seasonal differences in accessibility, but, overall the same amount of search time was devoted to each site each year. At all sites, great care was taken to avoid double counting and only the same experienced observers were used.
Population trend and rainfall
For long-term recovery planning, an index of population abundance that can indicate any trend is essential for gauging the ‘status’ of threatened fauna. There were suitable long-term datasets for two areas, all based on similar search effort in the same paddocks each year. First, for Torilla Plain, the October or November counts for the combined total of three sites: Torilla South, Torilla West plus the western portion of Torilla Central. Data from the eastern portion were not available in these months for 2004–2006. As the western portion accounts for approximately two-thirds of the site in area and is influenced by all the main creeks that run onto the marine plain at Torilla Central, it is representative of the site as a whole. Data were available from 2004 to 2009 plus 2012, 2013 and 2015.
Second, for the Fitzroy River delta, a sequence of eight years (2008–2015) in which standard counts of all known sites were undertaken mainly in October of each year (but including some data from late September or early November). These are comparable to the dry season counts assembled for Torilla Plain.
Relationships between chat numbers and previous rainfall were examined for the two continuous datasets, Torilla Plain and Fitzroy River delta. Rainfall stations were selected that lay within or near to the main catchment that influences CYCs in each area and had few or no missing data, the former from Samuel Hill and the latter from Rockley (Rainfall Stations 033308 and 039250 respectively, Bureau of Meteorology 2016). A multiple linear regression with backwards elimination was used. The dependent variable was the standard estimate of chat annual abundance. Independent variables were: (i) aggregated monthly rainfall for the latest wet season (i.e. rainfall that fell between October of the preceding year and May of the year in which the trend survey was undertaken - various iterations of the current wet season rainfall were tested: October to March, October to April, October to May, November to March, November to April, November to May, December to March, December to April, December to May) and (ii) two iterations of the previous year’s wet season rainfall, either November to March or December to March. Due to the relatively small sample sizes, only two independent variables could be tested simultaneously and thus it was necessary to undertake separate analyses for each of the various combinations of the current wet season rainfall paired with one of the two iterations of the previous year’s wet season rainfall.
Results
Seasonal habitat use
In the southern area, there was a consistent pattern of habitat occupancy with birds present in salt field sites during the drier months and absent or in low numbers during the wetter months (Figure 3). The reverse pattern was observed at the saltmarsh sites. The simplest explanation for these observations is that the majority of chats undertake seasonal movements between the confirmed breeding sites (Twelve-mile Ck and Inkerman Ck saltmarshes) and the dry season refuge salt field sites. This pattern of movement was supported with a sighting of a bird that had been banded at one of the salt field sites in July 2012 at the Twelve Mile Ck saltmarsh site in May 2013 (a distance of at least 7.5 km). This bird, a male, was observed to be in the known breeding area at Twelve Mile Creek and was accompanying immature birds which were still being fed, indicating a recent breeding event following an extended wet season.

Figure 3. Patterns of occurrence of Capricorn Yellow Chats at Fitzroy River delta sites based on pooled data for the two breeding sites (Twelve-mile Ck and Inkerman Ck) and the two dry season sites (Inkerman Ck Salt field and Pelican Ck Salt field) expressed as a percentage of the total monthly survey count for the delta between September 2008 and December 2011 (w indicates the 4 months of the year with greatest average rainfall).
In the Broad Sound area, chats were present all year round at the main Cyperus-dominated site (Torilla Central), confirming the importance of this site for the persistence of the subspecies on the Torilla Plain. Chats used the Schoenoplectus-dominated supratidal saltmarsh sites for breeding but did not always persist at these during the drier part of the year (Table 2). Pooling the data for the three Schoenoplectus-dominated sites showed that this pattern was significant (Kruskal-Wallis: H 2 = 6.169, P = 0.046, n = 9) and dry season occurrence was significantly lower than wet season occurrence (post-hoc Multiple Comparisons test). These findings suggest that chats retreat to the upper marine plain when conditions are drier; leaving the Schoenoplectus-dominated sites which become hypersaline as they dry. Distances between the three Schoenoplectus-dominated sites and the Cyperus-dominated Torilla Central site were all less than 10 km.
Table 2. Percentage of surveys for each season (wet, post-wet or dry) in which chats occurred at the five Torilla Plain sites.
Population size
From repeated surveys of sites over time, the average population size of CYCs is estimated at 251 +/-31 birds (SE) with almost two-thirds in one site complex, Torilla Plain (Figure 4). While it is possible that not all birds were counted, as some paddocks with marginally suitable habitat were not regularly surveyed, the effect on population size estimates is likely to be small given the marginal quality of the habitat.

Figure 4. Mean population size estimate (error bars indicate 1 SE) based on multiple surveys of sites (using standard effort at each) using data compiled 2004-2010 (numbers in brackets represent percentage contribution to the population size estimate by coastal plain).
* In the Southern Fitzroy Delta only the confirmed breeding sites (Twelve Mile Ck and Inkerman Ck) were used for the estimate as less data were available for the salt fields. Also, the latter sites were seasonally occupied and likely to be the same birds as at the two breeding sites as shown in this study.
There were occasional post-wet season counts well in excess of the long-term average, particularly at Torilla Central. In May 2005 there was a count of 484 chats for Torilla Central and in July 2008 a count of 254 birds suggesting a much larger population than the estimate of approximately 120 for Torilla Central based on the long-term average (Figure 4). However, in both cases, subsequent counts were substantially lower (74 and 91 in September and November 2005 respectively, and 147 in October 2008). Further, peak counts coincided with formation of large flocks (i.e. 20 or more) of mixed adults and young birds. A large proportion of birds in these flocks had plumage resembling that of immature birds but overlapping with adults (i.e. indeterminate). Subtracting indeterminate individuals from the total count, gives population estimates much closer to the long-term average of 120 for Torilla Central (145 and 142 respectively). Post-breeding flocks are common in many species of birds (Bibby et al. Reference Bibby, Burgess, Hill and Mustoe2000, Dowding and Greene Reference Dowding and Greene2012).
Population trend and rainfall
Numbers of CYCs fluctuated at Torilla Plain but with higher numbers sighted in the wetter period from 2008 to 2012 (Figure 5). To examine this, chat count was regressed against various combinations of aggregated monthly rainfall for the current year’s wet season immediately preceding the chat count plus the previous year’s wet season rainfall aggregated from November to March or December to March. Only the influence of the previous year’s wet season rainfall was found to be significant and this explained 76% of the variation in the chat abundance for Torilla Plain (Table 3, Figure 6a):
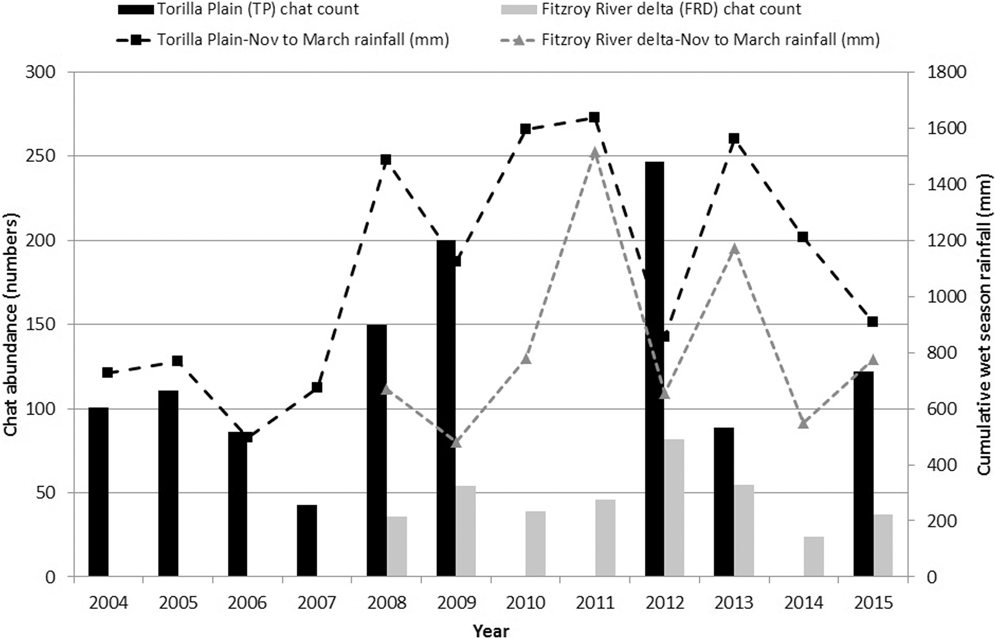
Figure 5. Population trend monitoring of CYCs at Broad Sound (2004 to 2009, plus 2012, 2013 and 2015) and Fitzroy River delta (2008 to 2015) based on dry season standard counts of selected sites plotted against cumulative Nov-March rainfall of the current year’s wet season (e.g. rainfall of 2007 is from the 2006-07 wet season) (TP rainfall data from Samuel Hill Station 033308, BOM; FRD rainfall data from Rockley Station 039250, BOM).
Table 3. Multiple linear regression (backwards stepwise) results for CYC abundance using wet season rainfall as the independent variables. The Fitzroy River delta data excludes an outlier in 2014.
(a) Torilla Plain: r= 0.871, r2= 0.759, Adjusted r2 = 0.724; F 1,7 = 22.015, P < 0.01
(b) Fitzroy River delta: r = 0.932, r2 = 0.868, Adjusted r2 = 0.842; F 1,5 = 32.979, P < 0.01
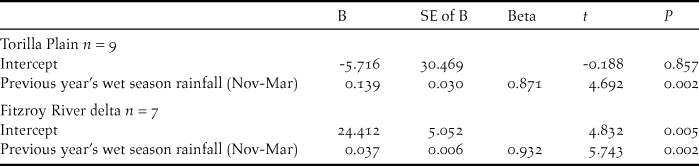
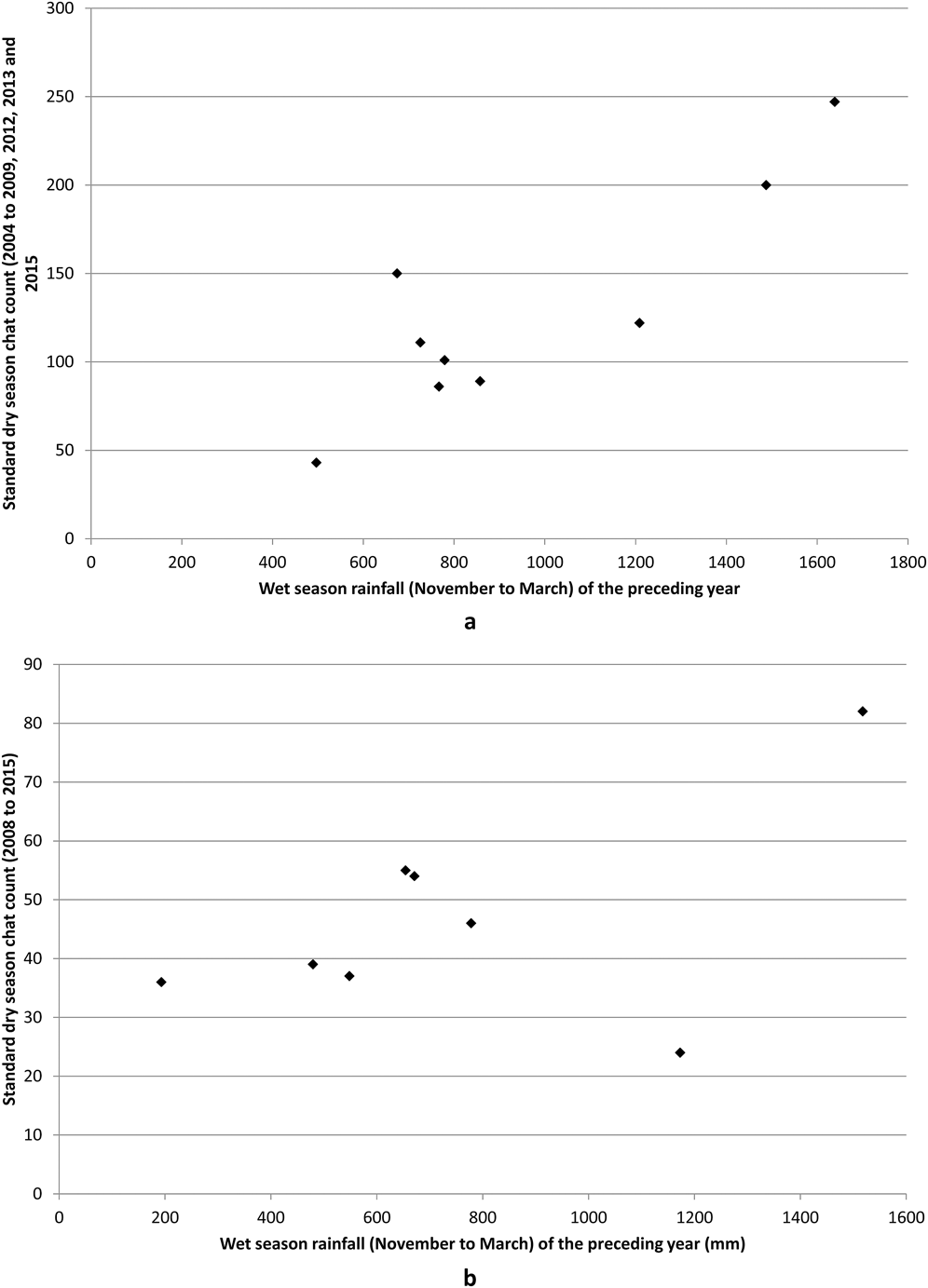
Figure 6. Scattergrams showing standard chat counts at (a) Torilla Plain and (b) Fitzroy River delta in relation to wet season rainfall of the previous year (e.g. rainfall of 2007 is from the 2005-2006 wet season).
Chat abundance = -5.716 + 0.139 (previous year’s wet season rainfall - November to March). Thus, CYC breeding success appears to be promoted by greater wet season rainfall of the previous year.
The 2008 to 2015 dataset for the Fitzroy River delta also showed substantial fluctuations in numbers with an almost two-fold increase from 2011 to 2012 (Figure 5). Similar to the Broad Sound data, the November to March rainfall of the previous wet season had the highest correlation with chat standard counts of abundance. However, the regression was not significant. While most yearly counts fluctuated synchronously with the previous year’s wet season rainfall, 2014 was an outlier (Figure 6b) and when this point was removed, a similar relationship to the Torilla Plain dataset was found. 87% of the amount of the variation in chat counts was explained by the previous wet season November to March rainfall (Table 3).
Chat abundance = 24.412 + 0.037 (previous year’s wet season rainfall - November to March)
Discussion
Status
This study has confirmed the low population size of the CYC with a long term average of approximately 250 birds based on seven years of data. While it is likely that the population estimate would be higher during wetter periods, an estimate undertaken during a mixture of dry and wet years, such as the period between 2004 and 2010, provides a preliminary benchmark upon which to base management decisions. Populations of less than 500 individuals are considered to be at risk of low genetic diversity and of not being able to maintain long term evolutionary fitness (Frankham et al. Reference Frankham, Bradshaw and Brook2014). Endemic species or subspecies with small ranges and highly fragmented patterns of distribution such as the CYC (Houston et al. Reference Houston, Black and Elder2013) are considered to be even more vulnerable (Harvey et al. Reference Harvey, Rix, Framenau, Hamilton, Johnson, Teale, Humphreys and Humphreys2011). Further, about two-thirds of the population is concentrated in one aggregation of sites (Torilla Plain), increasing vulnerability of the subspecies should this subpopulation decline.
The extensive nature of marine plains in the region suggests that more occupied habitat may be found in the future. However, CYCs have very specific requirements and suitable habitat is restricted (Houston et al. Reference Houston, Black and Elder2013). Currently available aerial mapping and satellite imagery allow for detailed investigation of potential CYC sites. All of these have now been surveyed. Any remaining sites are likely to be small and have few CYCs. As shown in this study, identification of ‘new’ sites does not necessarily equate with more birds as CYCs move small distances seasonally or opportunistically to inhabit breeding habitat when conditions are favourable. The breeding sites and dry condition sites are in juxtaposition and are part of the same ecological systems. Despite considerable search effort representing thousands of hours by researchers and local birdwatching groups (Houston et al. Reference Houston, Black and Elder2013), only five locations have been found where chats persist during the dry season – Torilla Central, Herbert Ck West, Southern Fitzroy River delta aggregation, Nankin Ck Oxbow and Curtis Island.
Although the substantial increase in population size at Broad Sound following a well above average wet season in 2008 (and an average one in 2009) is indicative of a robust population, population declines were equally rapid. In 2007, following a below-average wet season (approximately 52% of average wet season rainfall), less than 50 birds were counted on Torilla Plain which corresponded to less than half the long term average. The strong association of population size with preceding rainfall suggests that the population could diminish rapidly during several years of low rainfall. Climate change scenarios indicate such episodes are likely to be more intense with increasing temperatures and longer drought periods (Hughes Reference Hughes2003, McKechnie and Wolf Reference McKechnie and Wolf2010). While shown to be resilient to lengthy periods of below average rainfall, a greater frequency of extreme droughts may increase the vulnerability of CYCs to breeding failure and population decline. Effects of increasing flood frequency on saline-adapted vegetation and sea level changes impinging on the low lying marine plains, which are mostly less than 5 m above sea level and within storm surge range, may also impact on the habitat CYCs depend on and alter breeding and survival rates (Houston et al. Reference Houston, Black and Elder2013).
A discussion of status would not be complete without reference to the current listing of the CYC as ‘Critically Endangered’. Our findings are consistent with the evaluation of CYC by Garnett et al. (Reference Garnett, Szabo and Dutson2011) as ‘Endangered’. CYCs have a fluctuating population averaging around 250 but with a large standard error indicating that in some years the population may be well below 250 with a documented decline in numbers at one site. They occupy a small area of less than 7,000 ha and occur regularly in five or fewer locations (Houston et al. Reference Houston, Black and Elder2013). However, given the substantial threat posed to CYC persistence by the combined effects of climate change (e.g. sea level rise and gradual habitat loss, greater intensity of rainfall events causing prolonged flooding and more severe droughts) plus direct anthropogenic threats such as industrial expansion and shale oil mining (Houston and Melzer Reference Houston and Melzer2008, Houston et al. Reference Houston, Black and Elder2013), it is proposed that the CYC should remain as ‘Critically Endangered’.
Movements
Full understanding of movements and dispersal distances of Yellow Chats has not yet been achieved. However, we have demonstrated in this study that local movements across relatively well connected terrain do occur and are in the order of approximately 10 km. This does not apply to all chats as some remain at the most reliable sites throughout the year.
Population dynamics and rainfall
While standard annual counts of chat abundance fluctuated considerably (e.g. at Torilla Plain from 2007 to 2008 there was a three-fold increase in chat abundance from 43 to 150), much of this annual variation was explained by wet season rainfall of the previous year. In combination with the earlier research showing that breeding activity and the abundance of invertebrate prey peak one to two months following rainfall (Houston Reference Houston2013), these findings support the hypothesis that the amount of wet season rainfall is an important driver of breeding success in Yellow Chats (Williams Reference Williams1979, Strong and Fleming Reference Strong and Fleming1987).
The mechanism appears to be related to increases in spatial and temporal opportunities for breeding following above average rainfall events. These include the increased area available for breeding following inundation and a prolonging of the breeding season as the waters gradually recede, which progressively creates fresh muddy substrates for foraging. Marine plains in this region are very extensive with gentle gradients and vary considerably annually in the extent of inundation. As well as providing increased nesting habitat, these factors also provide increased invertebrate food resources and cover leading to enhanced survival of young (Houston Reference Houston2013).
Other contributing factors to increased chat numbers following above average rainfall may be the timing of wet season rainfall and the extent of the marine plain inundated as a function of the number of sub-catchments receiving good rainfall. As an example, in 2004-2005, a large breeding event was recorded on Torilla Plain with over 600 birds observed during the post-wet season period. This followed only average rainfall over the previous two wet seasons. However, a large portion of this was earlier than usual with a substantial rain event (> 190 mm) in spring (October) 2004. This may have extended the breeding season allowing multiple nesting and increased recruitment. Further, the Torilla Central site is influenced by three main creeks. In some years only one or two may flow and reach the plain, while in other years all three flow expanding the amount of suitable breeding habitat. Summarising, it may not simply be the amount of rainfall but when and where the rain falls.
Enhancing the capacity for rapid increases in numbers may be favourable life history attributes such as being able to undertake repeated breeding and a rapid maturation permitting the current year’s recruits to breed in the same year. Other chat species such as White-fronted Chats Epthianura albifrons are capable of breeding multiple times under conditions of prolonged inundation during wetter years (Major Reference Major1991). This species was found to be able to make five attempts in a season, with a capacity to rear up to three broods. While this has not been confirmed for Yellow Chats, observations suggest maturity within six months and CYCs were observed to breed on several occasions in the same year at some sites (Houston Reference Houston2010). Assuming favourable conditions with high food availability and survivorship of young, 50 adult birds (25 pairs) breeding twice in the same year and successfully rearing two young each time (Houston Reference Houston2010) gives numbers of at least 150 which is within the range observed in 2007–2008 when numbers increased three-fold. This number could be higher if offspring were capable of breeding in their first year.
However, rainfall may not be uniformly beneficial. A low count was obtained in 2014 in the Fitzroy River delta area. This followed well above average rainfall in the previous wet season of 2012-2013. Reasons for this are not fully understood. The high rainfall obtained in that year was concentrated in one rainfall event with over 300 mm falling in one day and approximately 600 mm over three days in late January 2013. As most CYC nests found have been less than 30 cm above ground level (Houston Reference Houston2010), it is possible that the concentrated volume of surface run-off may have been detrimental if the chats were nesting. Loss of nests due to submergence by flood waters is not unusual in ground-nesting wetland-associated birds (Greenberg et al. Reference Greenberg, Elphick, Nordby, Gjerdrum, Spautz, Shriver, Schmeling, Olsen, Marra, Nur and Winter2006, Poiani Reference Poiani2006). Other effects may relate to declines in food availability or changes in habitat structure (Poiani Reference Poiani2006). Both of these factors may have affected CYCs at some sites in the Fitzroy River delta post the 2013 flood event. At one site the dominant sedge, Schoenoplectus subulatus, died off following prolonged inundation. S. subulatus is salt tolerant (Espinar et al. Reference Espinar, García and Clemente2005) and, similar to other saltmarsh-associated species may be prone to mortality under such conditions (Laegdsgaard Reference Laegdsgaard2006). The prolonged inundation may also have affected food availability by altering the natural wet-dry cycles which are thought to promote invertebrate abundance levels in ephemeral wetlands ( Maher and Carpenter Reference Maher and Carpenter1984, Poiani Reference Poiani2006, Houston Reference Houston2013).
Management issues
Patterns of rainfall deficits over the 2000–2009 period were similar in all three areas where CYCs occur. However, while chats persisted at Broad Sound and the Fitzroy River delta, they declined substantially at Curtis Island from around 30 in 2002 to only four adults in 2008 and none in 2009 (Houston et al. Reference Houston, Porter, O’Neill and Elder2004b, W. Houston unpubl. data). There are some environmental differences between Curtis Island and the other two areas. Torilla Central, the Torilla Plain site where chats occur throughout the year, has permanent surface water on the marine plain. Second, the Fitzroy River delta, although lacking permanent surface water at breeding sites, has several large salt fields that CYCs occupy during the dry season (Houston Reference Houston2010). These have abundant food resources (W. Houston unpubl. data) and it is possible that food types such as midges may provide enough moisture to maintain metabolic water balance during the drier part of the year (Schodde Reference Schodde, Barker and Greenslade1982, Houston Reference Houston2010). In contrast, the Curtis Island Marine Plain lacks watering points and there are no salt fields in close proximity. It is possible that habitat attributes such as watering points or the presence of food-rich salt fields may enable chats to persist at Torilla Plain and the Fitzroy River delta during drought periods, although further data are needed to assess these hypotheses. Consideration could be given by land managers to provide watering points on the marine plain at Curtis Island. Ideally, these should be engineered to include shallow muddy edges with suitable emergent vegetation such as sedges.
The relationship with prior rainfall found in this study shows that land managers have to be mindful of the importance of previous wet season rainfall when developing population monitoring programmes and evaluating trends in population size. Falls in abundance are expected in drier years. For example, using the trend data from Torilla Plain the average count of the wetter period is almost double that of the average for the drought years (162 +/-28 birds for 2008 to 2015 compared with 85 +/-15 birds for 2004 to 2007 respectively).
Sites on Torilla Plain support the most CYCs of any location with approximately two-thirds of the known population. Further, the Cyperus-dominated upper marine plain habitat at this location is one of the few sites where CYCs persist in dry periods, provides extensive habitat for breeding birds, and is likely to be the source for breeding birds observed in the adjacent Schoenoplectus-dominated habitats fringing the lower marine plain. As such, this location should be the focus for conservation efforts which are reliant on a continued close relationship between researchers and the graziers who manage this land. The existence of several mining tenures, including shale oil, probably poses the greatest threat in this area. However, the nexus between the long-term perpetual benefits of highly productive grazing land and conservation benefits such as supporting substantial CYC numbers should provide a buffer against the short-term non-sustainable gains from extractive industries.
Conclusion
CYCs have developed a life history strategy capable of exploiting the “boom-bust” conditions stemming from the relatively high rainfall variability found in this region (Houston Reference Houston2013). The relationship of standard measures of abundance with rainfall clearly defines the importance of maintaining wet season run-off into their breeding habitat in order to ensure breeding success. Their small population size in combination with fragmented distribution and concentration of most numbers at one area all point to a subspecies vulnerable to chance events affecting key habitats. This is of particular relevance under scenarios of climate change where models predict increased intensity of disturbances (such as floods and droughts) and increased temperatures and evaporation rates, all with the capacity to affect breeding success. It is possible that these predicted increases in climatic extremes could exceed the resilience of this wetland adapted passerine to persist.
Acknowledgements
We thank the many landholders/managers who willingly allowed their properties to be surveyed including Ralph and Lorraine Bartlem, Craig and Latisha Mace, Lachlan and Trudi Mace, Lawson and Linda Geddes, Peter Naylor and his manager Gary Hall, Simon and Garry Nolan, Graham McCamley, Ian and Margaret Christiansen, Ray Smith, Frank Titmus, Cheetham Salt (Richard Segal in particular) and George and Joy Wilson. We wish to acknowledge the great support we have received from our colleagues and volunteers; they have always been enthusiastic with fieldwork, even in the face of adversity, and we would particularly like to thank Roger Jaensch, Leif Black, Allan Briggs and Lorelle Campbell (and all those not mentioned here who have assisted over the years) for their efforts. Funding was provided by several sources (Birds Queensland, Threatened Species Network and BirdLife Australia, Fitzroy Basin Association, Central Queensland University, Gladstone Area Water Board) and we are most grateful for the support of these organizations. Some of this study formed part of the first author’s Master of Applied Science at Central Queensland University that was supervised by Steve McKillup and Bob Newby; their advice and support was much appreciated; as were the constructive comments of the anonymous reviewers of this paper. Chats were sampled under Central Queensland University ethics permit A12/02-279, the Queensland Government Scientific Purposes Permit SP08039210 and an Australian Bird and Bat Banding Scheme banding authority no. 706 under the direction of Ian Carruthers.











