Introduction
The Wood Snipe Gallinago nemoricola is a threatened shorebird species, categorised as Vulnerable by the IUCN Red List due to its small and likely declining population (BirdLife International 2017), though little was known about its population and ecology. A migratory species in at least some locations, it breeds in alpine meadows above the treeline in the Himalayas and the Hengduan Mountains of south-western China, and winters at lower altitudes in South and South-East Asia (Nepal, India and Bhutan, Thailand, Myanmar and Vietnam) (BirdLife International 2001, Eaton and Duff Reference Eaton and Duff2013, Ludlow and Kinnear Reference Ludlow and Kinnear1944). Non-breeding birds considered to be vagrants have also been recorded in Sri Lanka, Bangladesh, and Laos (eBird 2022). Since the 1980s, sightings of this species have been sparse and restricted to a fraction of the locations with historical records suggesting a possible decline (BirdLife International 2001, eBird 2022). In China, the Wood Snipe has been recorded from a few locations in Sichuan and Yunnan provinces, including two that are known to support regular breeding populations, in the Wolong and Xuebaoding National Nature Reserves in Sichuan. The population in China is unclear, possibly declining since there have been very few records from Wolong in the last nine years (eBird 2022).
Information about Wood Snipe ecology is very limited. Only one formal study has been undertaken to assess its breeding population size and conservation threats in one national park in the Nepalese Himalayas (Basnet et al. Reference Basnet, Shrestha, Thakuri, Pun, Chaudhary and Baral2021). All existing information on the species’ breeding or non-breeding habitat use comes from an informal assessment of historical observations (BirdLife International 2001). During the breeding season, the Wood Snipe is reported to use alpine meadows with scattered bushes above the treeline, although no nest has been recorded nor more detailed habitat associations documented (Ludlow and Kinnear Reference Ludlow and Kinnear1937, Khatiwada and Chaudhary Reference Khatiwada and Chaudhary2008). The species’ habitat use during the non-breeding season is also poorly understood, with a mixture of records from grasslands and swamps near forests to marshy habitats some distance away from forest (BirdLife International 2001). Habitat degradation and disturbance linked to livestock grazing and tourism are suggested to be important threats at the species’ breeding ground (Basnet et al. Reference Basnet, Shrestha, Thakuri, Pun, Tamang and Chaudhary2020, BirdLife International 2017), while little is known about threats in its non-breeding range. Given the small and likely declining population of the Wood Snipe, knowledge on its basic ecology could help inform the design and implementation of conservation recovery actions.
Here we provide the first assessment of the habitat use of the Wood Snipe during the breeding season. We studied its habitat characteristics and food resource availability at a 4-km2 alpine meadow bordering the Xuebaoding National Nature Reserve in Sichuan province, which is the only location in China where this species was recorded during the breeding season each year for the past 10 years. We conducted population surveys of the Wood Snipe in the study area to identify locations where it occurred. Then we compared a range of biotic and abiotic attributes that might affect Wood Snipe habitat selection between occurrence sites and background sites. Based on observing birds probing the soil for prey, we also identified foraging sites to characterise the availability of potential food resources. We expected that the Wood Snipe would prefer sites with dense vegetation for cover, and those with high soil moisture and high densities of soil fauna for food access. Our study provides an important new piece of understanding on the ecology of this poorly known species.
Methods
Study area
We conducted our study in the breeding season (May–July) of 2021 in an alpine meadow area in Huya township (32.56° N, 103.98° E) of Sichuan province, China (Figure 1). Bordering the Xuebaoding National Nature Reserve inside the newly established Giant Panda National Park, our study area is located in the north-eastern Hengduan Mountains. It has a subtropical highland climate (Cwb) in Köppen’s climate classification system, characterised by an average annual temperature of 10.6–14.8°C and average annual precipitation of 950–1,130 mm (Peel et al. Reference Peel, Finlayson and McMahon2007, Fu et al. Reference Fu, Liu, Wang, Zhao and Ran2007). From the broadleaf forests by the Huya River at the bottom of deep gorges (1,400 m) to the nival belts on the top of Mount Xuebaoding (5,588 m), natural vegetation communities at Huya cover a wide range of conditions along the elevational gradient.
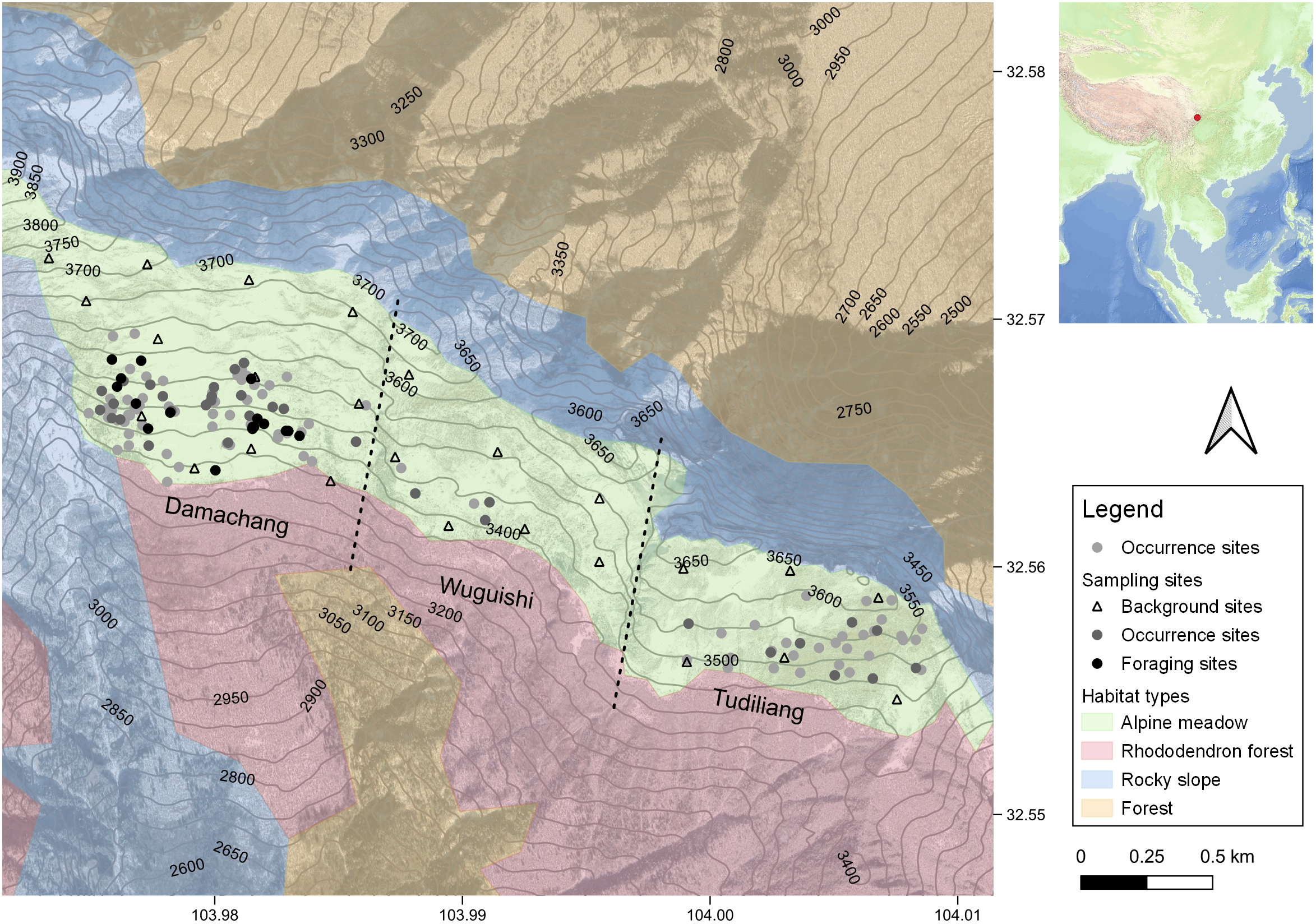
Figure 1. Map of study area. The map on the left with contour lines shows the study area, habitat types, sampling sites, and all the Wood Snipe occurrence sites during the fieldwork. Occurrence sites are represented by points (n = 123), while the background sites are represented by triangles. We sampled habitat variables at background sites and part of the occurrence sites, but food resources at background sites and foraging sites (see Methods). Habitat types are shown in different colours. The map on the top right corner shows the location of the study area (red dot). Satellite imagery: Google.
Based on historical sightings and consultation with local people (Wang pers. comm. 2020), we selected three neighbouring alpine meadows (Damachang, Tudiliang, and Wuguishi) totalling 4 km2 for fieldwork. These meadows are located at 3,300–3,800 m in elevation between the treeline and the mountaintop ridge on a south-facing slope, with plant communities dominated by Carex sp., Primula sp., and Deyeuxia scabrescens. The study area has no permanent human residents, although herders living in the nearby forests close to the treeline graze yaks, cattle, and horses in the meadows almost throughout the year. Other human activities in the study area included limited foot traffic of workers and visitors en route to a mine near the peak of Xuebaoding, along with limited wild herb harvesting especially for Fritillaria sp. during summer.
Breeding population surveys
We combined systematic transect surveys and casual encounter records, both conducted during the daytime, to quantify the breeding population size of the Wood Snipe in the study area. We designed both surveys around the locations of a grid randomly set up with roughly 400-m spacing covering the majority of the study area (Figure 1, ground distance). We chose this spacing as 200 m was the average distance that we could see or hear a Wood Snipe in the field. For the systematic transect survey, we walked the transects every eight days en route visiting all the points in the grid to cover the whole range of the study area, at an average speed of 1 km/h. In total, we conducted nine rounds of systematic surveys. For the casual encounter records, we recorded any Wood Snipe individuals we encountered outside the transect counts throughout our field season. For all Wood Snipe individuals seen or heard that were on the ground, we recorded their number along with the coordinates of the sites where they were first detected (later referred to as occurrence sites). As we seldom detected flying Wood Snipe and all the encounters with flying individuals happened during display flights before sunrise or after sunset when the light was too dim to count, these flying individuals were not included in the population surveys. We then made a best guess for the Wood Snipe population size at the study site based on our experience in the field combining all information.
Habitat characteristics measurement
We used the “use-available” design to investigate the fine-scale habitat selection of the Wood Snipe, comparing the habitat characteristics of its occurrence sites (“use” sites) with a set of randomly selected background sites (“available” sites) (Manly et al. Reference Manly, McDonald, Thomas, McDonald and Erickson2002), based on the locations of a grid set up for population survey in our study area. Due to the high density and non-independence of occurrence sites in some parts of the study area, we drew a random sub-sample of occurrence sites with a minimum spacing of 30 m for measurement of habitat characteristics. The final selection of sites comprised 34 occurrence sites and 25 randomly selected background sites (Figure 1), and we measured a set of biotic and abiotic characteristics at each site in mid-June.
Owing to the very limited information on Wood Snipe ecology, we considered a range of terrain, vegetation, soil, and human disturbance variables noted to be important to the habitat selection of other snipe species (Table 1) (Hoodless et al. Reference Hoodless, Ewald and Baines2007, Mongin Reference Mongin and Ferrand2006, Korniluk et al. Reference Korniluk, Bialomyzy, Grygoruk, Kozub, Sielezniew, Swietochowski and Tumiel2021, Løfaldli et al. Reference Løfaldli, Kålås and Fiske1992). At each site, we recorded elevation, slope aspect, and grade, as well as soil variables including moisture and penetrability. We estimated soil moisture on a scale from 0 (dry) to 5 (wet) using a soil moisture meter (model D2BTQ). We also measured soil penetrability by dropping a pointed iron pin (6 mm diameter and 80 g) at 1.5 m height within 0.5 m from the site three times and taking the average penetration depth. We also set up a 0.5 m × 0.5 m plot centred around each site to measure vegetation characteristics. Within each plot, we recorded a list of all plant species present, along with their average height (to the nearest centimetre), and percentage cover of each species (total cover summed to >100% due to vertical overlap among species). We additionally measured vegetation horizontal cover, shrub density, and the intensity of human disturbance around each plot. For vegetation horizontal cover, we set up a Robel Pole at each site (Robel et al. Reference Robel, Briggs, Dayton and Hulbert1970), and an observer stood 4 m away to visually assess the height at which the pole was blocked by vegetation, viewed at a height of 1 m from four ordinal directions. For shrub density, we used the point-quarter sampling method (Cottam et al. Reference Cottam, Curtis and Hale1953) to take three measurements for each site, one at the site, and the other two at 2 m away from the site to the north and east. For each measurement, we recorded the distance to the closest shrub individual, species name, and crown diameter in four quarters to calculate the shrub density. For the intensity of human disturbance, we counted the number of livestock manure piles within a 5 m × 5 m plot expanded from the centre to the north-east of each site.
Table 1. Habitat variables measured to investigate the Wood Snipe habitat selection at fine scale.

Food resources survey
We used the same “use-available” design as above to assess the foraging habitat associations of the Wood Snipe, by comparing its foraging sites (“use” sites) and a set of randomly selected background sites (“available” sites) (Manly et al. Reference Manly, McDonald, Thomas, McDonald and Erickson2002). We GPS-marked all foraging sites (n = 17) at which Wood Snipes were seen feeding in the study area during the last week of June, and also the previously used background sites (n = 25, same sites as for habitat characteristics measurement, but one site with sample missing), against which to compare potential prey density. We collected soil samples at all 41 sites (three samples around the centre of each site with 1-m spacing, 15 cm × 15 cm× 10 cm deep, about the length of Wood Snipe head-bill) (Mongin Reference Mongin and Ferrand2006), during the first week of July. We hand-sorted all potential food items (all the soil macroinvertebrates ≥3 mm and visible to the observer while hand-sorting were considered as food resources for Wood Snipe) from the soil samples in the field and stored them in 75% ethanol. We then expressed soil fauna abundance at each site as total body length of each identified order or family, ±1 mm. We used body length instead of biomass since we needed a non-destructive metric so that samples could be kept for further identification. Studies on earthworms and other soil macroinvertebrates have confirmed that body length is positively correlated with biomass (Greiner et al. Reference Greiner, Costello and Tiegs2010, Coulis and Joly Reference Coulis and Joly2017). We further separately calculated the body length of earthworms (Opisthopora) for each site, since we directly observed Wood Snipe feeding on earthworms in the field.
Statistical analyses
We used program R 4.0.2 (R Core Team 2020) to conduct all statistical analyses. As a preliminary, we covered a wide range of parameters (Table 1), but to avoid collinearity and given the large number of candidate covariates we sampled and the relatively small sample size, we focused on the six following uncorrelated covariates (Pearson’s correlation |r| <0.6; Figure S1) that we believed were the most ecologically meaningful to the habitat selection of the Wood Snipe (Hosmer et al. Reference Hosmer, Lemeshow and Sturdivant2013): elevation and slope grade representing topography; soil moisture and penetrability representing soil condition; amount of livestock manure representing disturbance level; sum of herbaceous coverage above 15 cm (calculated by summing the percentage cover of all the herbaceous species with average height larger than 15 cm in plots) representing vegetation cover. After data exploration, we transformed the amount of livestock manure on the log10 scale (on manure number+1) to achieve normality, and we included the quadratic term of soil moisture following studies on other snipes (Løfaldli et al. Reference Løfaldli, Kålås and Fiske1992). We fitted a Generalised Linear Model (GLM) using function glm in the stats package (version 4.0.2) (R Core Team 2020), using a logit link and a binary response variable representing use and background sites. We then selected the best single model with a stepwise method using the step function in the stats package by Akaike information criterion (AIC) and assessed the goodness of fit of the selected model with McFadden’s pseudo-R2 (McFadden Reference McFadden and Zarembka1974).
We also assessed the difference in the vegetation community of herbs between occurrence and background sites. We analysed species-level composition of herbaceous plants using a non-metric multidimensional scaling (NMDS) plot and analysis of similarities (ANOSIM) provided by the vegan package (version 2.5-7) (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn and Minchin2020). Shrub species composition was not compared between sites because the shrub community was simple with only 13 species from nine genera present in our study area.
To compare the amount of potential food resources at Wood Snipe foraging sites and background sites, we fitted a GLM to overall food/earthworm length using function glm in the stats package, using a logit link function, and with a binary response variable representing foraging and background sites. We also investigated the composition of food resources (on the level of orders or, if order information was not available, families) between foraging and background sites using NMDS plots and ANOSIM provided by the vegan package.
Results
Breeding population at study area
During the whole survey season, we recorded a total of 123 occurrence sites of the Wood Snipe in the study area (Figure 1), with most of them involving encounters with one individual snipe. A total of 82 encounters occurred in the Damachang region, 36 at Tudiliang, and only five at Wuguishi (Figure 1). Our field observations suggested to us that there was a minimum of six breeding pairs of Wood Snipe at Damachang, two pairs at Tudiliang, and one pair at Wuguishi.
Fine-scale habitat selection
The single best model for probability of occurrence of Wood Snipe included elevation, soil moisture, the quadratic term of soil moisture, and total herbaceous plant coverage above 15 cm (see Table S1 for model selection results). The model had a reasonably good fit with McFadden’s pseudo-R2 = 0.198. The predicted probability of Wood Snipe occurrence showed a clear preference for the lower part of the alpine meadow in the study area (Figure 2A, Table S2). A bell-shaped relationship between snipe occurrence and soil moisture was predicted by the model (Figure 2B, Table S2), indicating their preference for moderate soil moisture. The predicted probability of snipe occurrence showed a positive but not significant preference for higher total herbaceous plant coverage above 15 cm (Figure 2C, Table S2).

Figure 2. Predicted probability of Wood Snipe occurrence in relation to (A) elevation, (B) soil moisture, and (C) total herbaceous plant coverage in plot above 15 cm. Predictions and 95% confidence intervals (blue shadowed area) are from the selected model. (D) Non-metric multidimensional scaling (NMDS) ordination plot illustrating similarity of herb vegetation composition between Wood Snipe occurrence (“use”) sites and background (“available”) sites (three dimensions, stress = 0.192).
Vegetation structure
NMDS analysis on the herbaceous plant community reached convergence at a stress level of 0.19 with three dimensions. Based on the NMDS plot, we found no significant difference between the occurrence sites and background sites, although there was a considerable level of dispersion in the background points (Figure 2D, three dimensions, stress = 0.192), suggesting that the “use” sites occurred in a narrower subset of herbaceous plant communities than background sites. The ANOSIM test also indicated no significant difference between the occurrence sites and background sites (R = 0.051, P = 0.061).
Food resources
We found soil macroinvertebrates in 14 orders at both Wood Snipe foraging sites and background sites (Table S3). Sites with a greater total length of invertebrates were significantly more likely to be “use” sites (Figure 3A, Table S4), although total length of earthworms was not significantly different between foraging sites and background sites (Figure 3B, Table S4). The NMDS plot for total food composition (Figure 3C, three dimensions, stress = 0.152) showed some difference between background and foraging sites (see Table S5 for food composition at sites), while an ANOSIM test indicated a significant but small difference (R= 0.143, P = 0.009).
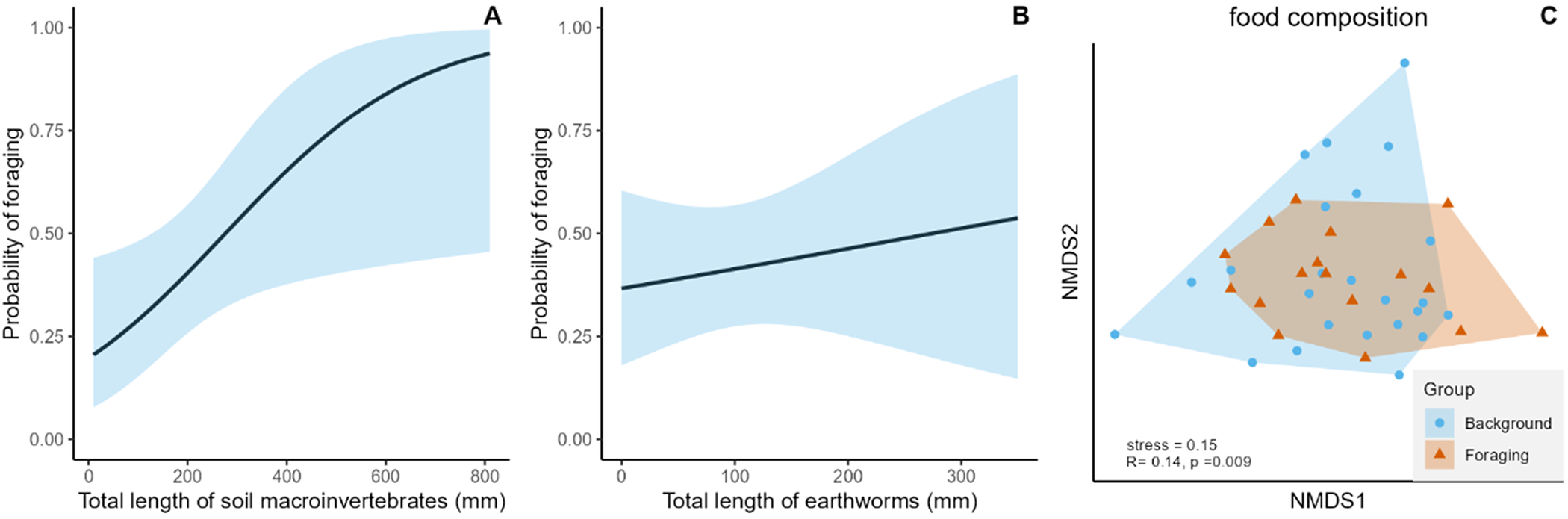
Figure 3. Predicted probability of Wood Snipe foraging in relation to (A) total length of food resources and (B) total length of earthworms; 95% confidence intervals for predictions are shown in the blue shadowed area. (C) Non-metric multidimensional scaling (NMDS) ordination plot illustrating the similarity of food resources composition between Wood Snipe foraging sites and background sites (three dimensions, stress = 0.152).
Discussion
By conducting the first formal assessment of the habitat selection of the Wood Snipe during the breeding season, we found that the Wood Snipe preferred alpine meadow habitat with lower elevation and moderate soil moisture during the breeding season, and that it preferred to forage at sites with more potential food resources. We found weak evidence for vegetation cover and no evidence for vegetation composition, human disturbance, or other abiotic habitat features (e.g. slope or soil penetrability) influencing Wood Snipe habitat selection.
Our findings that the Wood Snipe preferred habitat with a lower elevation, moderate soil moisture, and more potential food resources suggest that the actual distribution range of the Wood Snipe during the breeding season may be smaller than expected from the extent of apparently suitable habitat. In our study, the occurrence of the Wood Snipe in alpine meadows was notably selective, despite the homogeneity of the vegetation communities and their physical structure. Therefore, the occurrence of the Wood Snipe seemed to be largely driven by soil conditions and food density rather than vegetation variables. This reflects similar findings for the breeding Common Snipe (Gallinago gallinago) and Great Snipe (G. media), in which habitat selection and body condition are associated with certain soil conditions and higher food availability (Green et al. Reference Green, Hirons and Cresswell1990, Korniluk et al. Reference Korniluk, Bialomyzy, Grygoruk, Kozub, Sielezniew, Swietochowski and Tumiel2021, Witkowska et al. Reference Witkowska, Pinchuk, Meissner, Karlionova and Marynkiewicz2022). However, unlike the Great Snipe, we did not find strong evidence of Wood Snipe selecting habitat with moderate vegetation height and density to avoid potential predators (Løfaldli et al. Reference Løfaldli, Kålås and Fiske1992), even though predation attempts by raptors were observed in the field. There is also evidence from the Nepalese Himalayas of the Wood Snipe not occurring at the majority of apparently suitable habitats: the species was recorded at only half of all apparently suitable survey stations in one study (Basnet et al. Reference Basnet, Shrestha, Thakuri, Pun, Chaudhary and Baral2021).
There is little information on the diet of the Wood Snipe, except that it is said to consume worms, aquatic insects, and perhaps seeds (Baral et al. Reference Baral, Inskipp, Inskipp and Regmi1996). We observed individuals feeding on earthworms during the survey, but we still assumed that all visible soil macroinvertebrates would be potential food resources in our analysis since we can not rule out the possibility that other soil fauna are also consumed. Besides the amount of potential prey items present, soil conditions could also affect snipe foraging, and other shorebird studies have shown positive relationships between soil penetrability, soil moisture, and earthworm availability owing to its abundance and surfacing activity (Milsom et al. Reference Milsom, Hart, Parkin and Peel2002, Eggleton et al. Reference Eggleton, Inward, Smith, Jones and Sherlock2009, Onrust et al. Reference Onrust, Wymenga, Piersma and Olff2019).
Contrary to our expectation that the Wood Snipe would avoid higher levels of human disturbance including livestock grazing to avoid nest damage (Green Reference Green1988, Fondell and Ball Reference Fondell and Ball2004), we did not find evidence for this prediction. Firstly, this might be related to the long history of grazing at our study area so that the Wood Snipe has adapted to the grazing activities. Secondly, this might be related to the potential food benefit provided by livestock: the abundance of detritivorous invertebrates, potentially important food resources for the Wood Snipe, has been reported to be high in livestock manure (Filazzola et al. Reference Filazzola, Brown, Dettlaff, Batbaatar, Grenke, Bao and Peetoom Heida2020). While we did not observe any feeding behaviour of the Wood Snipe on or beside livestock manure, the Wood Snipe study in Nepal reported feeding marks of the snipe on Chauri dung (Basnet et al. Reference Basnet, Shrestha, Thakuri, Pun, Tamang and Chaudhary2020). Further studies are urgently needed to investigate the relationship between the intensity of grazing and Wood Snipe activities during the breeding season because a possible ban on grazing activities due to the establishment of protected areas (such as the Giant Panda National Park at our study site) might be implemented in part of Wood Snipe breeding sites.
Working with such a rare and poorly known species, our current study inevitably had a number of limitations. We investigated a large number of habitat variables for a limited number of occurrence, foraging, and background sites, which meant that we were not able to consider more than six variables in our analyses to fully explore the relevance of potentially important habitat variables. Thus, we call for further study to explore the possible relationship between the probability of Wood Snipe occurrence and foraging with other environmental factors to better understand the habitat preference of Wood Snipe so that important breeding sites can be identified and conserved. Furthermore, without studying the local movement of the Wood Snipe, we were not able to define the territories of breeding pairs, which limited our ability to accurately assess the breeding population size in our study area.
Our study provides the first formal assessment of the habitat selection of the Wood Snipe, and it considerably adds to our knowledge of the ecology of this enigmatic and threatened species. We concluded that the occurrence and foraging of Wood Snipe on the alpine meadow in the breeding season is uneven and it is significantly related to elevation, soil moisture, and the amount of food resources present in the soil. We therefore urge caution when evaluating potential habitat availability for Wood Snipe. The actual distribution range of Wood Snipe is likely to be much smaller than the amount of alpine meadow in a region, depending at a site scale on elevation, soil moisture, food availability, and potentially, vegetation cover at its height. Moreover, we urge further study of Wood Snipe ecology especially on the diet preference and change of habitat over time to inform effective conservation efforts across the distribution range.
Acknowledgements
We thank Xuebaoding National Nature Reserve for supporting our work at Huya township. We are grateful to local community members including Huaiping Zhang and Wulu Chi, volunteers including Bin Li, Hong Ren, Kar-Sin Katherine Leung, Keren Yang, Lifang Chen, Shaoping Zang, Shuangqi Liu, Weiyi Wang, Yanmei Zhang, Zimeng Zhao, and Zongzhuang Liu for invaluable help with fieldwork, and to Changda Wang who kindly provide his precious knowledge of the Wood Snipe at Damachang. Special thanks to Tong Mu for helpful discussions and much advice provided throughout the period of our work. This work was supported by the Zhilan Foundation (2021010291B, 2021–2022), Society of Entrepreneurs and Ecology (SEE Foundation, 2021–2022), and the East Asian-Australasian Flyway Partnership Small Grant Funds (2021–2022).
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S0959270923000047.






