Introduction
The African Penguin Spheniscus demersus is endemic to Southern Africa but its population is currently at the lowest level ever recorded (Crawford et al. Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). Direct exploitation by humans and reduction of suitable nesting habitat through guano exploitation for more than 150 years caused their population to decrease by 90% during the 20th century (Crawford et al. Reference Crawford, Williams, Hofmeyr, Klages, Randall, Cooper, Dyer and Chesselet1995). Protection of the species in 1976 to prevent further exploitation led to some recovery in numbers (Crawford et al. Reference Crawford, Williams, Hofmeyr, Klages, Randall, Cooper, Dyer and Chesselet1995). However, recent decreases in food availability, due to a shift in distribution and abundance of their main prey, caused a further 60% population reduction in South Africa between 2001 and 2009 (Crawford et al. Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). The species is now listed as ‘Endangered’ (BirdLife International 2011) and considerable efforts are currently being coordinated to identify the major threats impacting on African Penguins and to set objectives to address this decline - see the recently written African Penguin Biodiversity Management Plan (Waller and Shaw Reference Waller and Shaw2010). For example, experimental fishing exclusions have been implemented around key breeding localities to increase food availability for penguins (Pichegru et al. Reference Pichegru, Grémillet, Crawford and Ryan2010), the small-scale variability in distribution and abundance of their main prey is being investigated (J. C. Coetzee unpubl. data), as well as the consequences of industrial fishing over fine scales in the vicinity of their colonies (Pichegru et al. Reference Pichegru, Ryan, van Eeden, Reid, Grémillet and Wanless2012). The effects of predation and climate change on African Penguin reproductive output still remain to be addressed in the context of conservation management.
Historic guano exploitation still has an impact on penguins, as the lack of suitable substrate in which to burrow forces penguins to breed in surface nests (Figure 1a). African Penguins naturally dig burrows in guano that provide protection from aerial predators as well as providing a constant microclimate, with high relative humidity, buffered temperatures and little exposure to the wind (Frost et al. Reference Frost, Siegfried and Burger1976). Penguins are generally sensitive to heat stress while breeding (Frost et al. Reference Frost, Siegfried and Burger1976, Seddon and Davis Reference Seddon and Davis1989). If breeding adults are forced to leave their nests due to disturbance or heat stress, surface breeders are more vulnerable to predators, such as Kelp Gulls Larus dominicanus, than birds in burrows (Cooper Reference Cooper1974, Frost et al. Reference Frost, Siegfried and Burger1976, Yorio and Boersma Reference Yorio and Boersma1994).

Figure 1. Pictures showing African Penguins breeding in (a) a natural surface nest, (b) an artificial fibreglass burrow, and (c) a ‘pipe’ nest on Bird Island, Nelson Mandela Bay, South Africa (photo credits: L. Edwards, B. Dilley).
Populations of many large gulls Larus spp. are increasing worldwide (Crawford et al. Reference Crawford, Whittington, Martin, Tree and Makhado2009, Lisnizer et al. Reference Lisnizer, Garcia-Borboroglu and Yorio2011), due to cessation of control and/or increased human food subsidies, such as fisheries waste and open refuse tips (Bertellotti et al. Reference Bertellotti, Yorio, Blanco and Giaccardi2001, Yorio and Caille Reference Yorio and Caille2004). Gulls are often considered as an overabundant pest species (Blokpoel and Spaans Reference Blokpoel and Spaans1991, Yorio et al. Reference Yorio, Bertellotti, Gandini and Frere1998). When targeting seabirds’ eggs and chicks, gulls can decrease breeding success and affect dispersal and recruitment (Harris and Wanless Reference Harris and Wanless1997, Finney et al. Reference Finney, Harris, Keller, Elston, Monaghan and Wanless2003, Donehower and Bird Reference Donehower and Bird2008). Some gulls can be highly specialised and pose a serious threat to seabird colonies (Spears Reference Spears1993, Yorio and Quintana Reference Yorio and Quintana1997). Reducing the numbers of gulls has assisted the recovery of some seabird populations (e.g. Finney et al. Reference Finney, Harris, Keller, Elston, Monaghan and Wanless2003, Sanz-Aguilar et al. Reference Sanz-Aguilar, Martínez-Abraín, Tavecchia, Minguez and Oro2009), but this is not invariably the case (Harris and Wanless Reference Harris and Wanless1997). Management decisions pertaining to “pest” species should be based on appropriate scientific evidence (Sutherland et al. Reference Sutherland, Pullin, Dolman and Knight2004, Oro and Martínez-Abraín Reference Oro and Martínez-Abraín2007), as altering predator-prey interactions in human-modified environments may have more profound impacts than anticipated (Yodzis Reference Yodzis1998, Dickman Reference Dickman2008). Thus, decisions to remove predators from a system over a protracted period should be monitored extremely carefully and alternative solutions should also be considered.
To reduce Kelp Gull predation on burrowing seabirds eggs and chicks, an alternative to culling is to provide artificial nests. Such nests could also shelter penguins against extreme weather events. Indeed, small chicks raised in surface nests are also more exposed to mortality during severe rains (Randall et al. Reference Randall, Randall and Erasmus1986, Frere et al. Reference Frere, Gandini and Boersma1992, Renner and Davis Reference Renner and Davis2001, Demongin et al. Reference Demongin, Poisbleau, Strange and Quillfeldt2010). Artificial nests have proved successful in increasing breeding success of Little Penguins Eudyptes minor in New Zealand, providing shelter from predation (Perriman and Steen Reference Perriman and Steen2000) and potentially from rain (Renner and Davis Reference Renner and Davis2001). However, similar nests failed to increase the breeding success of the same species in Australia, due to high ambient temperatures inside the nests (Ropert-Coudert et al. Reference Ropert-Coudert, Cannell and Kato2004). The success of artificial nests is therefore site- and design-dependent (Kemper et al. Reference Kemper, Underhill, Roux and Kirkman2007).
In this study I compared the potential benefits of culling gulls and of implementing two types of artificial nests on the breeding success of African Penguins on Bird Island, Nelson Mandela Bay, South Africa. I compared the intensity of predation by Kelp Gulls on African Penguin nests, and breeding success of penguins in surface and artificial nests (Figure 1), one year before (2009) and two years (2010–2011) after culling. I hypothesised that (1) gull predation on penguin eggs and chicks would decrease after culling, (2) breeding success in surface nests would be lower than in artificial nests prior to gull control, and (3) this success would increase after culling gulls.
Methods
The study took place on Bird Island (33°50’S, 26°17’E) between January and August, the peak of the penguin breeding season in Nelson Mandela Bay (Hockey et al. Reference Hockey, Dean and Ryan2005) in 2009, 2010 and 2011. Bird Island currently supports > 10% of the global population of African Penguins (2,500 pairs; Crawford et al. Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). Most of its guano was removed between 1850 and 1950 (Urquhart and Klages Reference Urquhart and Klages1996). The island is flat and devoid of rocks or bushes and most penguins breed in surface nests (Figure 1a), where breeding success is generally poorer than in natural burrows (Seddon and van Heezik Reference Seddon and van Heezik1991). The island is permanently occupied by rangers from South African National Parks and receives regular visits by researchers and other groups, causing human disturbance. The Kelp Gull population in the Eastern Cape has increased since the 1970s, with currently 43 pairs on Bird Island and another 189 pairs on neighbouring islands in 2005/06 (Crawford et al. Reference Crawford, Whittington, Martin, Tree and Makhado2009). The gull population was not disturbed in 2009, but in 2010 SANParks culled 62 gulls between February and June, as part of an active adaptive management intervention. As numbers of gulls increased again on the island after some months, another 116 were culled after the peak of the penguin breeding season in 2010. From then on, < 10 gulls were seen at any given time during the 2011 penguin breeding season.
Observations of gull predation
Gulls often transport penguin eggs to a specific spot to consume them, forming middens of broken shells. Predated penguin eggs were counted daily during the penguin breeding season, along pathways that traverse roughly two thirds of the island’s surface. Egg shells were squashed to avoid repeated counts of the same egg. The island is free of rats and other land predators that could target penguins, so penguin eggs found predated can be attributed only to Kelp Gulls. Comparisons of numbers of eggs found daily before (February–July 2009) and after (February–July 2010) gull culling were made with a Mann-Whitney U-test. Predated chicks were excluded from the counts as they are often swallowed whole, leaving no remains.
Direct observations of gull behaviour were made from an 8 m-high observation tower from which > 200 occupied penguin nests could be observed. During 5–15 June 2009, 14–17 February 2010 (just prior to culling) and 24 June–7 July 2010 (after culling), one observer continuously recorded the number of gulls present, their activities, the number of attacks on penguin nests and the number of successful attacks. After a successful attack, gulls typically fly to consume their prey at a specific site. This behaviour attracted conspecifics, enhancing visibility. Observation sessions lasted 1–1.5 hours, with usually one session in the morning and one in the afternoon. I compared morning and afternoon sessions in 2009 and 2010 with Generalised Linear Models (GLMs) including number of gulls (average and maximum), number of attacks, frequency of attack (number per hour) and success rate (per hour) as explanatory variables, the time of the session (morning/afternoon) as a dependent factor, and year as a random factor. I also compared the same parameters between the two sessions prior to culling (June 2009 and February 2010). As none of these tests was significant (see Results), I pooled the data and compared predation parameters before and after culling with GLMs. The very low number of gulls on the island in 2011 precluded such observations.
Breeding success of artificial burrows and surface nests
In January 2009, 150 artificial nests were set up on Bird Island by South African National Parks, in response to observations of gulls predating penguin eggs and chicks (SANParks unpublished data). These artificial fibreglass burrows, manufactured by the Dyer Island Conservation Trust (http://www.dict.org.za/), are 60 cm long, 40 cm wide and 30 cm high, with an opening 25 cm wide and 28 cm high (Figure 1b). They have been used at several African Penguin colonies since the mid-2000s but no previous study has determined their effectiveness in increasing penguin breeding success. In 2010, sections of c.30 cm high and 70 cm long of cement pipes cut in half (hereafter referred to as ‘pipe nests’, Figure 1c) were added to the island, and 50 of these pipe nest were monitored in 2011. Both types of artificial nests were individually numbered with paint and their contents monitored weekly. In parallel, a sample of 70–100 randomly chosen natural surface nests were also monitored from 2009 to 2011, after marking each with a numbered stone placed next to the nest.
African Penguins typically lay a clutch of two eggs (Hockey et al. Reference Hockey, Dean and Ryan2005). Surface nests were selected during incubation and the nest contents (number of adults, eggs, chicks and size of chicks) were monitored every 7–10 days. If the eggs disappeared between successive checks, the nest was assumed to have failed at the incubation stage. After 6-8 weeks, the chicks leave their nests to join crèches (Seddon and van Heezik Reference Seddon and van Heezik1993) and are not reliably associated with individual nests. Hence, I assumed that a nest was successful (up to the post-guard stage) if the chicks were known to have reached eight weeks of age and the nest bowl was unoccupied. Each breeding attempt in a marked nest was considered independently, as most of the birds were not individually marked and more than one pair may occupy a nest site in a given season (pers. obs. on some marked pairs). Unfortunately, nest desertion due to disturbance by researchers was not systematically noted. However, African Penguins breeding on Bird Island are generally habituated to humans and only very rarely desert their nests temporarily after human disturbance. Nevertheless, their breeding success in this study might have been slightly underestimated due to a potential impact of researchers.
Breeding success was estimated using Mayfield’s method (1961, 1975), with number of nest days calculated as the mid-point between nest visits. Nest survival probabilities were compared using survival models with the “survreg” function in R v2.12.0 (R Development Core Team 2010), which treats the number of days to nest failure as the response variable. The maximum likelihood estimate of risk of failure (F) per sampling interval was defined following Sherley et al. (Reference Sherley, Ludynia, Underhill, Jones and Kemper2012):
where α and β are the intercept and coefficients from the regression and x is the value of the explanatory variable. Nest survival (S) at time t was defined as
with t the average time for incubation and fledging period (38 and 77 days respectively, Hockey et al. Reference Hockey, Dean and Ryan2005). Breeding success was determined as (1) hatching success (probability of nests with at least one egg hatching), (2) fledging success (probability of nests with at least one chick reaching the post-guard stage), (3) overall breeding success (product of hatching success and fledging success).Breeding success was likely to be slightly over-estimated, as late chick mortality (> 8 weeks) due to disease, starvation or hypothermia is still possible. Upper and lower 95% confidence intervals (CI) are
respectively, where n is the number of breeding attempt failures occurring during incubation or brooding. Nest survival was modelled with year and nest type as explanatory variables, and post-hoc Tukey comparisons were made. Subsequently, as there was no difference in egg or chick survival between 2010 and 2011 (see results), these years were combined to estimate the survival of surface nests before and after culling, in comparison with fibreglass burrows as a control for environmental variability between years.
Results
Observations of gull predation
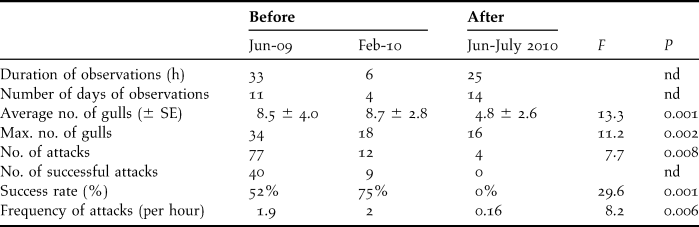
Prior to culling, 778 penguin eggs were found in gull eggshell middens over 173 days of observation (4.5 ± 4.3 eggs/day). In the same areas, these numbers decreased after culling to 121 predated eggs found over 62 days (1.9 ± 3.3 eggs/day, U = 23 060, P < 0.0001). Direct observations of gull predation lasted 39 hours over 15 days before culling, and 25 hours over 14 days after culling. There was no significant difference in predation patterns between morning or afternoon sessions (average number of gulls: F = 1.4, P = 0.26, maximum number of gulls: F = 0.79, P = 0.46, total number of attacks witnessed: F = 0.28, P = 0.75, frequency of attacks F = 0.35, P = 0.70, success rate: F = 0.78, P = 0.46), suggesting a constant predation rate by gulls on penguin nests over the day. There was also no difference between observations prior to culling, in June 2009 and February 2010, with similar numbers of gulls present and similar frequency of attacks and success (Table 1; average number of gulls: F = 0.47, P = 0.50, maximum number of gulls: F = 1.2, P = 0.20, total no. of attacks witnessed: F = 0.41, P = 0.53, frequency of attacks F = 0.07, P = 0.79, success rate: F = 0.05, P = 0.82). However, there were significant differences in predation patterns before and after culling. After culling, fewer gulls were observed, the numbers of attacks and their frequency decreased, and none of the attacks was successful (Table 1).
Table 1. Level of gull predation on penguin nests from direct observations before and after culling some predatory gulls, with level of significance (nd: not determined), SE = standard error.
Breeding success
From the 150 fibreglass burrows present on Bird Island, up to 257 breeding attempts have been recorded in a single breeding season (2009; Table 2). Some nests had up to four breeding attempts per season, suggesting that several pairs used the same artificial nest (Hockey et al. Reference Hockey, Dean and Ryan2005). Surface nests on Bird Island are made of grass, and are ephemeral once unoccupied, as neighbours rapidly steal the nest material, hence a smaller number of repeated breeding attempts in surface nests. Fewer surface nests were monitored in 2010 and 2011 than in 2009 to limit disturbance, as the penguin colony was much reduced.
Table 2. Hatching, fledging and breeding success from natural surface nests and two types of artificial burrows (fibreglass and cement pipes) on Bird Island, Nelson Mandela Bay, South Africa, between January-August 2009, 2010 and 2011.

Hatching success did not improve in surface nests after the removal of predatory gulls (z = 0.234, P = 0.82), but decreased in artificial burrows over the same period (z = 2.92, P < 0.01), which might suggest a potential deterioration of the environment during these years. However, chick survival significantly increased in surface nests after culling (z = -3.37, P < 0.001), resulting in breeding success in these nests twice as high after culling (Table 2).
Penguins that bred in fibreglass burrows consistently experienced lower hatching success than those breeding in both surface and pipe nests (Tables 2, 3). Chick survival, however, was similar in both types of artificial nests and consistently higher than in surface nests (Table 3), even after the removal of gulls. The combination of lower hatching success but higher fledging success in fibreglass burrows when compared to surface nests resulted in similar overall breeding success in both these nest types, although breeding success was slightly higher in surface nests after culling (Table 2). Finally in 2011 hatching success in pipe nests was as high as in surface nests, and fledging success as high as in fibreglass burrows (Table 3), which resulted in breeding success twice as high in pipe nests as both other nest types (Table 2).
Table 3. Results of survival and Tukey post-hoc analyses comparing hatching and fledging success between years (2009–2011) and nest types (surface nests, fibreglass burrows and cement pipe nests).

Discussion
Both types of artificial nests and the removal of predatory gulls increased African Penguin chick survival on Bird Island, although neither strategy improved hatching success, despite an observed decrease in numbers of penguin eggs found predated after culling. These results might suggest that a potentially important proportion of the penguin eggs counted as predated in this study before culling may have come from abandoned nests. Similarly, direct predation by Kelp Gulls on occupied Magellanic Penguin Spheniscus magellanicus nests in Argentina accounted for only a third of egg consumption, the rest coming from abandoned nests (Yorio and Boersma Reference Yorio and Boersma1994). On the other hand, the concomitant decrease in hatching success in fibreglass burrows suggests a potential deterioration of the environment in 2010 and 2011, which is confirmed by a parallel study on breeding penguins’ foraging effort (Pichegru et al. Reference Pichegru, Ryan, van Eeden, Reid, Grémillet and Wanless2012). During years of low food availability, adult seabirds favour their own survival and increasingly abandon their current breeding attempt (e.g. Monaghan et al. Reference Monaghan, Uttley and Burns1992, Chaurand and Weimerskirch Reference Chaurand and Weimerskirch1994). Poor environmental conditions in 2010 and 2011 could have masked a potential positive effect of gull removal on penguins’ hatching success, as observed direct predation by gulls decreased after culling.
The culling of Kelp Gulls significantly increased penguin chick survival in surface nests. Chicks may be an easier target for gulls than incubated eggs underneath an adult penguin, as well as being potentially more nutritious than eggs. Similarly, heavier predation by Greater Black-backed Gulls Larus marinus on Eider Duck Somateria mollissima chicks in Quebec, Canada, rather than on eggs, led to an almost complete breeding failure, despite a very high hatching success of > 80% (Donehower and Bird Reference Donehower and Bird2008). Seabird parental investment in chicks is generally higher than in eggs, and African Penguins are less likely to reattempt breeding after a failure at the chick-rearing stage than after a failure at the incubating stage (Hockey et al. Reference Hockey, Dean and Ryan2005). Increasing chick survival can therefore have an important impact on penguins’ final reproductive output, even more so during years of low food availability. Indeed, when food abundance decreases, seabird parents typically reduce their nest attendance to increase their foraging trip duration (Cairns Reference Cairns1987), leaving unattended offspring more vulnerable to predators. Moreover, predation can increase on individuals weakened by scarcity of food (Swennen Reference Swennen1989). Finally, high nesting densities in seabird colonies enhance nest defence, restricting predation to peripheral nests (e.g. Frere et al. Reference Frere, Gandini and Boersma1992), and declining colonies suffer greater predation rates (Gilchrist Reference Gilchrist1999). This Allee effect is likely to accelerate the decline of already vulnerable populations (Stephens and Sutherland Reference Stephens and Sutherland1999). Therefore, removal of predators is likely to be even more effective in enhancing seabird productivity during years of food stress.
Although gull-culling experiments sometimes showed contrasting results depending on the species targeted (see Wanless et al. Reference Wanless, Harris, Calladine and Rothery1996, Finney et al. Reference Finney, Harris, Keller, Elston, Monaghan and Wanless2003), they confirm that in order to be effective, culling should be continued over several years (Guillemette and Brousseau Reference Guillemette and Brousseau2001, Sanz-Aguilar et al. Reference Sanz-Aguilar, Martínez-Abraín, Tavecchia, Minguez and Oro2009). Gull predation can return to pre-culling levels the following year (Guillemette and Brousseau Reference Guillemette and Brousseau2001), as gull populations are regulated by density-dependent effects (Duncan Reference Duncan1978). Gulls are highly territorial, and will prevent other conspecifics from foraging on their part of the seabird colony (Spears Reference Spears1993) so new recruits will constantly fill territories opened by culling (Duncan Reference Duncan1978, Guillemette and Brousseau Reference Guillemette and Brousseau2001). Moreover, gulls targeting seabird chicks tend to be high-quality individuals that produce more fledglings per pair and faster growing chicks than other pairs (Watanuki Reference Watanuki1992, Spears Reference Spears1993), so there could be a positive selection to maintain that behaviour in a population. Similarly, Kelp Gull abundance on Bird Island increased a few months after the first culling session. Eliminating predatory gulls therefore requires ongoing management. In addition, systems where predators are removed should be monitored extremely carefully to detect potential effects of culling on the targeted predator population on a local and metapopulation scale, but also on the system itself. Predators usually target sick and/or weak individuals (Genovart et al. Reference Genovart, Negre, Tavecchia, Bistuer, Parpal and Oro2010), so systems where natural predators are removed can be more sensitive to disease outbreaks (e.g. Steen et al. Reference Steen, Olsson and Broman2005).
As an alternative to culling, artificial nests also improved African Penguin chick survival on Bird Island, although the success of such nests depended on their design. The consistently poor hatching success in fibreglass burrows revealed that these nests are far from mimicking conditions in natural burrows, which provide the birds with relatively constant humidity and temperature, and protect them from the wind (Frost et al. Reference Frost, Siegfried and Burger1976). Temperatures in fibreglass burrows are probably too high to allow most eggs to hatch, as was suggested by extreme temperatures (> 57°C) recorded in artificial nest boxes on Robben Island, another African Penguin colony on the west coast of South Africa (Griffin Reference Griffin2005). Simple aerated shelters made of cement pipes were more effective than fibreglass burrows in increasing penguin breeding success, although these results might be slightly overestimated due to a limited sample size, as well as the absence of gulls from the study site when this second type of nests were added: such nests might not protect penguins from gull predation as much as fibreglass burrows, and further studies might be necessary to answer that question. In Namibia, similar positive results were obtained from artificial nests made out of plastic bins cut in half and slightly buried in the ground (Kemper et al. Reference Kemper, Underhill, Roux and Kirkman2007). The authors suggested that plastic could prevent infestation by ticks, compared to nests made of rocks or cement. Therefore, the success of pipe nests needs to be confirmed over several years. Nevertheless, the high chick survival from both types of artificial nest suggests that they consistently provide shelter against extreme weather events, to which surface nests remain exposed without predators. Similar results were found on Robben Island, where chick survival in artificial nests was also higher than any other nest type (Sherley et al. in press). Mortality of small African Penguin chicks can be very high during severe rains (up to 80%; Randall et al. Reference Randall, Randall and Erasmus1986). Climate changes increase the frequency of extreme weather events (Parmesan et al. Reference Parmesan, Root and Willig2000), which can have dramatic events on some populations (McKechnie and Wolf Reference McKechnie and Wolf2010). In this context, appropriately designed artificial nests seem to provide the best solution to sustainably increase African Penguin breeding success.
However, where the use of such nests is logistically unrealistic, such as where the topography of the colony prevents it, culling predatory gulls can rapidly increase penguins’ reproductive output. The consistently low breeding success on Bird Island during this study (c. 0.2) when compared to the breeding success of penguins from the west coast of South Africa in the mid-1990s (0.486; Wolfaardt et al. Reference Wolfaardt, Underhill, Nel, Williams and Visagie2008), emphasizes the poor environmental conditions prevailing at this colony, largely as a result of anthropogenic influence (Crawford et al. Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). Although Kelp Gulls are natural predators of African Penguins and other seabirds, their impacts have been distorted through human activities. Kelp Gull populations in South Africa are presumably above historical limits (Steele and Hockey Reference Steele and Hockey1990), benefiting from human-induced food subsidies (Crawford et al. Reference Crawford, Whittington, Martin, Tree and Makhado2009). In such disturbed systems, human intervention is justified to assist the endangered species. Controlling gull numbers on breeding islands may also benefit non-burrowing seabirds, as Kelp Gulls can also target eggs and small chicks of Cape Cormorant Phalacrocorax capensis, Cape Gannet Morus capensis and Roseate Tern Sterna dougallii (Brooke and Cooper Reference Brooke and Cooper1979, Steele and Hockey Reference Steele and Hockey1990), all of which are now of conservation concern (BirdLife International 2011). The recent collapse in African Penguin numbers requires urgent management actions (Crawford et al. Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). The African Penguin Biodiversity Management Plan (Waller and Shaw Reference Waller and Shaw2010) recommends the introduction of artificial burrows in order to increase suitable breeding habitat for penguins as well as to limit gull predation. This study revealed that the design of artificial nests must be carefully thought through before installing these nests in penguin colonies, in order to be successful. The Biodiversity Management Plan also suggested pricking gulls’ eggs in order to control their population size and level of predation, but this study shows that removing Kelp Gulls from penguin colonies is probably a much more efficient way of increasing penguin production. However, the present results emphasise the need for ongoing management and large-scale coordination of such a strategy.
To significantly improve the conservation status of African Penguins, such conservation strategies should be concomitant with limiting oil spills and spatial management of fishing effort to increase food availability in their limited foraging range during the breeding season (Crawford et al. Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011, Pichegru et al. Reference Pichegru, Ryan, van Eeden, Reid, Grémillet and Wanless2012).
Acknowledgements
This work is mainly the fruit of weeks of patient data collection by the marine rangers of Addo Elephant National Park and I am indebted to them. I am also grateful to V. Lategan, S. Jones, H. Visser and N. Steele for their hours of observations. Finally, I warmly thank A. Gaylard, P. G. Ryan, P. Yorio, C. Loiseau, R. Sherley and R. van Eeden for their help at different stages of this manuscript. I thank Popi and another anonymous referee for their useful comments. During the study, LP was financed by “the African Penguin Species Champion” project of the Charles van der Merwe Trust.






