Introduction
Tinamus solitarius (Viellot 1819), popularly known as Solitary Tinamou or Macuco, is endemic to the Atlantic Rainforest (Naka and Rodrigues Reference Naka and Rodrigues2000). In Santa Catarina state there are only a few records of the species (Berlepsch Reference Berlepsch1873, Reference Berlepsch1874, Zimmermann Reference Zimmermann1991, Albuquerque and Brüggemann Reference Albuquerque and Brüggemann1996, Rosario Reference Rosário1996, Naka et al. Reference Naka, Rodrigues, Roos and Azevedo2002). Despite being widely distributed in Brazil, Solitary Tinamou is officially listed as an endangered species (Silveira and Straube Reference Silveira and Straube2007) and as a result of hunting and habitat loss (Naka and Rodrigues Reference Naka and Rodrigues2000) is currently found in only a few forest fragments (Bokermann Reference Bokermann1991, Sick Reference Sick2004).
Although it is known that some birds have the capacity to adapt to environmental disturbances (Lens et al. Reference Lens, Van Dongen, Norris, Githiru and Matthysen2002, Lindenmayer et al. Reference Lindenmayer, McIntyre and Fischer2003), nothing is known about the occurrence of Solitary Tinamou in different successional stages of vegetation. Very little is known, in fact, about the behaviour of Solitary Tinamou. With the exception of anecdotal data found in bird guides and handbooks, there are only a few detailed studies concerning aspects of its ecology (Bokermann Reference Bokermann1991, Brooks et al. Reference Brooks, Pando-Vasquez, Ocmin-Petit and Tejada-Renjifo2004, Schelsky Reference Schelsky2004, Sick Reference Sick2004, Sigrist Reference Sigrist2009). The almost complete absence of research is largely due to the difficulty of studying Solitary Tinamou (Brennan Reference Brennan2004): its secretive habitats and cryptic coloration make it difficult to observe in its natural habitat. Notwithstanding, there is no published study on Solitary Tinamou that uses camera traps, a technique that would be particularly suitable for species with this kind of behaviour.
Because of the lack of information on Solitary Tinamou, in this study we aimed to verify the use of habitats in different stages of plant succession by this species and its circadian activity pattern.
Methods
Study area
This study was carried out in part of the Serra do Tabuleiro State Park (27º44’S, 48º48’ W). The region is located in an Atlantic Rainforest area, covered by Dense Broadleaf Evergreen Atlantic Rainforest (Veloso et al. Reference Veloso, Rangel Filho and Lima1991), with diverse degrees of succession. The predominant climate in the region, according to the Köeppen-Geiger classification system, is humid mesothermal, with hot summers and without a definite dry season (Cfa).
Data collection
We collected physiognomic-structural data at each camera-trap site and performed surveys in rectangular parcels of 5 m x 20 m, positioned in parallel with the camera trap. Centres of the parcels were determined by the exact position of the camera-trap. Within each parcel, we assessed the canopy cover and litter fall height, counted all trees and shrubs and measured diameter at breast height (DBH = 1.3 m above ground) of trees (with diameters > 5 cm) to estimate the basal area.
The birds were recorded with the use of six digital camera traps (Tigrinus®) from August 2008 to September 2009. Altogether, 23 sites were sampled: seven sites in areas of primary forest, nine in secondary forest and seven in brush formations. Camera traps were set up and left at each sample site for two months, after which they were moved to a new site, a minimum distance of 100 m apart. The three sampled areas were continuously and simultaneously monitored by two camera traps each, resulting in a sampling effort of 1,458 trap-days.
Data analysis
Habitat use data were analysed using Fisher’s exact test, which compared the proportion of sampling points that did or did not record the occurrence of the species for each successional stage. As the early stage of succession did not produce any records of Solitary Tinamou, analysis of habitat use only considered primary and secondary forest. The relationship between vegetation structure and capture success was calculated by Spearman rank correlation analysis.
Each independent record (interval = 1 hour) of the species was classified as nocturnal, diurnal or crepuscular (after Van Shaick and Griffiths Reference Van Schaik and Griffiths1996). We collected times of sunrise and sunset throughout the year from the software Moonrise 3.5. The circadian pattern of the species was then determined according to Gómez et al. (Reference Gómez, Wallace, Ayala and Tejada2005).
Results and Discussion
Daily activity
Seventy-six independent records of Solitary Tinamou were obtained and the species showed a twilight pattern of activity. The majority of the records (38%) occurred at 07h00 and 8h00 (Figure 1). Half the records (n = 38) occurred during the period of twilight, 49% of the records (n = 37) were diurnal, and only one record took place at night.
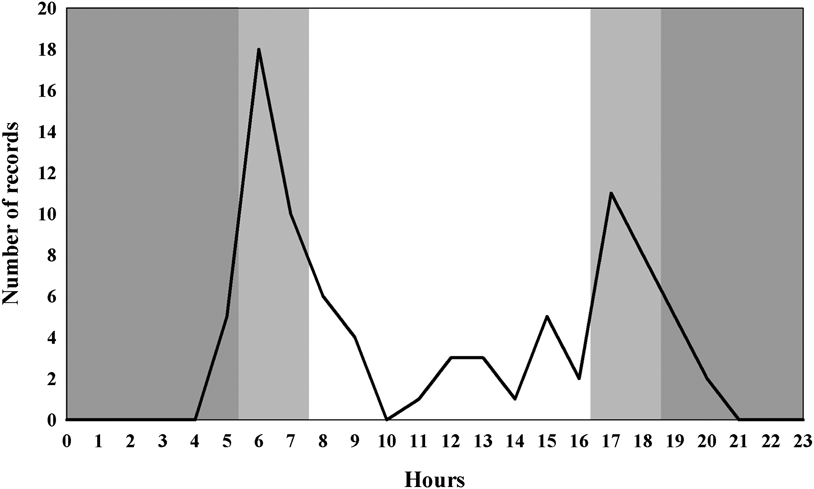
Figure 1. Hourly activity periods of Solitary Tinamou (dark grey = night; light grey = dusk and dawn; white = day).
The circadian cycle we observed for Solitary Tinamou was similar to that found for Tinamus tao, and T. major sampled in field trips in the Peruvian Amazon region (Brooks et al. Reference Brooks, Pando-Vasquez, Ocmin-Petit and Tejada-Renjifo2004), showing a possible pattern for the genus. As we used camera traps, we can be sure that disturbance to the animals during data collection was very low, if any.
Habitat use
The 76 independent records of Solitary Tinamou showed a higher occurrence of the species (P = 0.02) in secondary forest, where 54 independent records were obtained (0.09 records/day), compared with primary forest (22 independent records, 0.05 records/day). In the earliest stage of succession, Solitary Tinamous were not recorded. Among all vegetation structures analysed (Table 1), the occurrence of Solitary Tinamou was positively correlated only with canopy cover (P = 0.004), hence showing that regardless of the successional stage, the species tended to occur more frequently in areas with higher canopy cover.
Table 1. Minimum value, maximum value, average and standard deviation for each measured environmental variable of each one of the three successional stages. (Different letters within a line indicate statistical difference at the level of probability, indicated in the P column).
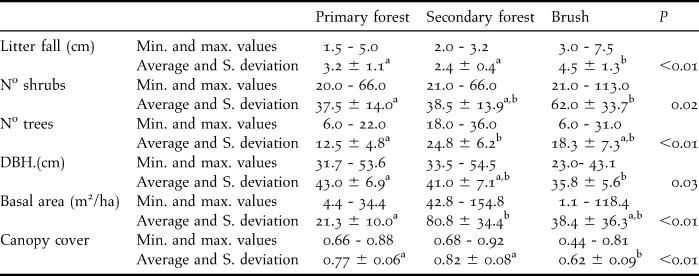
In other studies, Tinamus guttatus, T. tao, and T. major were mostly found in high ground forest, at the edges of forests (Brooks et al. Reference Brooks, Pando-Vasquez, Ocmin-Petit and Tejada-Renjifo2004), and also in mature floodplain forest, late succession forest and dense bamboo stands (Schelsky Reference Schelsky2004). However, no records were found in lowlands or secondary forest (Brooks et al. Reference Brooks, Pando-Vasquez, Ocmin-Petit and Tejada-Renjifo2004). Our findings are slightly different. Although no record of Solitary Tinamou was obtained in the most degraded areas, which may indicate a possible sensitivity of the species to removal of forest, they did appear in secondary forest. This habitat was at a much higher successional stage than brushwood (see Table 1). The absence of the species in the area of brush indicates that the Solitary Tinamou avoids non-forested areas.
The higher occurrence of Solitary Tinamou in secondary than in primary forest may be explained by the presence of a thick carpet of bromeliads on the primary forest floor, which could possibly hamper the search for food. The species’s occurrence in areas of higher canopy cover, independent of the successional stage, alongside the absence of trap recordings in areas of bush, corroborates the idea that established forest is vital for the occurrence of this particular bird species, hence indicating that the deforestation of the Atlantic Rainforest may also contribute to the local extinction of Solitary Tinamou, as fragmentation of tropical forest may cause similar impacts elsewhere (Schelsky Reference Schelsky2004).
The extinction of endemic bird species of the Atlantic Rainforest as a result of habitat loss has been widely demonstrated on small scales (Brooks et al. Reference Brooks, Tobias and Balmford1999). In Santa Catarina state, 78% of the Atlantic Rainforest has already been cleared (Medeiros Reference Medeiros, Schäffer and Prochnow2002). Endemic species that use secondary habitats can survive the complete removal of primary forest, whereas forest-obligate species cannot (Brooks et al. Reference Brooks, Tobias and Balmford1999). In our study, Solitary Tinamou proved to be well suited to intermediate successional stage environments. However, the extent to which their presence in secondary areas is temporary or conditional upon the remaining primary forest elsewhere remains unclear.
Solitary Tinamou may tolerate secondary habitats but its use of these habitats does not further protect against deforestation, nor reduce its chance of becoming a threatened species. Without immediate and comprehensive conservation actions to avoid deforestation and hunting, Solitary Tinamou which is threatened with extinction today, may yet become extinct in the near future.
Acknowledgements
We are grateful to Fernando Bruggemann for the opportunity and to Plaza Caldas da Imperatriz Resort for logistic support. We wish to thank CAPES for the scholarship to V.V.K., CNPq for the scholarship to R.E.M.L. and FAPESC for the financial support.




