Introduction
Pigeons and doves (Columbidae) are among the most successful and widespread bird families. They have colonised most of the world's oceanic islands, reaching eastern Polynesia and the Chatham Islands in the Pacific Ocean; Mauritius, the Seychelles and Réunion in the Indian Ocean, and the Azores in the Atlantic (Baptista et al. Reference Baptista, Trail, Horblit, del Hoyo, Elliot and Sargatal1997, Gibbs et al. Reference Gibbs, Barnes and Cox2001, Walker Reference Walker2007a). They are also highly vulnerable to extinction from hunting, introduced predators and habitat loss (Owens and Bennett Reference Owens and Bennett2000). Hence, nearly one third of the extant Columbidae now face some risk of extinction. Most threatened pigeons and doves are found in tropical and subtropical regions, are forest dependent, and a large number inhabit islands (Walker Reference Walker2007a).
The island of São Tomé, in the Gulf of Guinea, West Africa, is an important centre of bird diversity and endemism, and is inhabited by four pigeon species (Christy Reference Christy, Fishpool and Evans1991, Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998). Three of the four São Tomé pigeons are single island endemics (Maroon Pigeon Columba thomensis, São Tomé Green Pigeon Treron sanctithomae and the Lemon Dove Columba simplex). Another Gulf of Guinea species, the Bronze-naped Pigeon Columba malherbii, is also found on the islands of Principe and Annobon.
All São Tomé pigeons, except the Lemon Dove, are extensively hunted (Atkinson et al. Reference Atkinson, Peet and Alexander1991, Jones and Tye Reference Jones and Tye2005, Dallimer et al. Reference Dallimer, King and Atkinson2009, Carvalho et al. Reference Carvalho, Palmeirim, Rego, Sole, Santana and Fa2014). Three species are globally threatened, according to the IUCN Red List (IUCN 2013): the Maroon Pigeon is ‘Endangered’, the São Tomé Green Pigeon ‘Vulnerable’, and the Bronze-naped Pigeon ‘Near Threatened’. There is sufficient evidence available to show that populations of these birds have declined since the start of the 19th century (Bannerman Reference Bannerman1931, Snow Reference Snow1950, Peet and Atkinson Reference Peet and Atkinson1994, Jones and Tye Reference Jones and Tye2005, Olmos and Turshak Reference Olmos and Turshak2007).
All São Tomé pigeons are found within the island’s forested habitats (Atkinson et al. Reference Atkinson, Peet and Alexander1991, Dallimer et al. Reference Dallimer, King and Atkinson2009, de Lima et al. Reference De Lima, Dallimer, Atkinson and Barlow2012). The Maroon Pigeon is associated with old-growth and older secondary forests, its main population restricted to an area of around 320 km2 of mid- to high-altitude old-growth forest (Peet and Atkinson Reference Peet and Atkinson1994, Jones and Tye Reference Jones and Tye2005). The São Tomé Green Pigeon is also common in old-growth and secondary forest, but similarly inhabits forest-edge habitats, shaded coffee and cocoa plantations (Jones and Tye Reference Jones and Tye2005, Atkinson et al. Reference Atkinson, Peet and Alexander1991). It feeds high in the canopy that includes Ficus and Musanga fruits (Atkinson et al. Reference Atkinson, Peet and Alexander1991, Jones and Tye Reference Jones and Tye2005). Bronze-naped Pigeons are found in forests and forest scrub within the island’s northern savanna habitats. It forages in groups both on the ground and high up in trees (Atkinson et al. Reference Atkinson, Peet and Alexander1991, Jones and Tye Reference Jones and Tye2005). Lemon Doves are described as common to abundant, found singly or in pairs, wherever there is dense cover on and near ground in old-growth forest, secondary, and shade plantations of coffee and cocoa, from sea-level to high elevations (Christy and Clarke Reference Christy and Clarke1998, Jones and Tye Reference Jones and Tye2005).
In this paper, we present the first analyses of the abundance and distribution of all forest pigeons in São Tomé. We analyse: (a) the influence of environmental parameters on the distribution of pigeons; (b) whether fruiting patterns of trees affect their distribution; (c) the phenology of distribution patterns; and (d) whether hunting pressure influences abundance.
Methods
Study area
São Tomé (857 km2) is the larger of the two oceanic islands that constitute the República Democrática de São Tomé and Principe (Fig. 1). The island, located within the tropical belt, is about 250 km from the African coast. Annual rainfall ranges from less than 1,000 mm in the north-east to more than 6,000 mm in the south-west, and is marked by two main seasons: the rainy season between September and May and the dry season (or gravana) between June and August (Jones and Tye Reference Jones and Tye2005). Mean annual temperatures vary between a minimum of 18° to 21°C to a maximum of 30° to 33°C, with little seasonal variation and high humidity all year (Carvalho et al. Reference Carvalho, de Oliveira and Vaz2004). The island is characterised by rough relief, with numerous steep mountains and a maximum altitude of 2,024m at the Pico de São Tomé.

Figure 1. Map of São Tomé showing locations of the sampled transects. Elevation is shown as contour lines.
With no human presence before the Portuguese arrived in 1470, the original vegetation on the island comprised tropical lowland and montane forests on the wetter side of the island, and mossy forest at the highest altitudes (Jones and Tye Reference Jones and Tye2005). Native forest is distributed along a gradient from lower to higher altitudes (Fig. 1) with smaller trees (dbh < 2 m) predominating at the highest altitudes. A drier forest type, present in the north-east of the island, includes many deciduous trees, and is subject to frequent fires (Leventis and Olmos Reference Leventis and Olmos2009). This area has been extensively cleared for farmland and estates and much of it has been turned into an anthropogenic savanna.
Although some forest areas have been heavily modified in the past for planting cocoa and coffee, there has been significant regeneration to secondary forest since the time of independence in 1975 (Oliveira Reference Oliveira2002, Carvalho et al. Reference Carvalho, de Oliveira and Vaz2004). One third of the island, comprising areas of remaining lowland and mountain forests, was declared a protected area (Parque Natural do Obô) though there is little or no enforcement (Albuquerque and Cesarini Reference Albuquerque and Cesarini2009). Hunting legislation has been drafted since 1995, but is still to be promulgated.
The human population of the island is estimated at around 187,000 people (c.212 inhabitants/km2; INESTP 2012) and growth rate is 2.0% (CIA 2012). About 60% of the inhabitants of the island live in the capital and adjacent districts. Poverty levels are high, with 34.5% of the population affected (Alkire et al. Reference Alkire, Roche, Santos and Seth2011). Infrastructure is generally poor.
Sampled habitats
We sampled pigeon populations within four broad habitat types, ranging from least disturbed formations (old-growth forest), through secondary forest to shade plantations and non-forested habitats (INTERFOREST 1990, Salgueiro and Carvalho Reference Salgueiro and Carvalho2007). Old-growth forests are areas of natural vegetation, often remaining in inaccessible parts of the island, which may contain some introduced and human-favoured species (e.g. Bambusa vulgaris, Musanga cecropioides, Persea americana, Chinchona sp.). Secondary forests, derived from the cutting of old-growth forests, contain a large proportion of introduced species, but no cultivated plant species (Carvalho et al. Reference Carvalho, de Oliveira and Vaz2004). In contrast, shade plantation forests are characterised by the presence of coffee or cocoa, and a variable canopy cover of native and introduced species. Non-forested areas included in the sampling are mainly savannas but also include palm stands and open fields close to forests.
Bird surveys
We surveyed pigeons along 15 linear transects (total 35 km) in selected habitats (Fig. 1). Due to the difficult terrain, most transects followed existing trails. We walked five main (5-km long) transects; the maximum distance that could be sampled in one day in rough terrain without compromising census quality (Bibby et al. Reference Bibby, Marsden and Fielding2000). We also selected 10 shorter (1-km long) transects to increase sampling effort in habitats that were poorly represented within the main transects. All 15 transects were divided into 100-m sectors, within which we recorded habitat type (old-growth, secondary, shade or non-forested), altitude (measured with a GPS), and trail typology (forest trail, well marked footpath, old dirt road). In addition, the vegetation in each sector was characterised within two (10 m x 10 m) quadrats on each side of the initial point of each sector. In each quadrat we counted the number of trees (small: 30 cm to 2 m dbh, and large: > 2m dbh), estimated canopy height and cover, as well as grass and shrub cover (Bibby et al. Reference Bibby, Marsden and Fielding2000, Buckland et al. Reference Buckland, Marsden and Green2008). All estimates were conducted by M. C. With the support of local assistants, tree species with fruits consumed by our study species were counted along a 20 m wide band along each transect. Fruiting status of all counted trees was documented from presence and ripeness of fruits present.
Bird censuses were undertaken by two trained observers (G. S. and Aristides Santana) during early mornings (between 05h00 and 11h00). Transects were walked at a slow pace (1 km/hour) and repeated twice, in January and June 2010 (rainy and dry season, respectively). Pigeons seen or heard were recorded, except for individuals observed flying over the transects. Coordinates for each transect, the main habitat sections and number of birds recorded in each season are shown in Appendix S1 in the online supplementary materials.
Human presence and hunting pressure
To analyse the effect of anthropogenic factors on pigeons, an index of potential hunting pressure within each 500 m transect sector was calculated as the average of site accessibility, human population, and signs of hunting activity. Site accessibility was estimated as the mean distance from the sector to the nearest road according to three distance classes (1: > 5 km, 2: 2.5–5 km, 3: < 2.5 km) and another parameter representing access type (1: lightly marked forest trail, 2: well-trodden path, 3: old road). The human population within a 5-km radius was obtained from the 2001 and 2012 population censuses (INESTP 2012) and expressed as 1: < 350 persons, 2: 351–700 persons), and 3: > 700 persons. Signs of hunting activity (hunters, cartridges, piles of feathers, traps, and temporary hunting camps) were recorded during habitat surveys and bird counts as 1 (up to one sign) to 3 (more than 3 signs).
Fruit availability index
To analyse the effect of fruit availability on pigeon distribution and abundance patterns, we derived a seasonal fruit availability index for the three canopy-living species: São Tomé Green Pigeon, Bronze-naped Pigeon and Maroon Pigeon. The index was not applicable to the Lemon Dove as this species usually feeds on the ground and its diet is poorly known.
First, we identified 10 fruit-bearing tree species as important in the diets of the three canopy-living pigeons (Table 1), from observations in the field, suggestions from local informants, and available literature (Christy and Clarke Reference Christy and Clarke1998, Jones and Tye Reference Jones and Tye2005). We then assessed the seasonal fruiting pattern of each tree species. In the transect areas, we selected 10 individuals of each tree species to monitor. Each individual was visited twice in each season (January–February, June–July), and its fruiting status classified in three levels: 0 - no fruits, 1 – few fruits and/or raw, 2 – many fruits and ripe. We derived a seasonal fruiting index for each tree species (rainy season/dry season) by averaging the values obtained for the 10 individual trees monitored.
Table 1. Fruiting trees identified as relevant food sources for use in fruit availability indexes built for three species of pigeons: São Tomé Green Pigeon, Bronze-naped Pigeon and Maroon Pigeon. The observed fruiting refers to average number of fruits and their ripeness recorded in all identified trees located at sampled transects, and varies between 0 (no fruits or raw) and 2 (many ripe fruits).
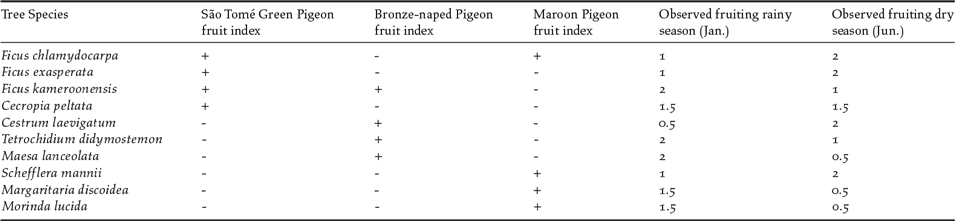
We then calculated a fruit availability index in each sampling sector for each bird species, per season, To achieve this, we multiplied the number of trees of each identified species in each sampling sector and by the estimated seasonal index of fruiting for that sector in each season. For each pigeon species and respective diet we estimated the fruit availability index by summing the values obtained for the four main species of trees of diet (Table 1) in every single sector.
Analysis
We used ordination techniques to develop an effective visual summary of site-specific changes in forest type structure, including all samples in the same ordination space (Borcard et al. Reference Borcard, Gillet and Legendre2011). We employed non-metric multidimensional scaling (NMDS) ordination based on Euclidean dissimilarities of standardised environmental variables. This procedure is considered robust for site-data analyses and is able to recover compositional dimensions associated with the underlying environmental gradients (Minchin Reference Minchin1987). To test the null hypothesis that habitat structure was similar in different forest types, a nonparametric analysis of similarity (ANOSIM) was conducted on the raw environmental data (Borcard et al. Reference Borcard, Gillet and Legendre2011). Envfit function was performed to test for the significance of sampled variables and its relation to the ordination axes (Borcard et al. Reference Borcard, Gillet and Legendre2011). These analyses were also used to select significant variables for the subsequent application of the statistical models.
An initial exploratory analysis was performed using the bird count data to determine if there were differences in abundance between habitats and seasons, for each species. General Linear Regression Models (GLM) were subsequently used to examine how canopy cover, number of large trees, habitat (old-growth, secondary, shade or non-forested), season (rainy or dry), indices of potential hunting pressure and fruit availability (for São Tomé Green Pigeon, Bronze-naped Pigeon and Maroon Pigeon) affected presence and abundance of each of the study species (Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009, Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). To identify which variables best explained the abundance of each pigeon species, we used model selection based on Akaike’s information criterion corrected for small samples sizes (AICc; Burnham and Anderson Reference Burnham and Anderson2002, Barlow et al. Reference Barlow, Louzada, Parry, Hernández, Hawes, Peres, Vaz-de-Mello and Gardner2010). We used the ‘dredge’ function from the ‘MuMIn’ package to test models defined by all possible variable combinations and rank them by their AICc-based model weight (Burnham and Anderson Reference Burnham and Anderson2002). All statistical procedures were carried out in R v. 2.10.0 (R Development Core Team 2009).
Results
Habitat structure
Variation in environmental variables among the sampled sectors was summarised using a non-metric multidimensional scaling ordination (NMDS). This graphic summary (Figure 2) shows that the greatest environmental contrasts in the study area are the different forest types, which to a great extent reflect the degree of habitat disturbance by humans. In fact, the first ordination axis (Stress = 0.18, ANOSIM statistics R = 0.54, P = 0.001) represented a transition in habitat structure and disturbance levels from native forest (on the left of the plot, with dense canopy cover) to increasingly open woodland types (Figure 2). The second axis indicated a gradient from forest with large trees and high canopies (at the top of the plot, usually shade forest, often abandoned) to a lower forest type with denser undergrowth (usually younger and regenerating secondary forest).

Figure 2. Two dimensional non-metric multidimensional-scaling ordination of transect sectors (as points; n = 347) based on nine environmental variables: habitat type, altitude (alt), % canopy cover (ccover), canopy height (cheight), slope (slope), number of large trees (ltrees), number of small trees (strees), % shrub cover (bush), % grass cover (herb) (Stress = 0.18). The diagram symbolises the sectors’ compositional dimensions associated with assessed environmental gradients and general habitat typology. Each habitat type is represented by a distinct symbol. Fitted environmental variables are represented as arrows, and correspond to their input for the ordination axes, both in direction and length of the arrow.
The two variables which explained most of the variance along the two axes were canopy cover (NMDS1) and canopy height (NMDS2). The ANOSIM analysis of similarities indicated that the habitat types have statistically distinct environmental characteristics (R = 0.54, P = 0.001).
Species abundance and habitat use
The most common pigeon species counted on all transects was the Lemon Dove; a total of 759 birds observed (389 in the rainy season, 370 in the dry season) in 44.4% of the 350 sampling units. The species was widespread in all forested habitats (from old-growth to shade forests) and a considerably lower presence in non-forested sampled sectors (Figure 3). São Tomé Green Pigeons were recorded in fewer sectors (17.3%) and were found at lower numbers than the Lemon Dove (394 individuals, 144 in the rainy season and 250 in the dry season). São Tomé Green Pigeons were observed mainly in mature forested habitats (old-growth and older secondary); non-forested areas were not important for this species (Fig. 3). In contrast, the Bronze-naped Pigeon was abundant in human-altered habitats, including shade forests and non-forested areas (such as savanna). This species was observed in 37.8% of sectors; 830 individuals (643 in the rainy season, 187 in the dry season). The species was observed significantly less during the dry season (Mann-Whitney U-test, V = 17,570, P < 0.001) (Fig. 3). The Maroon Pigeon was the rarest species, being observed mainly in old-growth forests and some isolated individuals in secondary growth and non-forested areas. This species was observed in just 0.5 % of sectors, totalling 37 individuals (Fig 3). The abundance of all species varied significantly among the four sampled habitat types (Kruskal-Wallis test, P < 0.05). Habitat use during the wet and dry seasons for each pigeon species is shown in separate ordination diagrams in Figure 4.
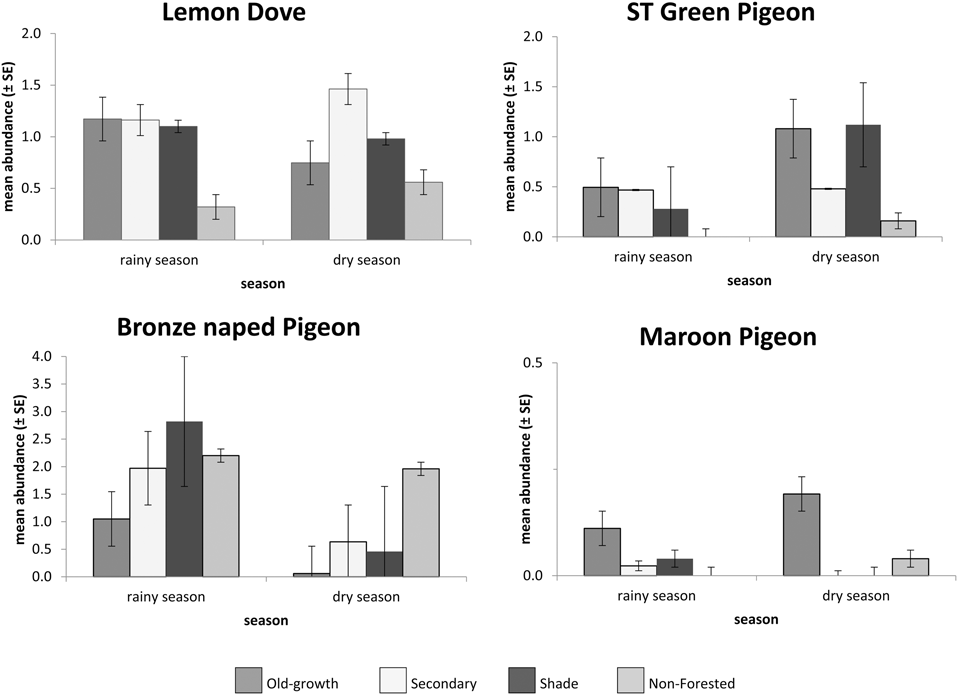
Figure 3. Mean abundance per sector of all four pigeon species in sampled habitats per sampling season: old-growth (n = 99), secondary (n = 171) and shade (n = 56) forests, and non-forested areas (n = 24).
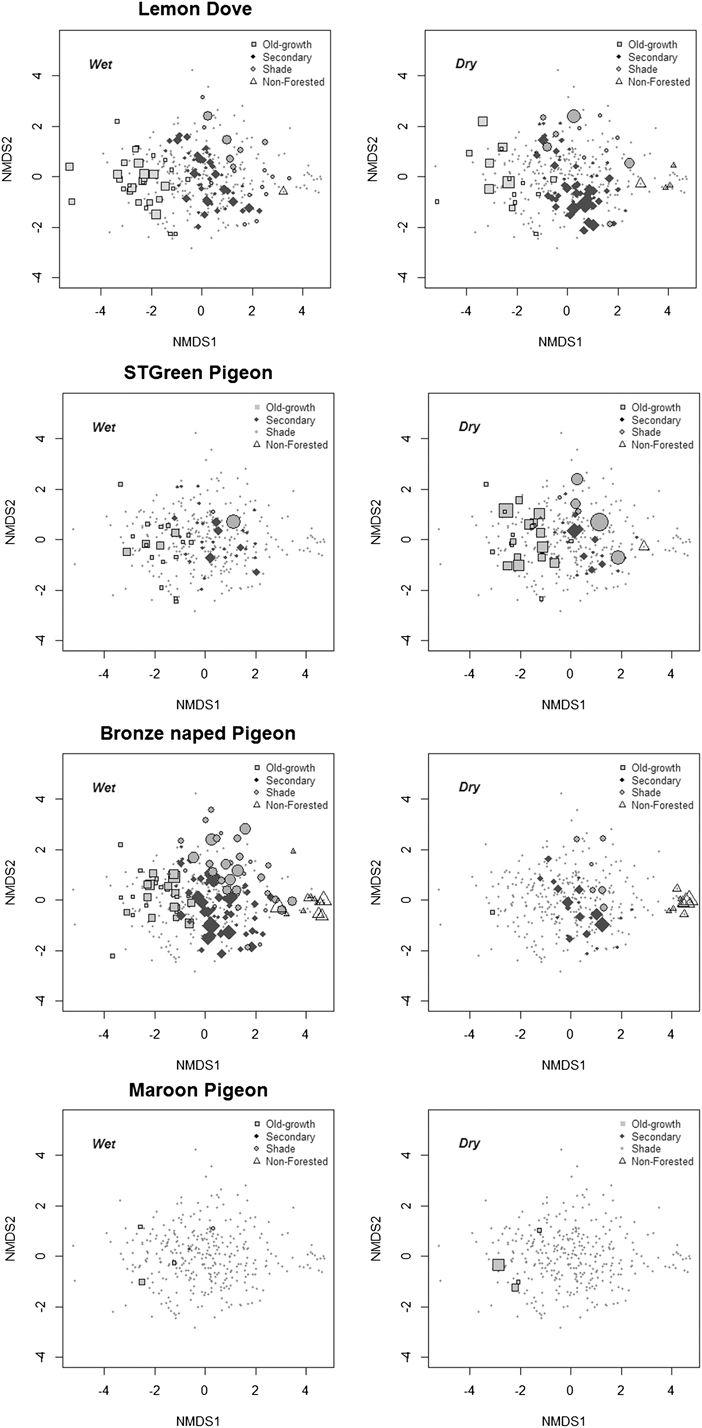
Figure 4. Use of ecological space by the four species of pigeons in rainy and dry seasons, using as a reference the transect sectors’ ordination, as in Fig. 2. Small dots are sampled sectors and symbols indicate the sectors where each pigeon species was observed. The size of the symbols reflects pigeon abundance, and its shape and colour the different habitats (as in Fig. 2).
Lemon Dove numbers were similar during both seasons, but frequencies per habitat varied during the dry season; Lemon Doves tended to concentrate in old-growth, shade and particularly in young or regenerating secondary forests (characterised by a lower tree canopy and denser undergrowth and shrub cover).
São Tomé Green Pigeons underutilised degraded habitats and young or regenerating forests, and were more common overall in more mature forests with higher canopies and greater canopy cover, during both seasons. They were seasonally present in shade forests, concentrating at this habitat during early dry season.
The Bronze-naped Pigeon was more abundant in lower altitude forests with denser shrub cover than in forest with high trees. It was not present in high-altitude forests. During the rainy season it was more common in lower, old-growth forests. Its distribution in shade forests is seasonal, as it uses this habitat type more extensively during the rainy season. It was observed in non-forested habitats in both seasons, but more in the rainy season.
The Maroon Pigeon data suggest a greater concentration in highland forests during the early dry season, with groups of up to 10 individuals being noted during this period. However, the species was also observed in low altitude old-growth forest, secondary forest and in a non-forested sector, mostly during the wet season, in pairs or as isolated individuals.
Factors associated with species abundance and distribution
Results of associations between environmental variables and pigeon abundance and distribution for the four São Tomé pigeon species are given in Table 2. Presence and abundance of the Lemon Dove were associated with habitat, especially secondary forest during the dry season (coef = 0.69, P = 0.03). Potential hunting pressure was also negatively correlated with species presence and abundance, significant during the rainy season (coef = -2.06, P = 0.05). Presence and abundance of the São Tomé Green Pigeon were significantly correlated with hunting pressure during both seasons (coef = -0.72, P < 0.001). Fruit availability was positively related to abundance of this species (coef = 0.05, P = 0.02). For the Bronze-naped Pigeon, season was linked to the distribution and abundance of the species (coef = -1.33, P < 0.001). There was a negative relation with altitude (coef = -0.001, P < 0.001) and canopy cover (coef = -0.01, P = 0.04), but a positive regression with canopy height (coef = 0.02, P = 0.05), associated with its seasonal use of shade forests. Species abundance was positively correlated with secondary forest (coef = 0.54, P = 0.02); this effect was stronger during the dry season (coef = 2.54, P < 0.001). Bronze-naped Pigeon abundance was not correlated with fruit availability although close to significance (coef = 0.028, P = 0.06), as this species tends to congregate in the available fruiting trees in closed forests. This effect is significant for the rainy season (coef = 0.040, P = 0.013).
Table 2. Results of the GLM models with significant variables selected by the best model and model averaging of top performing models (using dredge, delta < 2; RI – relative importance of the variable for best regression models). Significant values are in bold.
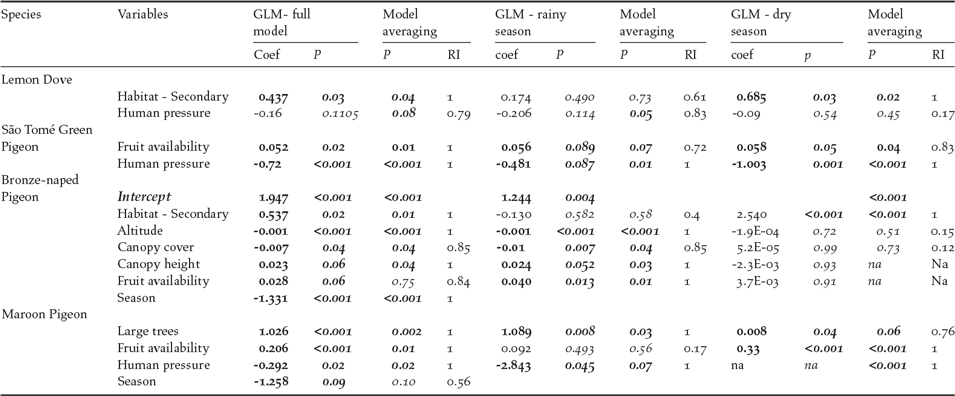
The number of large trees (coef = 1.03, P < 0.001), and fruit availability, particularly during the dry season (coef = 0.33, P < 0.001), were positively correlated with distribution and abundance of the maroon pigeon, while with human pressure the correlation is negative (coef = -2.92, P = 0.017).
Potential hunting pressure is negatively correlated with the abundance of three of the four species of pigeons (Fig. 5). The abundance of the Lemon Dove, São Tomé Green Pigeon and the Maroon Pigeon declined with increasing potential hunting pressure. This effect was not observed for the Bronze-naped Pigeon.
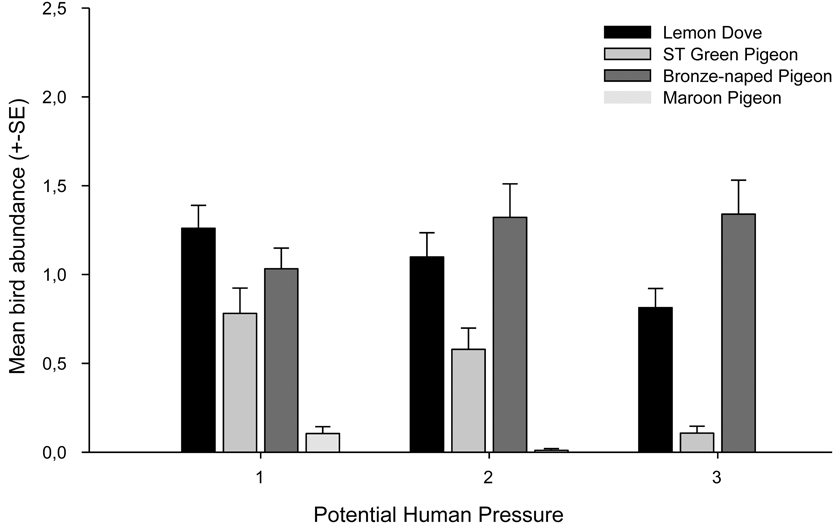
Figure 5. Average relative abundance of all four pigeon species in sites with light, medium or high potential human pressure.
Discussion
Distribution and abundance
The observed patterns of distribution and abundance of the four species of pigeons on São Tomé reflect habitat structure, food availability and hunting pressure, with a marked seasonal effect in most cases. Secondary forests were significantly associated with the distribution of at least three of the studied pigeon species. This was important for the Lemon Dove and Bronze-naped Pigeon, but we also found a significant association for the São Tomé Green Pigeon. Secondary forest is presently the most abundant forest type in São Tomé, but also the one most threatened by deforestation (selectively or intensively) and land-use intensification.
Old-growth forests found at higher altitudes seem to support a higher abundance of Maroon Pigeon and São Tomé Green Pigeon. However, these species’ association with these habitats may be the result of loss of populations of these species from lowland forests. In fact, both species were once noted as common and widespread, even within the coastal areas and Rolas Islet (Jones and Tye Reference Jones and Tye2005). Remote old-growth forests now probably constitute the core habitat for these pigeons because of their inaccessibility to bird hunters.
At lower altitudes, shade forests with high canopies as well as non-forested open habitats (mainly savanna) are linked to relatively large populations of the Bronze-naped Pigeon. However, its commonness in these human-altered areas makes the species more susceptible to hunting, so the regulation of this activity is vitally important.
Importance of fruit availability
Fruit availability in sampling sectors was identified as a significant correlate of the distribution and abundance of the Maroon Pigeon, São Tomé Green Pigeon and Bronze-naped Pigeon. As in other studies, forest pigeons were observed to respond to spatial differences in fruit abundance within fragments and habitats (Lambert Reference Lambert1989, Levey Reference Levey1988, Strong and Johnson Reference Strong and Johnson2001, Oliveira et al. Reference Oliveira, Menezes, Jones and Nogales2006). Although fruit availability was shown to affect bird distribution and abundance patterns, relating seasonal bird movements and fruit availability will require more research to clarify this point. Our study may have been limited by the fact that we did not monitor all species of fruiting tree species, but the four species recorded in our study represented significant food items for pigeons. This is so because São Tomé pigeons, like other frugivorous Columbidae, are opportunistic feeders that consume a large variety of fruits when available (e.g. Bancroft et al. Reference Bancroft, Bowman and Sawicki2000, Oliveira et al. Reference Oliveira, Menezes, Jones and Nogales2006, Walker Reference Walker2007b).
As shown in previous studies, the highest tree fruit diversity and abundance was typical of secondary and shade forests (e.g. Levey Reference Levey1988, DeWalt et al. Reference DeWalt, Maliakal and Denslow2003, Hart et al. Reference Hart, Grieve and Downs2013). In these habitats, a number of pioneer and transitional forest trees such as Musanga cecropioides and several Ficus species, as well as the introduced South American Cecropia peltata, are found. Fruits from all these tree species are important for canopy-living pigeons (Levey Reference Levey1988, Carvalho et al. Reference Carvalho, de Oliveira and Vaz2004). Figs, even though low in lipids and proteins, are very abundant seasonally and represent a large part of the diets of many tropical pigeons, up to 50% for Treron species in continental Africa (Lambert Reference Lambert1989, Walker Reference Walker2007b, Devi and Saikia Reference Devi and Saikia2012).
Old-growth forests generally have lower fruit availability than secondary forests, though this may be relatively constant throughout the year (Levey Reference Levey1988). Asynchronous fruiting patterns of the various tree species found in the different habitats ensure year-round food resources for the pigeons. Although we are certain that the São Tomé pigeons forage in a variety of habitats, information on this is limited. Further research is required to clarify these birds’ dietary patterns and their role as dispersers. Moreover, detailed studies of fruiting tree phenology and distribution will assist conservation managers on the island to ensure the long-term survival of its forests and biodiversity.
Impact of hunting
We show that hunting pressure is negatively correlated with the distribution and abundance of three pigeon species; Lemon Dove, Maroon Pigeon and São Tomé Green Pigeon. Although the Lemon Dove is not considered a game species on the island, we found that its presence and abundance were negatively correlated with potential hunting pressure. Children and rural inhabitants opportunistically take the species and even this infrequent human pressure may explain the correlation, although this needs further study. In contrast, there is evidence to support that hunting of the Bronze-naped Pigeon, São Tomé Green Pigeon and Maroon Pigeon is heavy (and may be increasing) given the considerable commercial value these species have in bars and restaurants in the main cities (Carvalho et al. Reference Carvalho, Palmeirim, Rego, Sole, Santana and Fa2014). The relatively low abundance and restricted range of the Maroon Pigeon and São Tomé Green Pigeon may be a function of hunting intensity, since both species have been intensively hunted for decades or even centuries (e.g. Bannerman Reference Bannerman1931, Snow Reference Snow1950, Jones and Tye Reference Jones and Tye2005). However, differences in each species’ reproductive rate and niche width may also explain the disparities observed in their ecological densities on the island. The Bronze-naped Pigeon, for example, despite heavy hunting pressure is more abundant, presumably because it has adapted to utilising human-altered habitats and a wider range of resources than the other species. In general, commercial hunting is a real threat to pigeons and other bird species on the island; they are relatively tame and easy to shoot at close range, which has enabled hunters to take large numbers of individuals in a short space of time (Carvalho et al. Reference Carvalho, Palmeirim, Rego, Sole, Santana and Fa2014).
Conservation measures
Previous studies on São Tomé have highlighted that human-modified habitats such as secondary forests and shade plantations are important as reservoirs for a significant numbers of the island’s endemics (e.g. de Lima et al. Reference De Lima, Dallimer, Atkinson and Barlow2012, Rocha Reference Rocha2008). In our study we also confirm this to be the case for most of the island’s endemic pigeons, at least seasonally. Although there is still much forest left on São Tomé, the fast growing human population and consequent urbanisation may change this; plans for infrastructure development and an intensifying agricultural pressure are ongoing (Dallimer et al. Reference Dallimer, King and Atkinson2009, Klueh et al. Reference Klueh, Pastor, Segura and Zarate2007, UNDP 2006). Combined with factors such as invasive species associated with human settlement, as well as increased illegal logging and exploitation of other natural resources, most endemic birds and particularly hunted pigeons are likely to be at increasing risk of extinction.
In our study we confirm that habitats and hunting pressure explain pigeon abundance and must be used to drive conservation actions for these species. The São Tomé Green Pigeon and the Maroon Pigeon are likely to be less threatened because of their association with the more inaccessible old-growth forests, though improved roads and continuous opening of agricultural fields are of major concern. Most pigeon hunting is conducted by commercial urban hunters coming from main towns to forest fringes (Carvalho et al. Reference Carvalho, Palmeirim, Rego, Sole, Santana and Fa2014), and increased accessibility of well-preserved forests will surely intensify hunting pressure over both pigeons. For these species, adequate habitat protection and the strong application of hunting restrictions within the national park are necessary, since the main habitats for these species are here.
The protection of the Bronze-naped Pigeon may be more problematic given that it is most often found in human-altered habitats, particularly in the dry season. This pigeon is known to use agricultural land where it feeds on the abundant fruits of the shrub Cestrum laevigatum along hedgerows. More research is needed on the drivers of seasonal movements of this species and its use of native and altered habitats.
All three exploited species are known to have declined in numbers during the last few decades and the main reason seems to be excessive hunting (Carvalho et al. Reference Carvalho, Palmeirim, Rego, Sole, Santana and Fa2014). Thus, enforcement of strong hunting regulations perhaps through the definition of restricted areas and periods for hunting, specific quotas for the more abundant species and the total ban of species such as the Maroon Pigeon would alleviate the current situation. A combination of hunting control and habitat preservation has resulted in the recovery of other forest pigeon species (e.g. Pierce et al. Reference Pierce, Atkinson and Smith1993, Oliveira et al. Reference Oliveira, Menezes, Jones and Nogales2006).
Supporting the conservation authorities on the island to implement the existing hunting legislation and enforce protection should also be accompanied by more targeted environmental education and awareness-raising among all islanders. São Tomé hunters and game consumers are not aware of the difference between endemic, native or exotic species, or the conservation problems resulting from overhunting. A main element of this strategy must be to reduce demand by consumers in town and city restaurants. Hunters, and particularly bird hunters, should also be involved in conservation efforts, both by capturing their knowledge on the species and as a critical point for the definition of livelihood alternatives which can ensure pigeon conservation.
Supplementary Material
The supplementary materials for this article can be found at journals.cambridge.org/bci
Acknowledgements
We particularly express gratitude to Eng. Arlindo Carvalho, the General Director for the Environment, and to Dr Vitor Bonfim and Eng. Salvador Pontes from the Direction for Conservation, for local support and integration in São Tomé. The Associação Monte Pico, particularly Luis Mário Almeida, provided technical support and advice. We warmly thank all the São Tomé citizens who facilitated field work, either by providing initial guidance, helping with overnight stays, meals and/or valuable company. This study was conducted within the scope of the project PTDC/BIA-BIC/115223/2009, a PhD grant SFRH / BD / 30171 / 2006 and a Post-doc grant SFRH/ BPD/ 91494/ 2012, all funded by the Portuguese Government (Foundation for Science and Technology - FCT/MCTES). M. C. was also supported by a Rufford Small Grant for Conservation of Nature.









